獭兔胃肠道乳酸菌的分离与鉴定
张菲菲 薛海涛 吴婕 任战军
doi:10.7606/j.issn.1004-1389.2024.07.003
https://doi.org/10.7606/j.issn.1004-1389.2024.07.003
收稿日期:2022-09-13 修回日期:2022-10-09
基金项目:陕西省农业科技创新驱动项目[NYKJ-2021-YL(XN)28]。
第一作者:张菲菲,女,学士,从事动植物检验检疫工作。E-mail:1181953766@qq.com
通信作者:任战军,男,博士,教授,主要从事经济动物教学、科研与推广工作。E-mail: renzhanjun@nwsuaf.edu.cn
摘 要 为探索獭兔胃肠道中固有的乳酸菌群,丰富乳酸菌应用,选择中性或偏酸性MRS培养基分离并培养健康獭兔胃肠道中的乳酸菌、16S rDNA测序对所获菌株进行种属鉴定、基因组DNA荧光定量PCR检测其在40、80日龄的獭兔胃肠道中的相对含量。结果从獭兔胃、空肠、盲肠、结肠、直肠中均分离出1株粪肠球菌;胃、十二指肠、空肠、回肠、盲肠中均分离出1株短乳杆菌;胃、十二指肠、回肠、盲肠、结肠、直肠中均分离出1株植物乳杆菌;十二指肠、空肠、盲肠、结肠中均分离出1株空肠肠球菌。所获菌株在獭兔胃肠道中普遍分布,并且其相对水平随獭兔日龄的增长而升高,这些菌类有待成为獭兔内源乳酸菌饲料添加剂的备用选择。
关键词 乳酸菌;獭兔;16S rDNA
獭兔是单胃草食性哺乳动物,其消化模式依赖盲肠、结肠中大量菌群的发酵作用[1]。在规模化生产中獭兔常因管理不善、致病菌侵染等多种因素诱发多种肠道疾病[2],典型症状为肠道上皮粘膜受损、免疫屏障被破坏、菌群结构平衡失调[3-4]。养殖户多用广谱抗生素防治獭兔腹泻,但抗生素易在动物体内长期残留,破坏肠道菌群的固有平衡并大幅提高病菌的抗药性,不利于獭兔肠道疾病的长期防治[5-7]。
乳酸菌作为肠道益生菌,有利于维持肠黏膜的菌群结构与免疫稳态,抑制大肠杆菌、沙门氏菌等致病菌的生长,提高宿主的免疫力,是理想的抗生素替代物[8-10]。腹泻幼兔肠道中的乳酸菌含量减少,及时补充乳酸菌可改善腹泻幼兔的肠道环境、有效治疗幼兔腹泻[11-12]。外源补充的乳酸菌往往缺乏黏附在家兔肠壁的能力,难以持续、充分地发挥益生作用[13-14]。宿主胃肠道固有的乳酸菌有潜力保持对宿主的益生功能[15],故獭兔肠道中附殖的乳酸菌具备重要的利用价值。为此,本试验采集獭兔肠道内容物,对所含乳酸菌进行分离、鉴定,为生产可用性高、效果出色的外源饲料添加剂提供依据与选择。
