两性离子中间层调控的纳滤膜制备及其脱盐性能












摘 " "要: 为了打破纳滤膜的渗透性-选择性上限,制备兼具高渗透通量和高截留性能的薄层复合纳滤膜,首先在水解聚丙烯腈(HPAN)基膜表面沉积聚多巴胺/聚甲基丙烯酰乙基磺基甜菜碱(PDA/PSBMA)中间层,然后利用哌嗪(PIP)和均苯三甲酰氯(TMC)进行界面聚合,制备聚酰胺(PA)层;对膜的化学结构、形貌和表面特性进行表征,研究纳滤膜的性能。结果表明:中间层亲水且带负电,对膜孔未造成严重堵塞,优化了界面聚合反应界面;聚酰胺层的厚度仅为65 nm左右,聚酰胺层内部及其与支撑层之间存在大量通道,使纳滤膜纯水渗透性高达21.82 L/(m2·h·bar) (1 bar = 100 kPa);纳滤膜对盐的截留顺序为Na2SO4(98.87%) gt; MgSO4(96.92%) gt; MgCl2(50.14%) gt; NaCl(30.60%),Na2SO4截留率基本不受操作压力和盐浓度的影响,NaCl和Na2SO4的分离因子为61.42;此外,纳滤膜具有良好的抗污染性和长期稳定性,在海水淡化、饮用水净化等领域具有应用潜力。
关键词: 聚多巴胺(PDA);聚甲基丙烯酰乙基磺基甜菜碱(PSBMA);两性离子中间层;纳滤膜;脱盐
中图分类号: TS102.54;X703.1 " " " " " "文献标志码: A " " " " " " " "文章编号: "1671-024X(2024)05-0016-08
Preparation and desalination of nanofiltration membrane regulated by
zwitterionic interlayer
WANG Xiaolei1,2,3, YU Jiangtao2, WANG Qi2, KONG Zhiyun1,2,3, WEI Junfu1,3
(1. State Key Laboratory of Separation Membranes and Membrane Processes, Tiangong University, Tianjin 300387, China; 2. School of Environmental Science and Engineering, Tiangong University, Tianjin 300387, China; 3. Cangzhou Institute of Tiangong University, Cangzhou 061000, Hebei Province, China)
Abstract: In order to overcome the upper bound of permeability-selectivity of nanofiltration (NF) membrane and prepare thin-film composite NF membranes with high permeability and high rejection, a polydopamine/poly(sulfobetaine methacrylate)(PDA/PSBMA) interlayer was deposited on the surface of hydrolyzed polyacrylonitrile (HPAN) substrate. Then polyamide (PA) layer was prepared by interfacial polymerization (IP) of piperazine (PIP) and trimesoyl chloride(TMC). The chemical structure, morphology and surface properties of membranes were characterized. The performance of NF membrane was discussed. The results indicated that the interlayer was hydrophilic and negatively charged, and did not cause serious blockage of the membrane pores, which led to the optimization of the reaction interface for IP process. The thickness of the PA layer was only about 65 nm, and a large number of channels were inside and underneath the PA layer, thus the pure water permeance of NF membrane was as high as 21.82 L/(m2·h·bar) (1 bar=100 kPa). The salt rejections of the resulted NF membrane followed an order of Na2SO4 (98.87%) gt;MgSO4 (96.92%) gt;MgCl2 (50.14%) gt;NaCl (30.60%), and the Na2SO4 rejection was basically not affected by the operating pressure and the salt concentration. Meanwhile, the separation factor of NaCl/Na2SO4 was 61.42. In addition, the NF membrane showed excellent antifouling property and long-term stability, which had potential for practical applications such as sea water desalination and drinking water treatment.
