醋酸甲地孕酮联合盐酸二甲双胍作为早期子宫内膜样腺癌和子宫内膜非典型增生患者保留生育功能治疗:一项前瞻性研究
关键词:子宫内膜样腺癌;盐酸二甲双胍;子宫内膜非典型增生;保留生育功能治疗;醋酸甲地孕酮;胰岛素样生长因子结合蛋白相关蛋白1
INTRODUCTION
Endometrial adenocarcinoma (EAC) is the mostcommon gynecological cancers, and 4%-14% of thepatients are below the age of 40 years at diagnosis,among whom 70% are either 1iparous or plan to havechildren[1] , amd therefore fertility preservation is animportant concern for both the physicians and thepatients. Progestin therapy is a common treatment optionfor fertility preservation in patients with grade 1 EAC oratypical endometrial hyperplasia (AEH), but whileachieving high remission rates, this therapy isassociated with a high recurrence rate[2] , and newtreatment options with low recurrence rate are urgentlyneeded.
The risk factors including obesity, diabetes, andpolycystic ovary syndrome (PCOS) all contributes to theoccurrence of type 1 EAC, suggesting a possibleassociation of an increased level of insulin resistancewith the risk of EAC development. Insulin resistance isprimarily caused by post-insulin receptor signalingdisorder involving two main signaling pathways, namelythe phosphatidylinositol 3-kinase (PI3K) pathway andthe Ras-mitogen-activated protein kinase (Ras-MAPK)pathway, which are associated with hyperinsulinemiaand play crucial roles in tumorigenesis. Metformin iswidely used for first-line treatment of type 2 diabetesand also for various other conditions involving insulinresistance, such as PCOS and metabolic syndromes.Population-based studies suggest that metformin candecrease cancer incidence and cancer-related mortalityin diabetic individuals[ 3], and oral metformin combinedwith progestin can effectively treat young patients withEAC and AEH
Metformin-activated adenosine monophosphatedependentkinase reduces the production of skeletalmuscle glycogen and promotes glucose absorption, thuslowering insulin levels and regulating cancer cellgrowth[5]. Metformin has been shown to inhibit thegrowth of breast, ovarian, endometrial, and prostatecancer cells, potentially by suppressing MAPK, cyclinD1, and mammalian target of rapamycin (mTOR)activity[ 6, 7]. Studies have also demonstrated theprotective effect of metformin against EAC developmentand recurrence[8]. In women with PCOS, metformin wasreported to increase clinical pregnancy rates bypromoting ovulation[9]. A clinical trial showed that inpatients with EAC, preoperative use of metforminlowered the expression levels of tumor cell proliferationmarkers likely by suppressing MAPK and mTORactivity, as a result of reduced levels of the humoralfactors including insulin-like growth factor 1 andleptin[10]. Consistently, a phase II clinical trialconducted in patients with AEH and EAC demonstratedthat medroxyprogesterone acetate (MA) plus metforminas a fertility-sparing treatment resulted in a low tumorrecurrence rate, and the use of metformin improvedinsulin sensitivity of the patients and prevented weightgain caused by MA[11].
The insulin-like growth factor binding protein (IGFBP) superfamily serves essential roles in theinsulin-like growth factor (IGF) system by modulatingthe bioavailability of IGFs and/or insulin [12]. IGFBPsdivide into two categories: those having a high affinityfor IGFs (IGFBP1-6), and those with strong bindingaffinity for insulin (IGFBP7-15, reclassified as IGFbinding protein-related proteins, or IGFBP-rPs)[12].IGFBP-rP1 (or IGFBP7) is the most important IGFBPrPs,which has a 500-fold higher affinity for insulin thanIGFBP1-6 and can strongly lower serum level of freeinsulin, and is thus thought to be closely associatedwith insulin resistance
All these evidence suggest the value of metforminas a promising therapeutic option for preservingreproductive function while controlling EAC recurrencefollowing remission. In this prospective observationalstudy, we aimed to further evaluate the clinical benefitsof MA combined with metformin as a fertility-sparingtreatment for patients with AEH and early-stage EAC.