1 材料与方法
1.1 试验材料
1.1.1 獭兔胃肠道内容物的采集 乳酸菌分离及纯化所用的样品来自4月龄健康无病的獭兔12只(公母各半),乳酸菌含量测定所用的样品来自40、80日龄獭兔母兔各5只,均由西北农林科技大学试验兔场提供。獭兔于清晨空腹处死后,采集胃、十二指肠、空肠、回肠、盲肠、结肠、直肠,用生理盐水冲洗干净肠道外部表面,用灭菌手术刀刮取消化道的内容物置于1.5 mL无菌离心管,4 ℃保存。
1.1.2 培养基 中性MRS培养基、酸性MRS培养基均购自青岛海博生物技术有限公司,按菌株培养所需配置成中性固体CaCO3-MRS培养基(NS)、偏酸性固体CaCO3-MRS培养基(AS)、中性液体MRS培养基(NL)、偏酸性液体MRS培养基(AL)。
1.2 试验方法
1.2.1 菌株的初筛 无菌条件下,取1 g肠道内容物,加入无菌水至10 mL,振荡混匀并经纱布过滤;用无菌水梯度稀释至10-6、10-7 g/mL;取200 μL稀释菌液均匀涂布于NS、AS固体培养基,37 ℃恒温厌氧倒置培养48 h。判断菌落的颜色、形态及溶钙圈的存在与否,确定进一步纯化的菌落。
1.2.2 菌株的复筛 无菌条件下,挑取初筛后的单菌落,采取平板涂布法接种到NL、AL液体培养基中,37 ℃恒温厌氧培养12~16 h;在NS、AS固体平板上划线分离菌落,并于37 ℃厌氧倒置培养24~48 h;挑取平板上的单菌落,根据采样部位编号标记,并分别接种至NL、AL液体培养基中进行纯培养,37 ℃恒温厌氧培养12~16 h,所得单菌落扩繁菌液于4 ℃保存。将200 μL扩繁菌液接种至固态平板,挑取平板上的单菌落进行革兰氏染色、镜检,最后确定纯化的试验菌株。
1.2.3 菌株的生理生化鉴定及16S rDNA测序 生理生化鉴定:按照说明书的操作方法,使用细菌微量生化鉴定管(海博生物)检测菌株的相关指标。