Key words: polydopamine(PDA); poly(sulfobetaine methacrylate)(PSBMA); zwitterionic interlayer; nanofiltration membrane; desalination
随着水资源需求量的不断增加,开发水资源刻不容缓。近年来,众多研究者将目光聚集在海水淡化方面。纳滤膜可利用孔径筛分和Donnan效应有效截留二价及以上的离子,允许一价离子透过,从而实现离子的选择性分离。此外,纳滤技术具有能耗低、效率高、无二次污染等优点,因此倍受关注。
纳滤膜的制备方法包括界面聚合法、涂覆法、层层自组装法、表面接枝法等。其中界面聚合法最为常用,但存在着通量较低的问题,原因在于聚酰胺层的厚度一般在100 nm左右[1],水传输阻力大。众多的研究者对传统的纳滤膜进行改进处理,常见的方法主要包括将ZIF-8、UiO-66-NH2等纳米颗粒添加到铸膜液中对基膜进行改性[2]、在水相或有机相中添加亲水性物质[3]、选择新的水相或者有机相单体[4]、在基膜表面通过表面涂覆或共沉积等方式构建中间层优化水分子传输通道[5]等。
多巴胺(DA)中含有酚羟基和氨基等官能团,可通过共价或者非共价作用黏附在各种材料表面。一般情况下,DA首先被氧化成多巴胺醌,然后通过单电子交换过程形成半醌自由基,最后通过自由基偶联进一步聚合形成聚多巴胺(PDA)[6]。甲基丙烯酰乙基磺基甜菜碱(SBMA)属于两性离子中的一种,同时具有阳离子季胺基(N+)和阴离子磺基(SO3-),可与8个水分子紧密结合形成水化层。SBMA不能自聚合,但可通过典型的自由基反应进行聚合,因此本文利用DA氧化产生的自由基实现DA和SBMA聚合,得到聚多巴胺/聚甲基丙烯酰乙基磺基甜菜碱(PDA/PSBMA)。PDA可以增强分离层和基膜之间的粘合力,提高膜的稳定性;PSBMA的强亲水性可优化界面聚合反应界面,增强对哌嗪的吸附。文中首先将聚丙烯腈(PAN)超滤膜进行水解,然后沉积PDA/PSBMA中间层,最后利用哌嗪(PIP)和均苯三甲酰氯(TMC)进行界面聚合,制备复合纳滤膜,并对其脱盐性能、抗污染性和长期稳定性进行研究。
1 实验部分
1.1 试剂与仪器
试剂:聚丙烯腈(PAN,Mw=80 ku),分析纯,杜邦有限公司;聚乙二醇1000(PEG 1000),分析纯,天津市科密欧化学试剂有限公司;N,N-二甲基甲酰胺(DMF)、氢氧化钠(NaOH)、盐酸多巴胺、三(羟甲基)氨基甲烷(Tris)、过硫酸铵(APS)、哌嗪、均苯三甲酰氯、正己烷、硫酸钠(Na2SO4)、硫酸镁(MgSO4)、氯化镁(MgCl2)、牛血清白蛋白(BSA),均为分析纯,上海阿拉丁生化科技有限公司;氯化钠(NaCl),分析纯,上海麦克林生化科技有限公司;甲基丙烯酰乙基磺基甜菜碱(98%),上海源叶生化科技有限公司;盐酸(HCl),分析纯,天津市风船化学试剂科技有限公司。
仪器:Nicolet 6700型傅里叶变换红外光谱仪,美国Thermo Fisher有限公司;DSA25型全自动接触角测量仪,德国KRUSS仪器公司;SurPass-3型固体表面Zeta电位仪,奥地利Anton Paar公司;MIRA LMS型场发射扫描电子显微镜,捷克TESCAN公司。
1.2 复合纳滤膜的制备
1.2.1 基膜的制备
通过相转化法制备PAN超滤膜,具体步骤如下:将PAN粉末于烘箱(60 ℃,12 h)中烘干;取1.6 g PEG 1000溶于68.8 g DMF中,待溶解完全再加入9.6 g PAN粉末溶解,60 ℃加热搅拌4 h,静置脱泡24 h得到均匀的铸膜液;将铸膜液倾倒在干净的玻璃板上,利用250 μm的刮刀均匀地涂覆在玻璃板上,迅速放入去离子水中进行相转化;待膜与玻璃板分离,得到PAN超滤膜。PAN膜储存在去离子水中,使用前多次更换去离子水使相转化完全。
将PAN膜在55 ℃的NaOH溶液(1 mol/L)中水解1.5 h,得到水解聚丙烯腈(HPAN)膜,将HPAN膜放入去离子水中浸泡,多次更换去离子水,直至去离子水约为中性。