METHODS
Study design and participants
The trial was initiated in August, 2019 and conductedunder approval from the Ethics Committee and theInstitutional Review Board of the First AffiliatedHospital of Zhengzhou University prior to patientrecruitment. Patients who were admitted to the FirstAffiliated Hospital of Zhengzhou University and theFirst Affiliated Hospital of Xinxiang Medical Universitybetween August 1, 2019 and August 31, 2023 wereconsidered eligible if they met the following inclusioncriteria: (1) Patients with a histologically confirmeddiagnosis of AEH or well-differentiated grade 1 EAC,stage IA disease as per the International Federation ofGynecology and Obstetrics, and EAC limited to theendometrium as confirmed pathologically followingendometrial tissue sampling by hysteroscopy anddilatation and curettage; (2) Patients aged between 20and 42 years who expressed a strong desire for fertilitysparingtherapy; (3) Those who tested positive forprogesterone receptor by immunohistochemical staining;and (4) those with normal serum cancer antigen (CA)125 levels
The patients were excluded if they had (1)suspected myometrial invasion or extrauterinemetastasis of EAC based on pelvic ultrasound ormagnetic resonance imaging (MRI); (2) presence ofspecific pathological types, such as low-differentiatedadenocarcinoma, serous papillary carcinoma, and clearcell carcinoma; (3) contraindications to MA ormetformin; (4) abnormalities in blood coagulation testsor a history of thromboembolism
Patients who fulfilled the inclusion criteria wereprovided with detailed information about the pros andcons of conservative treatment, following whichinformed consent was obtained from them. Afterenrollment, the patients were required to undergohysteroscopy plus curettage, and the obtainedendometrial curettage samples were evaluated by atleast two experienced gynecological pathologists (including at least one deputy chief pathologist) at theObstetrics and Gynecology Hospital of ZhengzhouUniversity to confirm the diagnosis of early G1 EAC orAEH. Immunohistochemistry was used to determine theexpression of estrogen and progesterone receptors in theendometrial specimens. Patients transferred from otherhospitals were required to have their final diagnosisconfirmed by at least two experienced gynecologicalpathologists.
Treatment methods
The enrolled patients were randomized into controlgroup and the combined treatment group using a randomtable method. The patients in the combined treatmentgroup were given a daily oral dose of 160 mg of MA andoral metformin at 0.85 g twice daily [11]. The patients inthe control group received only oral MA treatment at adaily dose of 160 mg. The patients who achieved acomplete response (CR) would continue with theoriginal treatment regimen for 3 months, after whichthey could choose to either undergo pregnancypreparation or receive maintenance treatment. Patientswho did not achieve remission after 1 year of treatmentwould need to undergo radical surgery. In the controlgroup, when remission was not achieved after 6 monthsof treatment, the patients could choose to have thecombined treatment with metformin. Follow-upassessments were conducted regularly at a 3-monthinterval during the treatment.
Follow-up of the patients
During follow-up, hysteroscopy was performed every 3months in all the patients, and the treatment responsewas evaluated by histological examination of thecurettage specimens. The primary endpoint of the studywas pathological CR rate, defined as the percentage ofpatients achieving CR among all the patients. Thesecondary endpoints included remission rate,pregnancy rate, and pregnancy outcome. Treatmentresponse was categorized as: CR, defined as absence ofany cancerous or hyperplastic lesion; partial response (PR), defined as the presence of residual hyperplasiaor carcinoma without total degeneration or atrophy of theendometrial glands; stable disease (SD), defined aspersistence of AEH or EAC; and progressive disease (PD), including progression to a higher-grade lesion orclinically progressive disease such as extrauterinedisease and lymph node metastasis.