16S rDNA测序:使用OMEGA细菌DNA提取试剂盒(Bacterial DNA Kit D3350-01)提取待测菌株的总DNA,用16S通用引物(27 F:5′-AGAGTTTGATCCTGGCTCAG-3′;1492 R:3′-CGGTTACCTTGTTACGACTT-5′)扩增V4可变区,经10 g/L琼脂糖凝胶电泳、OMEGA胶回收试剂盒[Gel Extraction Kit (100) D2500-01] 检测并回收扩增产物。将纯化产物送上海生工生物工程股份有限公司测序,测序结果在GenBank数据库中进行比对,同时用MEGA-X软件以最大似然法构建系统发育树。
1.2.4 不同日龄獭兔胃肠道中乳酸菌的相对含量 根据测序所鉴定的乳酸菌种属,设计16S区域保守序列引物(F:5′- AAGCCTGATGGAGCAAC -3′;R:3′- AATCCGGATAACGCTTG -5′)。提取40、80日龄獭兔胃肠道内容物总DNA,以预变性95 ℃ 5 min;变性94 ℃ 30 s,退火55 ℃ 30 s,延伸72 ℃ 30 s,终延伸72 ℃ 5 min,34个循环的反应程序。PCR mix 12.5 μL、上下游引物各1 μL,总DNA模板2.5 μL,ddH2O补足至25 μL的反应体系进行扩增。
经胶回收并纯化PCR扩增产物、构建T载体、导入大肠杆菌感受态细胞、蓝白斑筛选、阳性克隆子测序鉴定后,将重组质粒浓度梯度稀释至102~109拷贝/μL,作为标准品并计算质粒拷贝数。扩增选择含SYBR Ⅱ Green PCR Master mix 10 μL、总DNA 2 μL、正反向引物各1 μL的反应体系,以95 ℃预变性10 min;变性95 ℃ 10 s,退火55 ℃ 20 s、延伸72 ℃ 20 s,循环40 次的反应程序进行荧光定量。乳酸菌的拷贝数通过Ct值计算,并以拷贝数的对数值表示。
2 结果与分析
2.1 乳酸菌的初筛结果
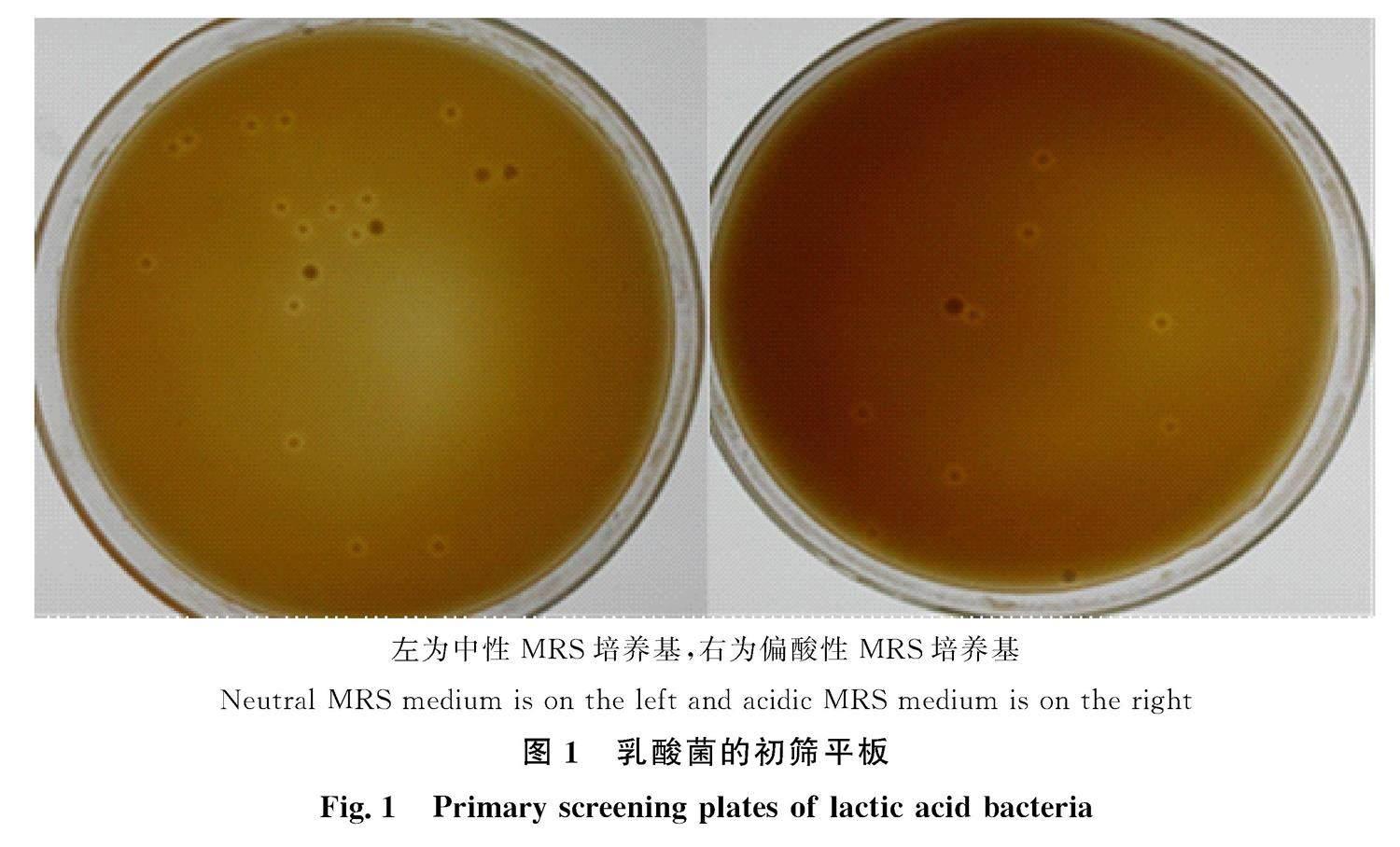
初筛结果中,稀释至10-7 g/mL的菌液涂布后形成的菌落总体稀疏合理,有表面光滑、边界整齐、生成溶钙圈的乳白色圆形菌落出现(图1,左为中性MRS培养基,右为偏酸性MRS培养基)。透明圈是乳酸菌产生的乳酸与MRS平板中的不溶性碳酸钙反应在菌落外周形成的乳酸钙圈,是形态鉴定中常用的筛选依据。肠道乳酸菌普遍适宜pH为5~7,不同菌种乳酸菌的最适pH环境存在差异。