1.2.2 中间层的制备
配制Tris溶液(50 "mmol/L),利用HCl溶液(0.1 mol/L)调至pH=8.5,得到Tris缓冲溶液。将DA溶于缓冲溶液(2 g/L,温度为50 ℃),然后加入APS(1.5 g/L)和SBMA(20 g/L)溶解,得到PDA/PSBMA沉积液。PDA/PSBMA形成的反应机理如图1所示[6]。
将HPAN膜浸泡在上述溶液中振荡沉积1.5 h,取出后使用去离子水浸泡和超声清洗,以去除膜表面结合不牢固的聚合物。涂覆PDA/PSBMA中间层的HPAN膜命名为HPAN-PDA/PSBMA膜。
1.2.3 聚酰胺层的制备
将HPAN-PDA/PSBMA膜固定于实验室自制的聚四氟乙烯框架中晾干,将0.15%的PIP倾倒在膜表面单侧,浸泡3 min后倒出。将膜在空气中晾晒20 min左右,以去除膜表面流动的水相。取0.2%的TMC/正己烷溶液浸泡膜表面,与PIP反应1 min后倒出,生成聚酰胺层。最后将膜放入60 ℃烘箱中热处理20 min以促进交联,得到复合纳滤膜,命名为HPAN-PDA/PSBMA-PA膜,具体制备过程及反应机理如图2所示。
1.3 复合纳滤膜的表征
膜表面的官能团通过傅里叶变换红外光谱仪(FTIR)进行分析;膜表面的亲水性通过全自动接触角测量仪测量水接触角进行表征。膜表面的电荷性质通过固体表面Zeta电位仪测量pH=7时的Zeta电位进行表征;采用场发射扫描电子显微镜(FE-SEM)观察膜表面和断面的形貌。
1.4 膜的过滤性能测试
使用微型膜分离装置进行错流过滤来评估膜的过滤性能,膜池的有效面积为7.065 cm2。所有膜在测试前均使用纯水在测试压力下预压30 min,使膜的性能达到稳定。在无特殊说明的情况下测试压力为5 bar(1 bar=100 kPa)。
根据式(1)、式(2)分别计算膜的通量和渗透性:
式中:J为通量(L/(m2·h));V为过滤液体积(L);A为膜的有效过滤面积(m2);T为过滤时间(h);P 为渗透性(L/(m2·h·bar));ΔP为操作压力(bar)。
根据式(3)计算膜的截留率:
式中:R为截留率(%);Cf和Cp分别为进料液和渗透液的电导率(μS/cm),通过电导率仪测量。
根据式(4)计算膜的分离因子:
式中:α为分离因子;RA为A截留率;RB为B截留率。
1.5 膜的抗污染性测试
将牛血清白蛋白作为典型污染物,用来测试纳滤膜的抗污染性。首先,纳滤膜过滤去离子水2 h获得初始通量Jw0,然后过滤0.1 g/L BSA溶液2.5 h,接下来再过滤去离子水过滤2 h。过滤2 h去离子水和2.5 h BSA溶液为1个循环,总共进行2.5个循环,测试压力为5 bar。根据式(5)计算纳滤膜的通量恢复率:
FRRi = ■×100%(5)
式中:FRRi为通量恢复率;Jwi为第i次去离子水清洗后的最终通量;Jw0为膜的初始水通量。
1.6 膜的长期稳定性测试
纳滤膜在5 bar的压力下连续过滤1 g/L Na2SO4溶液72 h,测试其长期稳定性。膜的渗透性和截留率分别根据式(2)和式(3)进行计算。
2 结果与讨论
2.1 膜的表征
2.1.1 红外光谱图分析
PAN、HPAN、HPAN-PDA/PSBMA和HPAN-PDA/PSBMA-PA膜的红外光谱图如图3所示。由图3可见,PAN膜在2 245 cm-1处出现CN的伸缩振动峰。相比之下,HPAN膜在3 343、1 665、1 565和1 410 cm-1处出现新峰,分别为O—H、CO、N—H和C—O的伸缩振动峰[7]。在HPAN膜表面沉积PDA/PSBMA中间层后,3 352 cm-1处的峰为PDA/PSBMA中N—H和O—H的伸缩振动峰[8],1 620 cm-1处出现的新峰属于DA苯环中的CC伸缩振动峰[9]。除此之外,1 729 cm-1和1 042 cm-1处分别出现SBMA的O—CO和SO伸缩振动峰[10]。以上峰的变化证明了PDA/PSBMA中间层在基膜表面成功构建。HPAN-PDA/PSBMA-PA膜在3 400 cm-1处的峰来自于酰胺中的N—H伸缩振动[11];1 620 cm-1处的峰显著增强是由于酰胺中N—CO和苯环中CC的伸缩振动[11-12],两者位置相近,发生了重合;在1 420 cm-1处增强的峰则来自于酰胺中C—N的伸缩振动[12]。这些峰的变化说明聚酰胺层被成功制备。