The patients who achieved CR and desiredpregnancy were referred to in vitro fertilization (IVF) orencouraged to conceive immediately based on theirclinical and gynecological history as well as thecondition of their partners. According to the NationalComprehensive Cancer Network guidelines for EAC,the patients were advised to undergo surgical treatmentfollowing delivery. Patients who achieved CR but wishedto delay pregnancy were recommended to continue withtheir original maintenance therapy to prevent cancerrecurrence. The patients with PR or SD at 3 and 6months during follow-up continued the treatment till the9th month. In cases where no treatment response wasobserved, the longest duration of treatment wasrestricted to 12 months, after which surgical treatmentwas recommended. The patients with SD in the controlgroup could either switch to the combined treatment orundergo surgery immediately. The women whoattempted natural conception or IVF underwenthysteroscopy every 12 weeks until pregnancy, or theywould be advised to stop trying to conceive.
Immunohistochemistry for IGFBP-rP1, p-Akt and p-AMPK
The paraffin-embedded endometrial tissue blocks weresectioned at 3-5 μm thickness for immunohistochemicalstaining using standard protocols. For antigen retrieval,the tissue sections were heated in citrate-EDTA antigenretrieval solution (pH9.0; Dako, USA) using a pressurecooker for 3 min. After cooling at room temperature, thesections were washed under running water followed byimmersion in citric acid buffer (pH6.0) and heatingusing a microwave oven. After cooling, the slides werewashed in PBS (pH7.4), shaken on a decolorizingshaker for 15 min, incubated with 3% hydrogenperoxide (Dako, USA) for 5 min, and then rinsed withdistilled water. The tissue sections were blocked with3% bovine serum albumin (BSA) for 30 min andincubated with the primary antibodies against IGFBPrP1,p-Akt and p-AMPK (1∶250; s-6064, Santa CruzBiotechnology, USA) at room temperature overnightand then with horseradish peroxidase-conjugated rabbitanti-mouse antibody (Sigma-Aldrich, USA) for 50 min.The reaction products were visualized using 3,3-diamiobenzidine (DAB)( Dako, USA). The sections werethen washed under running water twice andcounterstained with hematoxylin for 3 min followed bybluing in ammonia solution and dehydration in 75% and85% alcohol( 5 min each). After immersion in xylene Iand II (5 min each), the sections were mounted withDPX (Sigma-Aldrich, USA) and examined under anoptical microscope (Nikon Eclipse E600, Japan). Theexpression intensity the of the proteins in the cytoplasmor nuclei were graded on a 4-grade scale[14], in whichgrades 0 to 1 indicate negative or low expression, andgrades 2 to 3 indicate positive or high expression.
Statistical analysis
Statistical analyses of the data were conducted usingSPSS 20.0 software. The categorical data were comparedbetween the two groups using Chi-square and Fisher'sexact tests. Data are presented as Mean±SD forcontinuous variables and compared between the groupsusing Student's t-test. A P value less than 0.05 wasconsidered to indicate a statistically significantdifference.
RESULTS
Patient characteristics
Of the total of 60 patients initially enrolled, two patientsdropped out of the study after inclusion, and theremaining 58 patients completed the study. Thecombined treatment group comprised 30 patients (median age 32 years, range 23 to 39 years), including20 patients with AEH and 10 patients with EAC; thecontrol group comprised 28 patients (median age 31years, range 22 to 42 years), including 18 patients withAEH and 10 patients with EAC. In the combinedtreatment group, 28 patients were married, and 10 (35.7%) of them had a history of infertility; 27 patientsin the control group were married, and 7 had a historyof infertility. Two patients in the control group failed toachieve CR after 6 months of treatment, and they weresubsequently transferred to the combined treatmentgroup and achieved CR after 3 months of the combinedtreatment.