左为中性MRS培养基,右为偏酸性MRS培养基
Neutral MRS medium is on the left and acidic MRS medium is on the right
2.2 乳酸菌的复筛结果
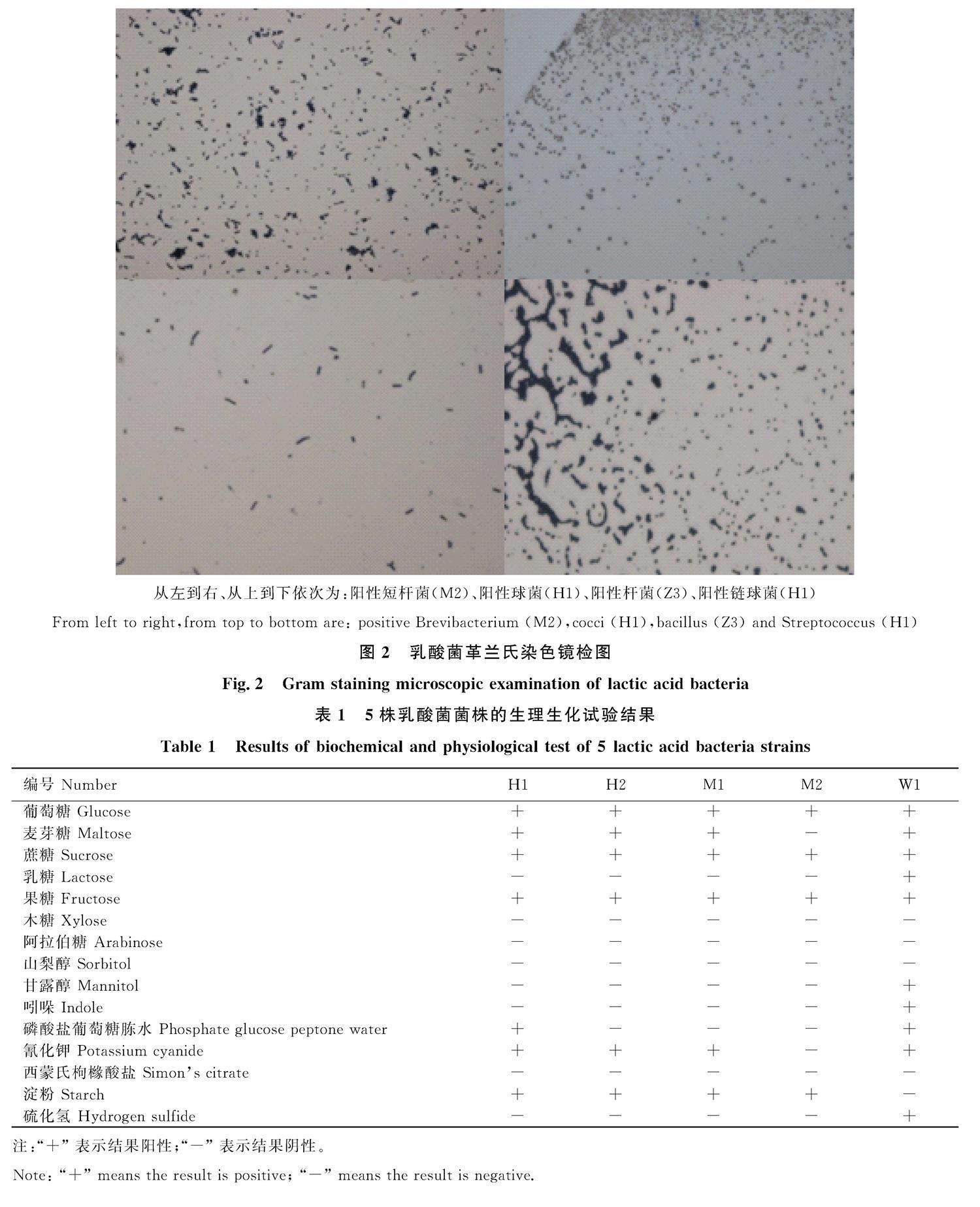
纯化培养后的单菌落革兰氏染色结果均呈阳性,所检乳酸菌具球菌、短杆菌、杆状菌、链球菌等不同形态(图2)。
2.3 乳酸菌的生理生化鉴定及16 S rDNA测序结果
由表 1 可知,各菌株的碳源利用与生理生化情况总体相近。
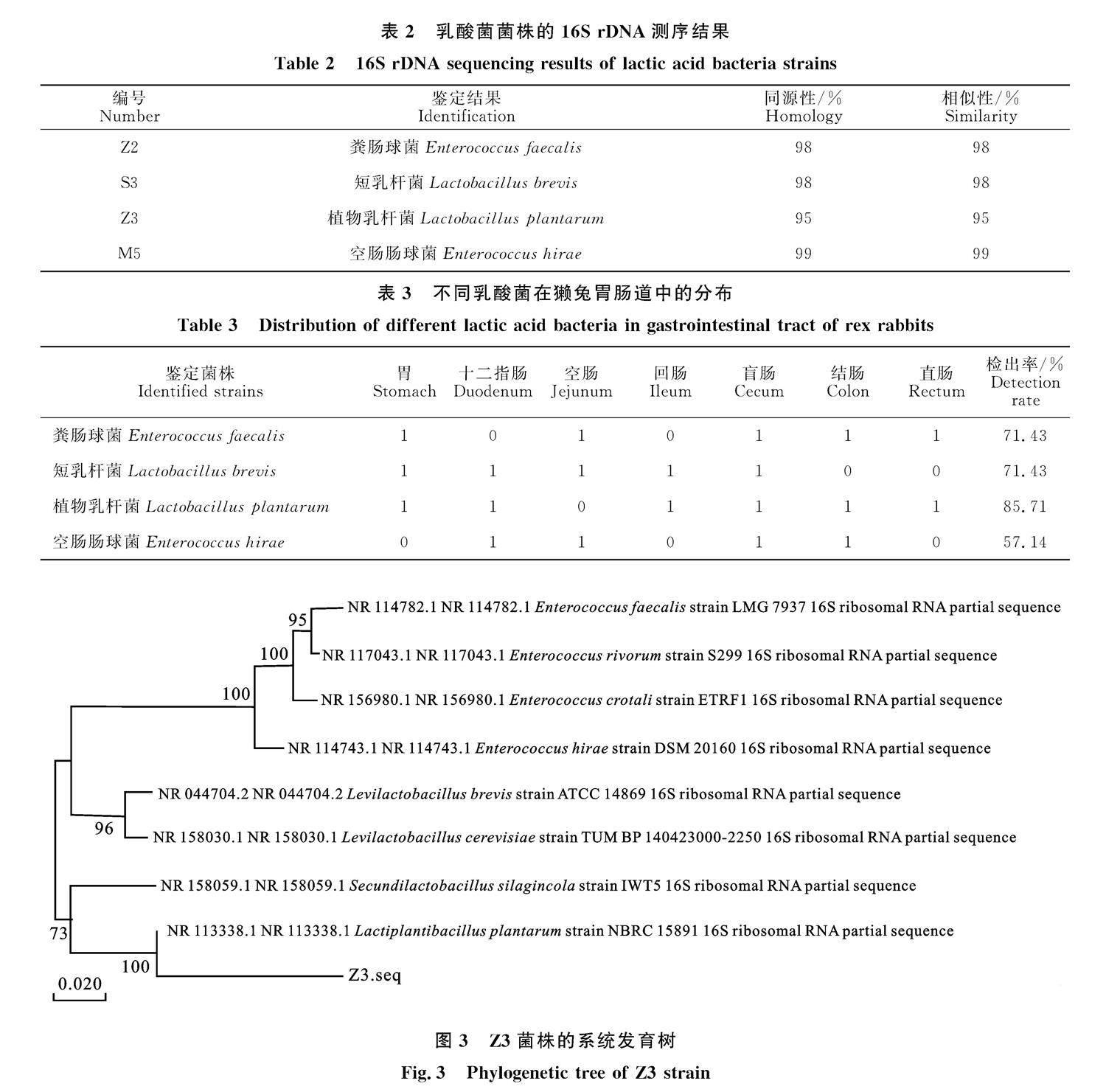
由表 2 可知,经培养基分离纯化后,从獭兔胃肠道7处共检测到4种乳酸菌,包括粪肠球菌(Enterococcus faecalis)、短乳杆菌(Lactobacillus brevis)、植物乳杆菌(Lactobacillus plantarum)和空肠肠球菌(Enterococcus hirae);各菌种在胃肠道中的检出结果见表 3,其中植物乳杆菌在7
从左到右、从上到下依次为:阳性短杆菌(M2)、阳性球菌(H1)、阳性杆菌(Z3)、阳性链球菌(H1)
From left to right,from top to bottom are: positive Brevibacterium (M2),cocci (H1),bacillus (Z3) and Streptococcus (H1)
个部位中的检出率最高,为85.71%。
由图 3可知,Z3菌株与植物乳杆菌NBRC 15891(Gen Bank登录号:NR 113338.1)聚于一枝,亲缘关系最近,证实该菌株为植物乳杆菌。
2.4 不同日龄獭兔胃肠道中乳酸菌的相对含量
由表 4可知,Z3等乳酸菌广泛定植于不同日龄獭兔的胃肠道中,相对水平总体以胃<小肠<大肠的趋势提升;随着獭兔日龄的增长,乳酸菌在胃、十二指肠、空肠、回肠、结肠中的相对水平也显著升高(P<0.05)。
3 讨 论
乳酸菌多附殖在动物肠道上皮,可产生乳酸[16]、乙酸[17]等有机酸,降低肠道的pH,抑制致病菌的繁殖,改善肠道菌群结构[18-19];也可通过分泌胞外多糖[20]、γ-氨基丁酸[21]等产物,减少血脂与胆固醇的含量,与肠道上皮屏障相互作用提升宿主的免疫力与生长性能[22]。