2.1.2 FE-SEM分析
为了研究中间层的作用,观察了HPAN、HPAN-PDA/PSBMA和HPAN-PDA/PSBMA-PA膜的表面和断面形貌,如图4所示。
由图4(a)可见,HPAN膜表面平整光滑,孔隙分布均匀,且存在大量指状孔。由图4(b)可见,沉积中间层后,膜表面的孔隙变小,出现了分布均匀的小颗粒,这直接证明了PDA/PSBMA的成功沉积。比较HPAN膜和HPAN-PDA/PSBMA的断面形貌,发现PDA/PSBMA中间层较薄,与基膜之间结合紧密,无明显界限,这有助于提高复合膜的稳定性。进一步界面聚合后,HPAN-PDA/PSBMA-PA膜表面的聚酰胺层呈现“脊-谷”结构和“结节”结构相结合的形貌(图4(c)),厚度约为65 nm,且在聚酰胺层内部以及聚酰胺层与支撑层之间存在许多缝隙,较薄的聚酰胺层减小了水分子的传输阻力,缝隙的存在增加了水分子的传输通道,这都有利于纳滤膜的渗透性提高。
2.1.3 水接触角分析
PAN、HPAN、HPAN-PDA/PSBMA和HPAN-PDA/PSBMA-PA膜表面的水接触角如图5所示。由图5可知,PAN和HPAN膜的水接触角分别为56.5°和48.7°,HPAN膜的亲水性有所提高,主要原因是腈基水解产生大量亲水性的羧基[7]。在沉积中间层后,HPAN-PDA/PSBMA膜表面的水接触角大幅度降低,稳定在26.6°,这是由于DA中有亲水的羟基和氨基官能团,SBMA的阴阳离子基团则可以与水分子高度结合形成水化层[13]。HPAN-PDA/PSBMA-PA膜的水接触角为32.1°,亲水性的表面有利于提高纳滤膜的渗透性和抗污染性。
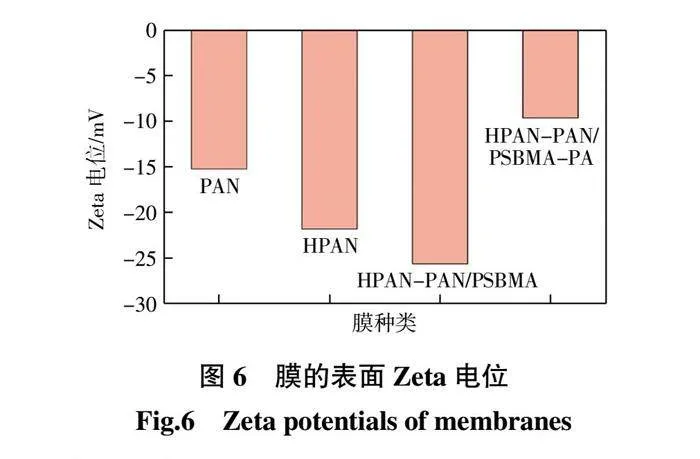
2.1.4 Zeta电位分析
PAN、HPAN、HPAN-PDA/PSBMA和HPAN-PDA/PSBMA-PA膜表面的Zeta电位如图6所示。由图6可见:PAN膜的Zeta电位为-15.24 mV;HPAN膜的Zeta电位为 -21.83 mV,电负性增强,这是由于水解产生的羧基带负电[14];HPAN-PDA/PSBMA膜的Zeta电位降低至 -25.64 mV,原因在于PDA/PSBMA带负电[8,15];HPAN-PDA/PSBMA-PA膜表面Zeta电位为-9.65 mV,负电性的膜表面有利于纳滤膜利用静电排斥作用截留阴离子。
2.2 膜的过滤性能
2.2.1 基膜的过滤性能
膜的纯水渗透性如图7所示。由图7可知,在1 bar压力下过滤纯水5 h后,PAN、HPAN和HPAN-PDA/PSBMA膜的渗透性分别稳定在452.94、552.02、537.86 L/(m2·h·bar)。HPAN膜的渗透性相对于PAN膜有所提高,原因在于PAN膜水解后,C—N键断裂导致基膜的结构变化,膜的孔径增加,同时亲水性提高[7,14]。沉积PDA/PSBMA中间层后,HPAN-PDA/PSBMA膜的渗透性相对于HPAN膜仅有小幅度降低,这是因为PDA/PSBMA亲水性较强且未对膜孔造成严重堵塞。
2.2.2 纳滤膜的过滤性能
分别以1 g/L的Na2SO4、MgSO4、MgCl2和NaCl作为进料液,评估HPAN-PDA/PSBMA-PA膜的过滤性能,如图8所示。由图8可见,纳滤膜对无机盐的截留率依次为:Na2SO4(98.87%)gt;MgSO4(96.92%)gt;MgCl2(50.14%)gt;NaCl(30.60%)。纳滤膜对Na2SO4和MgSO4的截留率远大于MgCl2和NaCl,原因在于其表面带负电,对SO42-的静电排斥大于Cl-。由于纳滤膜对Mg2+的静电吸引强于Na+,膜表面负电荷会产生静电屏蔽,所以纳滤膜的Na2SO4截留率大于MgSO4。除此之外,无机盐的截留率还受空间位阻效应的影响,由于Mg2+的斯托克斯半径(0.347 nm)大于Na+(0.184 nm),纳滤膜对MgCl2的截留率大于NaCl。HPAN-PDA/PSBMA-PA膜对Na2SO4、MgSO4、MgCl2和NaCl的渗透性分别为15.63、16.99、17.66和19.02 L/(m2·h·bar),与截留率排序相反,这是由于无机盐的渗透压不同。