Treatment responses in the two groups
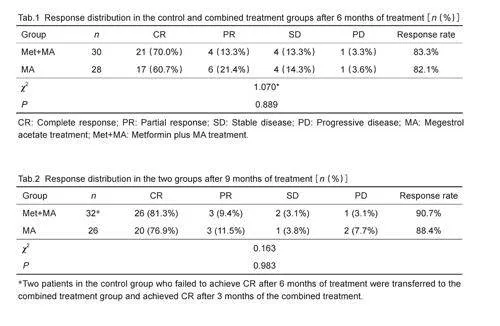
After 6 months of treatment, 17 (60.7%) out of the 28patients in the control group achieved CR, while 6patients (21.4%) achieved PR; 4 patients had SD, andone patient had PD. In the combined treatment group,21 (70.0%) out of the 30 patients achieved CR, and 4 (13.3%) achieved PR; 4 patients (13.3%) had SD andone patient (3.3%) had PD (Tab. 1). The overallresponse rate and CR rate were both higher in thecombined treatment group than in the control group, butthese differences were not statistically significant. Tab.2shows the response rates in the two groups after 9months of treatment. The CR rates in the control andcombined treatment groups were 81.3% and 76.9%,respectively, but this difference was not statisticallysignificant( P=0.983).
Adverse events
Throughout the treatment period, none of the patientsexhibited severe adverse effects associated with thetreatment. Of the 28 patients in the control group, 10 (35.7%) experienced significant weight gain of a meanof 5.7±6.1 kg, whereas none of the patients in thecombined treatment group showed significant changes inbody weight. Mild liver dysfunction caused by fatty liverwas observed in 5 patients, who had alanineaminotransferase and aspartate aminotransferase levelsabove the upper limit of normal range but less than twicethe normal value. All the other patients showed normalliver and kidney functions without coagulationabnormalities. None of the patients hadthromboembolism in either of the lower limbs asdetected by color ultrasound. Three patients in thecombined treatment group reported mild nausea aftertaking the medication, but it was tolerable and did notaffect the treatment.
Treatment response onset time
Comparison of the PR time (defined as the durationfrom the start of the treatment till the onset ofpathological remission) and CR time (duration from thestart of treatment till CR) showed that the control groupand combined treatment group had similar mean PRtime (3.98±1.36 vs 3.59±1.26 months; P=0.288). Themean CR time and total treatment time did not differsignificantly between the two groups either (Pgt;0.05),possibly due to the effect of metformin on the treatmenttime( Tab.3).
Follow-up and relapse
The mean follow-up time in the combined treatmentgroup was 13.99±6.86 months (range, 2.7-25.4months). Out of the 26 patients who achieved CR, only5 (19.2%) experienced a relapse. Three of these 5patients received further treatment with MA andmetformin due to their strong desire to preserve fertility,and they also achieved CR by the time of analysis. Themean follow-up time in the control group was 13.08±7.48 months (range, 2.1-23.9 months). Of the 20patients who achieved CR, 6 (30.0%) experiencedrelapse. One patient experienced relapse after 13months of MA treatment and underwent staged surgery,which revealed moderately to well differentiated EACwithout lesions in the remaining bilateral addendum orpelvic lymph nodes. The remaining 6 patients who failedto achieve CR received further MA and metformintreatment and eventually achieved remission. Onepatient was still under treatment at the time of analysis.
In the combined treatment group, 23 (88.46%)patients attempted pregnancy, resulting in 14 pregnancies(pregnancy rate of 60.86%) and 9 live births,including one delivered vaginally and 8 by caesareansection. In the control group, 17 (85%) patientsattempted pregnancy, resulting in 6 pregnancies (pregnancy rate of 35.29%) and 4 live births, includingone delivered vaginally and 3 by caesarean section.There was no significant difference in the pregnancy ratebetween the two groups( χ2=2.558, P=0.11).、
Of the total of 58 patients analyzed in this study, 3patients were lost after treatment, leaving 55 patientsavailable for further follow-up for an average of 15.92±5.63 months. None of the patients died during the followupand all those who experienced recurrence achievedremission again after treatment.