乳酸菌作为微生物制剂,替抗价值较为理想,试验条件下提升兔生产性能与免疫水平[23-25]、改善肠道环境的效果显著[26]。但獭兔乳酸菌补充剂的制备受以下条件制约:(1)外源补充乳酸菌对外界储存环境的耐受性差[27];(2)异源乳酸菌无法长久生存于宿主肠道中[28];(3)对獭兔养殖有利的乳酸菌研究较少。
本试验从獭兔胃肠道中分离出的粪肠球菌、短乳杆菌、空肠肠球菌和植物乳杆菌是《饲料添加
剂品种目录(2013)》中允许的微生物添加剂,在猪、禽及水产养殖中的应用效果出色[29-31],在獭兔胃肠道中普遍分布,有待进一步分析该菌株对獭兔腹泻与生产性能的影响。
4 结 论
从獭兔胃肠道内容物中分离并纯化得到粪肠球菌、短乳杆菌、空肠肠球菌和植物乳杆菌,所检乳酸菌在獭兔胃肠道中的相对水平在不同日龄獭兔胃肠道普遍分布,随獭兔日龄的增长而升高。
参考文献 Reference:
[1] 晓 敏,弓 剑,袁 博.家兔消化生理特性及寡糖在兔饲粮中的应用[J].中国饲料,2018(15):19-23.
XIAO M,GONG J,YUAN B.Physiological characteristics of rabbit digestive system and application of oligosaccharides in rabbit diets[J].China Feed,2018(15):19-23.
[2] 许国洋,沈克飞,徐登峰,等.重庆地区兔腹泻病原菌分离鉴定及检测方法的建立[J].西北农业学报,2018,27(8):1097-1103.
XU G Y,SHEN K F,XU D F,et al.lsolation and identification of pathogenic bacteria of rabbit diarrhea in Chongqing and establishment of its detection method[J].Acta Agriculturae Boreali-occidentalis Sinica,2018,27(8):1097-1103.
[3] 杜冬华,利 凯,周 静,等.白头翁素对E.coli诱导断乳腹泻仔兔肠道组织病理变化、NF-κB p65 mRNA及蛋白表达的影响[J].中国兽医学报,2018,38(6):1201-1206.