HPAN-PDA/PSBMA-PA膜的纯水渗透性高达21.82 L/(m2·h·bar),Na2SO4截留率为98.87%,α(NaCl/Na2SO4)为61.42,与其他研究中的纳滤膜相比[16-29],具有较强的竞争力,如图9所示。
2.2.3 操作条件对纳滤膜过滤性能的影响
以1 g/L的Na2SO4溶液为进料液,探究操作压力对HPAN-PDA/PSBMA-PA膜过滤性能的影响,如图10所示。由图10可见,在1~6 bar的压力范围内,HPAN-PDA/PSBMA-PA膜的通量随操作压力的增加几乎呈线性增加,当压力为6 bar时,通量达到93.42 L/(m2·h)。Na2SO4截留率随压力增加稍有增加,这是因为水通量增加的同时盐通量保持不变。这种现象说明HPAN-PDA/PSBMA-PA膜对高压具有较强的耐受性。
Na2SO4浓度对HPAN-PDA/PSBMA-PA膜过滤性能的影响如图11所示。由图11可见,随着Na2SO4浓度增加,膜的渗透性从17.15 L/(m2·h·bar)下降至12.23 L/(m2·h·bar),主要是因为Na2SO4浓度增加会导致渗透压增加,有效驱动力降低。但是膜对Na2SO4的截留率几乎不变。
2.3 膜的抗污染性
HPAN-PDA/PSBMA-PA膜的抗污染性如图12所示。
由图12可见,经过2次污染后,纳滤膜具有高的通量恢复率,FRR1和FRR2分别为96.15%和92.31%,这说明HPAN-PDA/PSBMA-PA膜具有良好的抗污染性,主要原因为纳滤膜具有亲水且荷负电性的表面,污染物不易在膜表面沉积。
2.4 膜的长期稳定性
HPAN-PDA/PSBMA-PA膜的长期稳定性测试结果如图13所示。由图13可见,随着过滤时间的延长,纳滤膜的渗透性略微下降,当过滤时间为72 h时,渗透性为14.68 L/(m2·h·bar),而Na2SO4截留率基本保持在97.80%~99.00%的范围内,表现出优良的长期稳定性。
3 结 论
本文在HPAN基膜表面沉积PDA/PSBMA中间层,然后利用PIP和TMC进行界面聚合制备聚酰胺层,得到了兼具高渗透通量和高截留率的复合纳滤膜。
(1) 亲水且带负电荷的PDA/PSBMA中间层优化了界面聚合反应界面,最终形成了厚度约65 nm的聚酰胺层,聚酰胺层内部及其与支撑层之间存在大量水通道。
(2) HPAN-PDA/PSBMA-PA膜的纯水渗透性为21.82 L/(m2·h·bar),对4种盐的截留排序依次为:Na2SO4(98.87%)gt;MgSO4(96.92%)gt;MgCl2(50.14%)gt;NaCl(30.60%),α(NaCl/Na2SO4)为61.42,有望用于海水淡化。
(3) 纳滤膜的通量随着压力的增加呈线性增加,对Na2SO4的截留率稍有增加,具有较强的耐压性;随着Na2SO4浓度的增加,膜渗透性逐渐下降,截留率基本不变,说明纳滤膜具有处理较高浓度盐溶液的能力。
(4) 纳滤膜第1次和第2次BSA污染后的通量恢复率分别为96.15%和92.31%,具有良好的抗污染性。此外,在72 h的连续过滤中,渗透性略微下降,对Na2SO4的截留率基本不变,具有长期稳定性。
参考文献:
[1] " "HU R R, ZHANG R J, HE Y J, et al. Graphene oxide-in-polymer nanofiltration membranes with enhanced permeability by interfacial polymerization[J]. Journal of Membrane Science, 2018, 564: 813-819.