Endometrial expression of IGFBP-rP1, p-Akt and p-AMPK
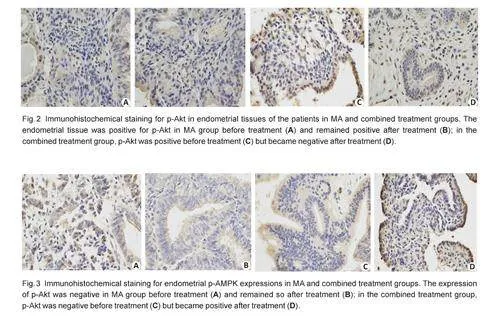
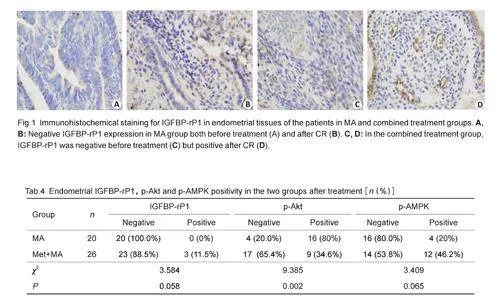
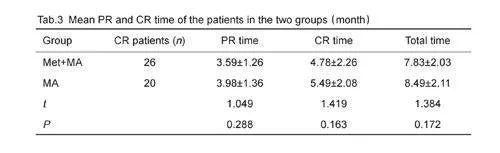
Immumohistochemical staining showed that in theendometrial tissues of the patients, IGFBP-rP1 wasexpressed mainly in the cytoplasm, and its expressionlevel was slightly higher in metformin-treated patientsthan in the control group, although the difference wasnot statistically significant (χ2=3.584, Pgt;0.05; Fig. 1,Tab.4). To explore the possible mechanism of metformininducedtumor growth inhibition, we analyzed theactivities of the p-Akt and p-AMPK pathway in the twogroups before and after the treatment. Fig. 2 showspositivity of p-Akt expression in the endometrial tissuesbefore treatment in both groups, but after the treatment,its expression became negative in the combinedtreatment group and remained positive in the controlgroup, showing a significant difference between the twogroups (χ2=9.385, Plt;0.05;Tab. 4). Before treatment,immunohistochemical staining was performed in 28 and30 patients in the control and combination groups,respectively, and the expression of p-AMPK was negativein both groups. After treatment, immunohistochemicalstaining was performed in patients who achieved CR inboth groups, and positive expression of p-AMPK wasdetected in the combined treatment group but not in thecontrol group, but this difference was not statisticallysignificant( χ2=3.409, Pgt;0.05; Fig.3 and Tab. 4)
DISCUSSION
The incidence of EAC is estimated to be 23.5 cases per100 000 women, and about 25% of the patients arepremenopausal[15, 16]. One of the common symptom ofEAC is abnormal uterine bleeding, which facilitates itsearly diagnosis via diagnostic curettage. About 84% ofcases of type I endometrioid adenocarcinoma have welldifferentiatedlesions[17]. In young women with AEH andwell differentiated early-stage EAC, progestin-basedfertility-sparing treatment has shown encouragingresults, but the recurrence rate and drug resistance rateremain high after progesterone monotherapy. Metforminwas reported to inhibit EAC recurrence and prolongrecurrence-free survival (RFS) of EC patients after MAtherapy[11].
In this study, the combined treatment with MA andoral metformin achieved more favorable outcomes thanoral MA alone in patients with grade 1 early-stage EACand AEH. The combined treatment for 6 and 9 monthsresulted in slightly higher CR rates than MA alone, andthe lack of statistical significance might be due to therelatively small sample size. The patients who achievedCR following the combined treatment had a recurrencerate of 22.7% (mean follow-up time 13.99±6.86months), lower than the rate of 30% in the controlgroup (mean follow-up time 13.08±7.48 months), andvery likely, this difference between the two groups canbe more distinct by increasing the number of patientsand extending the follow-up period. Nonetheless, thesefindings suggest the clinical benefits of oral metformintherapy, which warrants further investigation with alarger sample size and longer follow-up period.