DU D H,LI K,ZHOU J,et al.Effect of anemonin on NF-κB p65 expression and pathologic changes of intestines in weanling rabbit diarrhea induced by E.coli[J].Chinese Journal of Veterinary Science,2018,38(6):1201-1206.
[4] BIAN X,SHI T,WANG Y,et al.Gut dysbiosis induced by antibiotics is improved by tangerine pith extract in mice[J].Nutrition Research,2022,101:1-13.
[5] 胡献刚,罗 义,周启星,等.固相萃取-高效液相色谱法测定畜牧粪便中13种抗生素药物残留[J].分析化学,2008(9):1162-1166.
HU X G,LUO Y,ZHOU Q X,et al.Determination of thirteen antibiotics residues in manure by solid phase extraction and high performance liquid chromatography[J].Chinese Journal of Analytical Chemistry,2008(9):1162-1166.
[6] 张义楠,袁文焕,兰力伟,等.抗生素耐药与肠道菌群的研究进展[J].饲料研究,2021,44(1):139-142.
ZHANG Y N,YUAN W H,LAN L W,et al.Research progress on antibiotic resistance and intestinal flora[J].Feed Research,2021,44(1):139-142.
[7] 石 磊,蒲芳芳,张瑜杰,等.植物乳酸菌LP45株对抗生素诱导肠道菌群、免疫及脑部功能损伤的改善作用[J].现代预防医学,2021,48(21):3945-3949,3959.
