镧系锑硒化合物[Ln(en)4]SbSe4·0.5en(Ln=Dy,Ho)的溶剂热合成与晶体结构
王 娇 潘迎利 陈江芳 张 勇 顾建胜 贾定先*,
(1苏州大学材料与化学化工学部,苏州 215123)(2苏州大学分析测试中心,苏州 215123)
王 娇1潘迎利1陈江芳1张 勇2顾建胜1贾定先*,1
(1苏州大学材料与化学化工学部,苏州 215123)(2苏州大学分析测试中心,苏州 215123)
本文研究了Ln2O3/Sb/Se/en溶剂热反应体系,合成了2个镧系锑硒化合物[Ln(en)4]SbSe4·0.5en[Ln=Dy(1),Ho(2)],用元素分析和红外光谱对化合物进行了表征,并用X-射线单晶衍射测定了化合物的单晶结构,两者都属于单斜晶系,P21/n空间群。结构对比研究发现,在en溶剂中,SbSe43-离子可以与半径较大的La3+和Nd3+离子配位,而不与半径较小的Dy3+和Ho3+离子配位,可见镧系收缩效应对SbSe43-离子与镧系金属离子Ln3+的配位有重要影响。
溶剂热合成;镧系配合物;锑硒化合物;晶体结构
0 Introduction
Lanthanide chalcogenides have attracted increased attention in recent years because of their promising photo-,thermo-and electroluminescence,magnetic and nonlinear optical properties[1-3].These compounds are generally prepared by the flux method at high temperature[4].Recently,the mild solvothermal synthesis,in which the reaction is performed in the presence of a structure-directing agent such as an organic amine,has been proven to be a versatile route to the preparation of chalcogenides containing such Main Group elements as germanium,tin,arsenic,and antimony[5-7].When transition metal(TM)ions were introduced into the amine solution,the transition metal complex cations formed in situ act as space fillers and/or charge compensating ions in the chalcogenometalate compounds and the ternary chalcogenometalates containing transition metals are obtained[8-12].
Recently,we have developed a solvothermal route to the preparation of lanthanide chalcogenometalates in the light of the solvothermal synthesis of above ternary transition metal chalcogenometalates in amine solution.We introduced lanthanide metals into the solvothermal synthetic system Sb/S/en,and had successfully synthesized two series of lanthanide thioantimonates[Ln(en)3(H2O)x(μ3-x-SbS4)](x=0,1;Ln=La,Nd,Sm)and[Ln(en)4]SbS4·0.5en(Ln=Eu,Dy,Yb)[13-14].The structural types of these lanthanide thioantimonates are related with the entity of the lanthanideseries,and the structure dividing point locates at the samariumion[14].Recently,we extended the solvothermal method to the synthesis of lanthanide selenidoantimonates,and the novel compounds[Ln(en)4(SbSe4)](Ln=La,Nd)and[Sm(en)4]SbSe4·0.5en were first prepared by the solvothermal route in en[15].Now we study on the synthetic system Ln2O3/Sb/Se/en under solvothermal conditions in detail to investigate the relationship between the structural feature of the lanthanide selenidometalates and the entity of lanthanideions across the lanthanide series,and two new lanthanide selenidoantimonates[Dy(en)4]SbSe4·0.5en and [Ho(en)4]SbSe4·0.5en were prepared from the synthetic system.
1 Experimental
1.1 Materials and instruments
All reagents were obtained commercially and used without further purification.Elemental analysis was conducted on a MOD 1106 elemental analyzer.FTIR spectra were recorded with a Nicolet Magna-IR 550 spectrometer in dry KBr discs in the 4 000~400 cm-1range.