[2] " "YUAN B B, WANG N, ZHAO S H, et al. Polyamide nanofiltration membrane fine-tuned via mixed matrix ultrafiltration support to maximize the sieving selectivity of Li+/Mg2+and Cl-/SO42- [J]. Desalination, 2022, 538: 115929.
[3] " "WU X N, YANG L, MENG F B, et al. ZIF-8-incorporated thin-film nanocomposite (TFN) nanofiltration membranes: Importance of particle deposition methods on structure and performance [J]. Journal of Membrane Science, 2021, 632: 119356.
[4] " "ZHANG S Y, ZHANG R N, LI R L, et al. Guanidyl-incorporated nanofiltration membranes toward superior Li+/ Mg2+ selectivity under weakly alkaline environment[J]. Journal of Membrane Science, 2022, 663: 121063.
[5] " "YANG Z, WANG F, GUO H, et al. Mechanistic insights into the role of polydopamine interlayer toward improved separation performance of polyamide nanofiltration membranes[J]. Environmental Science amp; Technology, 2020, 54(18): 11611-11621.
[6] " "ZHANG C, MA M Q, CHEN T T, et al. Dopamine-triggered one-step polymerization and codeposition of acrylate monomers for functional coatings[J]. ACS Applied Materials amp; Interfaces, 2017, 9(39):34356-34366.
[7] " "AUSTRIA H F M, LECAROS R L G, HUNG W S, et al. Investigation of salt penetration mechanism in hydrolyzed polyacrylonitrile asymmetric membranes for pervaporation desalination[J]. Desalination, 2019, 463: 32-39.
[8] " "DE GUZMAN M R, ANDRA C K A, ANG M B M Y, et al. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation[J]. Journal of Membrane Science, 2021, 620: 118881.
[9] " "ZHANG C, LI H N, DU Y, et al. CuSO4/H2O2-triggered polydopamine/poly(sulfobetaine methacrylate) coatings for antifouling membrane surfaces[J]. Langmuir, 2017, 33(5): 1210-1216.
[10] "ZHANG C, MA M Q, CHEN T T, et al. Dopamine-triggered one-step polymerization and codeposition of acrylate monomers for functional coatings[J]. ACS Applied Materials amp; Interfaces,2017,9(39):34356-34366.
[11] "ZHANG T H, ZHANG H, LI P Y, et al. Highly permeable composite nanofiltration membrane via Γ-cyclodextrin modulation for multiple applications[J]. Separation and Purification Technology, 2022, 297: 121541.
[12] "WU M Y, YUAN J Q, WU H, et al. Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability[J]. Journal of Membrane Science, 2019, 576: 131-141.
[13] "DUONG P H H, DAUMANN K, HONG P Y, et al. Interfacial polymerization of zwitterionic building blocks for high-flux nanofiltration membranes[J]. Langmuir, 2019, 35(5): 1284-1293.
[14] "WANG X L, DONG S Q, QIN W, et al. Fabrication of highly permeable CS/NaAlg loose nanofiltration membrane by ionic crosslinking assisted layer-by-layer self-assembly for dye desalination[J]. Separation and Purification Technology, 2022, 284: 120202.