Our findings are consistent with the results ofprevious studies[ 18, 19], which reported that in patientswith EAC and AEH, the combined treatment with MAand metformin resulted in remission rates of 76.2% and85.6% and recurrence rates of 40.6% and 26.0%,respectively[ 2]. Although we failed to demonstrate thatthe combined treatment resulted in a significantreduction of EAC relapse rate, our results still haveclinical relevance as they were obtained after followingthe patients for nearly two years after the treatment,which can be a high-risk period when 68% of relapsesoccurr (the median recurrence time is 20 months,ranging from 4 to 81 months)[20-24]. The length of thefollow-up time in this study was sufficient to record mostof the cases of relapse. According to Gallos et al[2], thepatients with AEH receiving oral progestin andmetformin had a response rate of 85.6% within a followupperiod of 11-76.5 months, and another studyreported that the CR rate could be even as high as97%
While progesterone therapy alone can achieve highCR rates, the high incidences of adverse side effectsincluding weight gain and liver function abnormalitieshave raised much concern[20]. The combination ofmetformin provides additional benefits apart fromimproving insulin resistance, inhibiting cancer cellproliferation, and preventing diabetes[26]. Metformin wasreported to inhibit tumor cell growth[27] and reduceprotein phosphatase 2A (PP2A) expression, which isassociated with insulin resistance and type 2 diabetes inpatients with EAC[28]. Metformin can also improveendothelial function and enhance ovulation even inpatients with PCOS and endothelial dysfunction withoutcausing glucose metabolism changes, dyslipidemia orprediabetes[29]. Of additional clinical interest is theeffect of metformin for preventing weight gain, acommon adverse effect of MA treatment
We found that the primary adverse effect in thecontrol group was significant weight gain, occurring in10 (35.7%) of the patients but in none of the patientsreceiving the combined treatment. In both of the groups,some patients had asymptomatic elevations of liverenzymes caused by fatty liver. According to previousreports, most of the EAC and AEH patients with astrong willingness for fertility-sparing treatment hadconcurrent metabolic syndrome, including obesity andinsulin resistance, which underscores the safety andefficacy of metformin in fertility-sparing treatment of thepatients
IGFBPs are important components of the IGFsystem, and among them IGFBP7-15 have strongbinding affinity with insulin. In a case-control study of206 patients with endometrial cancer and 350 normalwomen, Zhan et al[33] found that insulin concentrationwas significantly higher while IGFBP-rP1 expressionlevel was lower in patients with endometrial cancer thanin normal women, indicating that high insulin level is arisk factor while high IGFBP-rP1 level is a protectivefactor for endometrial cancer, and IGFBP-rP1 mayserve as a tumor suppressor in endometrial carcinoma.Gao et al[34] revealed that overexpression of IGFBP-rP1reduced p-AKT levels in endometrial cancer cells bysuppressing the activation of the PI3K/AKT signalingpathway, which is known to regulate tumor cellproliferation, apoptosis and metastasis[35]. In thispresent study, we found that metformin treatmentdownregulated p-Akt expression and upregulated theexpressions of IGFBP-rP1 and p-AMPK in theendometrial tissues, suggesting the possibility thatmetformin inhibits tumor cell growth by promoting theexpression of IGFBP-rP1 via inhibiting the AKTpathway.
Conclusion
The combined treatment with MA and metformin can bean effective fertility-sparing treatment option for AEHand grade 1 stage IA EAC, and the use of metforminbrings additional benefits for controlling weight gaincaused by MA. Nevertheless, the efficacy and clinicalbenefits of this combined treatment warrants furtherstudy with large sample sizes, and the underlyingmolecular mechanism mediating the effect of adjuvantmetformin treatment awaits clarification.