1.2 Synthesis
Compound 1 was synthesized by a solvothermal reaction of Dy2O3(187 mg,0.5 mmol),Sb (122 mg,1 mmol),and Se (316 mg,4 mmol)in 6 mL en.The reactantmixture wasloaded into a Teflon-lined stainless steel autoclave with an inner volume of 15 mL,and then the sealed autoclave was heated under autogenous pressure at 160 ℃ for 6 d.Upon cooling to ambient temperature,orange block crystals of 1 were obtained in ca.48%yield (based on Sb)and stored under a vacuum.Anal.Calcd.for C9H36N9Se4DySb(%):C,12.42;H,4.17;N,14.48.Found(%):C,12.24;H,4.06;N,14.31.IR data (KBr pellet,cm-1):3 328vs,3218vs,3088vs,2871vs,1582s,1571vs,1490s,1380 s,1328s,1156m,972s,826m,781m,589m,and 480m.Chip crystals of 2 were prepared with a procedure similar to the synthesis of 1 except that Ho2O3was used instead of Dy2O3with 56%yield (based on Sb).Anal.Calcd.for C9H36N9Se4HoSb(%):C,12.38;H,4.16;N,14.44.Found(%):C,12.25;H,4.02;N,14.28.IR data(KBr pellet,cm-1):3303vs,3282s,3245s,3132s,2930 vs,2 882s,1 570vs,1 511s,1 386m,1 331s,1 007s,866w,814w,776w,662w,498m.
1.3 X-ray crystallography
The intensity data were collected on a Rigaku Mercury CCD diffractometer at 293(2)K using graphite-monochromated Mo Kα radiation (λ =0.071 073 nm)with a ω-scan method to a maximum 2θ value of 50.70°for 1 and 2.An absorption correction was applied for all the compounds using multi-scan method.The structures were solved with direct methods using the SHELXS-97 program[16]and refinement was performed against F2using the SHELXL-97 program[17].All the non-hydrogen atoms were refined anisotropically.The hydrogen atoms were positioned with idealized geometry and refined with fixed isotropic displacement parameters using a riding model.Technical details of the data collection and refinement are summarized in Table 1.CCDC:777749,1;777750,2.

Table 1 Crystallographic data collection and structure refinement details for 1 and 2
2 Result and discussion
2.1 Synthesis
Compounds 1 and 2 were synthesized with a onepot process by the reaction of Dy2O3(or Ho2O3),Sb and Se in en at 160℃.In the solvothermal reaction,the lanthanideoxides Ln2O3(Ln=Dy,Ho)were transferred to lanthanidecomplex cations[Ln(en)4]3+to act as counter ions of the SbSe43-anions.When en-H2O mixed solution was used instead of en in the synthesis,an unknown amorphous grey powder was obtained.It is well known that lanthanideions are typically hard acid and preferentially hydrolyze to form hydroxyl precipitate in basic solution.So the waterless en is needed in the solvothermal synthesis to avoid the hydrolysis of lanthanideion.
2.2 Crystal structure
Compounds 1 and 2 are isostructural crystallizing in the monoclinic space group P21/n with four formula units.They are composed of isolated[Ln(en)4]3+(Ln=Dy,Ho)and SbSe43-ions and free en molecule.The crystal structures of 1 and 2 are depicted in Fig.1.The atom C(8)of 1 and 2 are disordered(Fig.1)and the occupancies of disordered C/C′are assigned as 57%and 43%for 1,and 59%and 41%for 2.The lanthanideion is coordinated by four bidentate en ligands,and the coordination geometry can be described as a distorted bicapped trigonal prism,with three nitrogen atoms N(1),N(7),and N(8)forming one face,and N(4),N(5),and N(6)forming the opposite face.The capping positions are occupied by N(2)and N(3)atoms form DyN8(Fig.1(a)).The Ln-N bond lengths range from 0.249 3(5)to 0.254 2(6)nm for Dy-N,and from 0.246 1(11)to 0.2518(11)nm for Ho-N(Table 2).Both bond lengths are consistent with those in the reported dysprosiumand holmiumcomplex with amino ligand respectiv-ely[14,18].The tetraselenidoantimonate SbSe43-anion can be described as a distorted tetrahedron,as evidenced by the Se-Sb-Se angles ranging from 105.32(3)°to 113.96(3)°for 1,and from 105.11(6)°to 114.03(7)°for 2.The Sb-Se bond lengths are between 0.24525(8)and 0.24768(8)nm for 1,and 0.24545(17)and 0.24777(16)nm for 2(Table 2).Both bond lengths and angles are in the range of those observed in other compounds containing the SbSe43-tetrahedral anion[15,19-20].