[15] "HARESCO C K S, ANG M B M Y, DOMA B T, et al. Performance enhancement of thin-film nanocomposite nanofiltration membranes via embedment of novel polydopamine-sulfobetaine methacrylate nanoparticles[J]. Separation and Purification Technology, 2021, 274: 119022.
[16] "WANG Z H, XIA D W, WANG B T, et al. Highly permeable polyamide nanofiltration membrane incorporated with phosphorylated nanocellulose for enhanced desalination[J]. Journal of Membrane Science, 2022, 647: 120339.
[17] "GONG G H, WANG P, ZHOU Z Y, et al. New insights into the role of an interlayer for the fabrication of highly selective and permeable thin-film composite nanofiltration membrane[J]. ACS Applied Materials amp; Interfaces, 2019, 11(7): 7349-7356.
[18] "ZHANG X, ZHANG Y F, WANG T C, et al. A thin film nanocomposite membrane with pre-immobilized UiO-66-NH2 toward enhanced nanofiltration performance[J]. RSC Advances, 2019, 9(43): 24802-24810.
[19] "GUO Y S, JI Y L, WU B, et al. High-flux zwitterionic nanofiltration membrane constructed by in-situ introduction method for monovalent salt/antibiotics separation[J]. Journal of Membrane Science, 2020, 593: 117411.
[20] "WANG T, WANG J, ZHAO Z Z, et al. Bio-inspired fabrication of anti-fouling and stability of nanofiltration membranes with a poly(dopamine)/graphene oxide interlayer[J]. Industrial amp; Engineering Chemistry Research, 2021, 60(41): 14868-14883.
[21] "LAN H L, LI P F, WANG H, et al. Construction of a gelatin scaffold with water channels for preparing a high performance nanofiltration membrane[J]. Separation and Purification Technology, 2021, 264: 118391.
[22] "CHEN L L, ZHANG C X, GAO A L, et al. Nanofiltration membrane embedded with hydroxyapatite nanowires as interlayer towards enhanced separation performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 626: 127001.
[23] "WANG Z H, GUO S, ZHANG B, et al. Hydrophilic polymers of intrinsic microporosity as water transport nanochannels of highly permeable thin-film nanocomposite membranes used for antibiotic desalination[J]. Journal of Membrane Science, 2019, 592: 117375.
[24] "YANG S M, WANG J Q, WANG Y, et al. Interfacial polymerized polyamide nanofiltration membrane by demulsification of hexane-in-water droplets through hydrophobic PTFE membrane: Membrane performance and formation mechanism[J]. Separation and Purification Technology, 2021, 275: 119227.
[25] "XIA D W, ZHANG M X, TONG C C, et al. In-situ incorporating zwitterionic nanocellulose into polyamide nanofiltration membrane towards excellent perm-selectivity and antifouling performances[J]. Desalination, 2022, 521: 115397.
[26] "YANG Z, LI L T, JIANG C, et al. Tailored thin film nanocomposite membrane incorporated with Noria for simultaneously overcoming the permeability-selectivity trade-off and the membrane fouling in nanofiltration process[J]. Journal of Membrane Science, 2021, 640: 119863.
[27] "CHEN Y H, SUN H X, ZHANG H B, et al. Fabrication of high performance nanofiltration membranes based on the interfacial polymerization regulated by the incorporation of dextran nanoparticles[J]. Desalination, 2021, 519: 115308.
[28] "LIU C C, ZUO X T, WU Q Y, et al. Novel highly stable guanazole-incorporated ultrathin loose nanofiltration membrane with superior permeability for water desalination and purification[J]. Desalination, 2021, 520: 115335.
[29] "XIA M J, ZHANG W T, XU Y C, et al. Polyamide membranes with a ZIF-8@Tannic acid core-shell nanoparticles interlayer to enhance nanofiltration performance[J]. Desalination, 2022, 541: 116042.
本文引文格式:
王晓磊,余姜涛,王齐,等. 两性离子中间层调控的纳滤膜制备及其脱盐性能[J]. 天津工业大学学报,2024, 43(5): 16- 23.
WANG X L, YU J T, WANG Q, et al. Preparation and desalination of nanofiltration membrane regulated by zwitterionic interlayer[J]. Journal of Tiangong University, 2024, 43(5): 16-23(in Chinese).