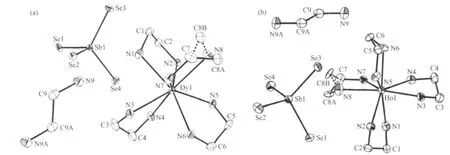
Fig.1 Crystal structures of 1(a)and 2(b)with the labelling scheme

Table 2 Selected bond lengths(nm)and angles(°)of compounds 1 and 2
Continued Table 2
In 1,all Se atoms of SbSe43-anion are involved in hydrogen bonding with NH2groups of en ligands.Each of the SbSe43-anion contacts with four[Dy(en)4]3+cations and two en molecules forming N-H…Se hydrogen bonds with N…Se lengths ranging from 0.3404(6)to 0.3727(6)nm and N-H…Se angles ranging from 141.4°to 171.6°(Fig.2).Between the free en molecule and coordinated en ligand,hydrogen bond N(3)-H(3B)…N(9)is observed with N(3)…N(9)length of 0.3114(8)nm and N(3)-H(3B)…N(9)angle of 157.8°(Tabe 3).The lengths and angles of hydrogen bonds are in agreement with the values reported in literatures[15,21].The N-H…Se and N-H…N hydrogen-bonding interactions lead to a 3-dimensional network structure of[Dy(en)4]3+,SbSe43-,and en moieties(Fig.3).A similar H-bonding network is observed in compound 2(Tabe 3).
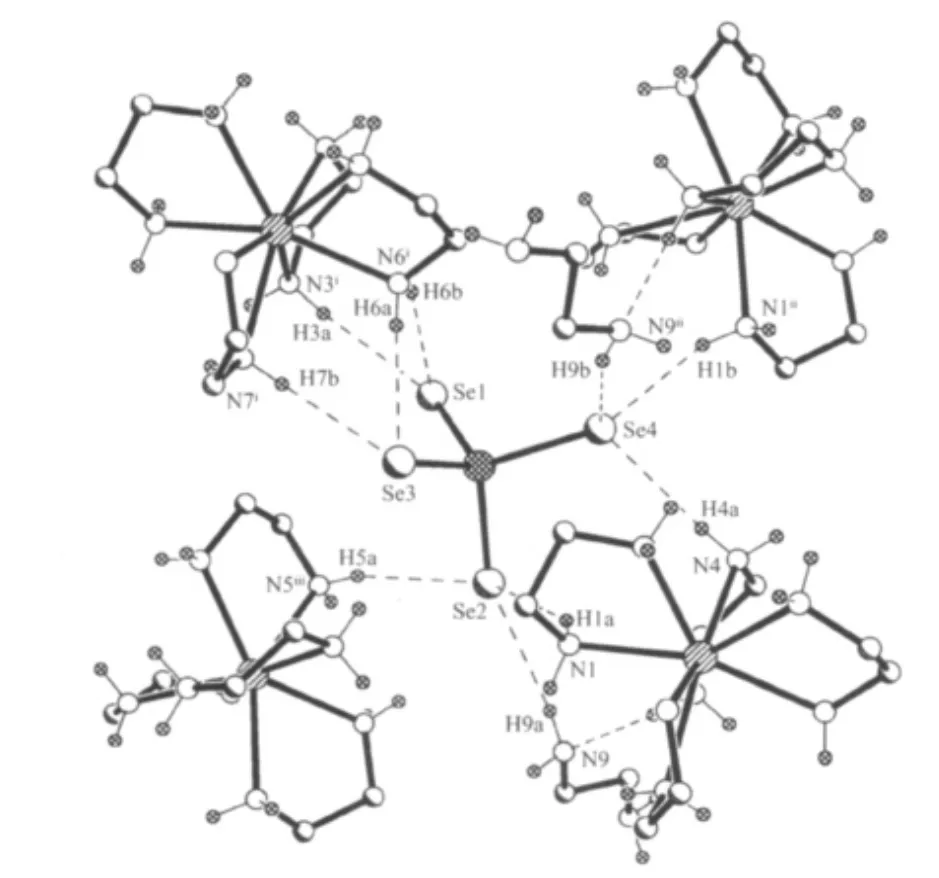
Fig.2 Environment of a SbSe43-anion in 1
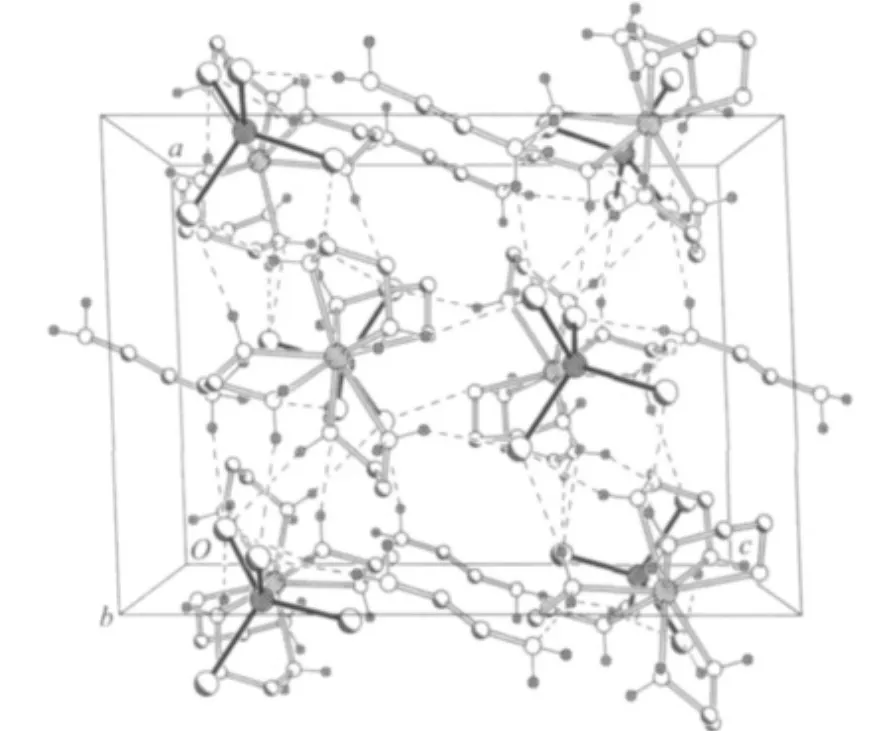
Fig.3 Crystal packing of 1 viewed along b axis
Under the same synthetic conditions,the reactions of La2O3or Nd2O3,Sb,and Se in en produced compounds[La(en)4(SbSe4)]and[Nd(en)4(SbSe4)]respectively[15].In[La(en)4(SbSe4)]and[Nd(en)4(SbSe4)],the SbSe43-ion coordinates to[La(en)4]3+and[Nd(en)4]3+ions as a monodentate ligand.The reaction of Sm2O3,Sb,and Se gave compound[Sm(en)4]SbSe4·0.5en[15]which is isostructural with 1 and 2.This structural difference can be interpreted in terms of the coordination number of lanthanideions.It is commonly observed that the lighter lanthanide ions prefer coordination number of nine and heavier ones prefer eight in solution[22].Because en is a bidentate ligand,the[La(en)4]3+and[Nd(en)4]3+ions are bonded to the monodentate ligand of SbSe43-to maintain the coordination number of nine for La3+and Nd3+,and[La(en)4(SbSe4)]and[Nd(en)4(SbSe4)]are formed.While the coordination number of Sm3+,Dy3+and Ho3+have been saturated by four en ligands,SbSe43-anion has no opportunity to coordinate to the metalcenters and the ionic compounds[Sm(en)4]SbSe4·0.5en,[Dy(en)4]SbSe4·0.5en and [Ho(en)4]SbSe4·0.5en are formed.

Table 3 Hydrogen bonding of compounds 1 and 2
[1]Choi K S,Iordanidis L,Chondroudis K,et al.Inorg.Chem.,1997,36:3804-3805
[2]Gitzendanner R L,DiSalvo F J.Inorg.Chem.,1996,35:2623-2626
[3]Mitchell K,Haynes C L,McFarland A D,et al.Inorg.Chem.,2002,4:1199-1204
[4]Mitchell K,Ibers J A.Coord.Chem.Rev.,2002,102:1929-1952
[5]Li J,Chen Z,Wang R J,et al.Coord.Chem.Rev.,1999,190-192:707-735
[6]Sheldrick W S.J.Chem.Soc.,Dalton Trans.,2000:3041-3052
[7]JIA Ding-Xian(贾定先),DAI Jie(戴 洁),ZHAO Qian-Xin(赵前信),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22(2):193-200
[8]Bensch W,Näther C,Schur M.Chem.Commun.,1997:1773-1774
[9]Stähler R,Mosel B D,Eckert H,et al.Angew.Chem.Int.Ed.,2002,41:4487-4489
[10]Vaqueiro P,Chippindale A M,Powell A V.Inorg.Chem.,2004,43:7963-7965
[11]Jia D X,Zhang Y,Zhao Q X,et al.Inorg.Chem.,2006,45:9812-9817
[12]Fu M L,Guo G C,Cai L Z,et al.Inorg.Chem.,2005,44:184-186
[13]Jia D X,Zhu Q Y,Dai J,et al.Inorg.Chem.,2005,44:819-821
[14]Jia D X,Zhao Q X,Zhang Y,et al.Inorg.Chem.,2005,44:8861-8867
[15]Jia D X,Zhao Q X,Zhang Y,et al.Eur.J.Inorg.Chem.,2006:2760-2765
[16]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[17]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[18]Fernandes A,Jaud J,Dexpert-Ghys J,et al.Polyhedron,2001,20:2385-2391
[19]Park C W,Pell M A,Ibers J A.Inorg.Chem.,1996,35:4555-4562
[20]Blachnik R,Fehlker A,Reuter H.Z.Kristallogr,2001,216:211-212
[21]Dehnen S,Zimmermann C Z.Anorg.Allg.Chem.,2002,628:2463-2469
[22]Cossy C,Barnes A C,Enderby J E,et al.J.Chem.Phys.,1989,90:3254-3259
Solvothermal Syntheses and Crystal Structures of LanthanideSelenidoantimonates[Ln(en)4]SbSe4·0.5en(Ln=Dy,Ho)
WANG Jiao1PAN Ying-Li1CHEN Jiang-Fang1ZHANG Yong2GU Jian-Sheng1JIA Ding-Xian*,1
(1College of Chemistry,Chemical Engineering and Materials Science,Suzhou University,Suzhou,Jiangsu 215123)(2Instrumental Analysis&Research Center,Suzhou University,Suzhou,Jiangsu 215123)
Organic hybrid lanthanideselenidoantimonates[Dy(en)4]SbSe4·0.5en(1)and[Ho(en)4]SbSe4·0.5en(2)(en=ethylenediamine)were synthesized under mild solvothermal conditions and characterized by elemental analysis,IR spectra and single-crystal X-ray diffraction.1 and 2 are isostructural-crystallizing in the monoclinic space group P21/n.Crystallographic data for 1:a=1.14069(14)nm,b=1.31419(16)nm,c=1.6283(2)nm,β=92.324(3)°,V=2.4390(5)nm3,Z=4,Mr=870.56,Dc=2.371 g·cm-3,F(000)=1624,the final R=0.0386 and wR=0.073 3 for observed reflections 3 874 with I>2σ(I).For 2:a=1.140 20(17)nm,b=1.314 70(18)nm,c=1.623 2(2)nm, β=92.358(4)°,V=2.431 1(6)nm3,Z=4,Mr=872.99,Dc=2.385 g·cm-3,F(000)=1 628,the final R=0.070 1 and wR=0.1219 for observed reflections 3471 with I>2σ(I).1 and 2 consist of tetrahedral anion SbSe43-,lanthanide complex cation[Ln(en)4]3+(Ln=Dy,Ho)and free en molecule.The lanthanide Ln3+ion is in an eight coordinated environment involving in eight N atoms of four en ligands forming a bicapped trigonal prism.In the crystal structure of 1 and 2,extensive N-H…Se hydrogen bonding interactions lead to a 3-dimensional network structure of the cations and anions.CCDC:777749,1;777750,2.
solvothermal synthesis;lanthanidecomplex;selenidoantimonate;crystal structure
O614.342;O614.343
A
1001-481(2010)08-1339-06
2010-01-07。收修改稿日期:2010-04-06。
国家自然科学基金资助项目(No.20771077)。
*通讯联系人。 E-mail:jiadingxian@suda.edu.cn
王 娇,女,24岁,硕士;研究方向:主族金属硫属化合物的合成及结构研究。