SHI L,PU F F,ZHANG Y J,et al.Protective influences of Lactobacillus plantarum LP45 on antibiotic-induced impairment of intestinal microbiota,immunity and brain function[J].Modern Preventive Medicine,2021,48(21):3945-3949,3959.
[8] 任大勇.益生乳酸杆菌的黏附及免疫调节作用研究[D].长春:吉林大学,2013.
REN D Y.Research on adhesion and immunoregulation of probiotic Lactobacillus strains[D].Changchun: Jilin University,2013.
[9] 刘艳姿.乳酸菌的生理功能特性及应用的研究[D].河北秦皇岛:燕山大学,2010.
LIU Y Z.The investigation on physiology functional characteristics of lactic acid bacteria and its applications[D].Qinhuangdao Hebei: Yanshan University,2010.
[10] CROSS M L.Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens[J].FEMS Immunology and Medical Microbiology,2002,34(4):245-253.
[11] 刘莲莲,张应龙,杨海莹,等.不同治疗条件下对仔兔大肠杆菌病肠道显微结构的影响[J].江西农业大学学报,2013,35(2):238-244.
LIU L L,ZHANG Y L,YANG H Y,et al.Effect of colibacillosis on intestine ultrastructure of rabbits under different treatment conditions[J].Acta Agriculturae Universitatis Jiangxiensis,2013,35(2):238-244.
[12] ZHOU Y,NI X,WEN B,et al.Appropriate dose of Lactobacillus buchneri supplement improves intestinal microbiota and prevents diarrhoea in weaning rex rabbits[J].Beneficial Microbes,2018,9(3):401-416.
[13] 李亚力.乳酸菌对幼龄獭兔生产性能与免疫性能的影响[D].陕西杨凌:西北农林科技大学,2013.
LI Y L.Effects of lactic acid bacteria on growth performance and immune capability of juvenile rabbits[D].Yangling Shaanxi: Northwest A &F University,2013.
[14] 占 萌,李 春,李慧臻,等.乳酸菌粘附肠道上皮细胞的研究进展[J].中国食品学报,2020,20(11):341-350.
ZHAN M,LI CH,L H ZH,et al.The research progress on the adherence of lactic acid bacteria to intestinal epithelial cells[J].Journal of Chinese Institute of Food Science and Technology,2020,20(11):341-350.
[15] 陈新亮,邵启兵,王 超,等.唾液乳杆菌的分离鉴定及生物特性研究[J].食品科学,2016,37(13):157-161.
CHEN X L,SHAO Q B,WANG CH,et al.Isolation and identification of Lactobacillus salivarius from chicken intestine and its biological characteristics[J].Food Science,2016,37(13):157-161.
[16] ARSOY E S,GVL L B,ON A H.Characterization and selection of potential antifungal lactic acid bacteria isolated from Turkish spontaneous sourdough[J].Current Microbiology,2022,79(5):148.
[17] NOPPARATMAITREE M,NAVA M,CHUMSANGCHOTISAKUN V,et al.Effect of trimmed asparagus by-products supplementation in broiler diets on performance,nutrients digestibility,gut ecology,and functional meat production[J].Veterinary World,2022,15(1):147-161.
[18] CHEN T,WANG L,LI Q,et al.Functional probiotics of lactic acid bacteria from Hu sheep milk[J].BMC Microbiology,2020,20(1):228.
[19] SEDDIK H A,BENDALI F,GANCEL F,et al.Lactobacillus plantarum and its probiotic and food potentialities[J].Probiotics and Antimicrobial Proteins,2017,9(2):111-122.
[20] FAN Y,LI X,TIAN R,et al.Characterization and biological activity of a novel exopolysaccharide produced by Pediococcus pentosaceus SSC-12 from silage[J].Microorganisms,2021,10(1):18.
[21] DAHIYA D,MANUEL J V,NIGAM P S.An overview of bioprocesses employing specifically selected microbial catalysts for γ-aminobutyric acid production[J].Microorganisms,2021,9(12):2457.
[22] SOKOVIC B S,DJOKIC J,DINIC M,et al.GABA-producing natural dairy isolate from artisanal Zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro[J].Frontiers in Microbiology,2019,10:527.
[23] 曹玮娜,任战军,李亚力,等.乳酸菌对獭兔生长性能、肉品质及肌肉胰岛素样生长因子-1、胰岛素样生长因子1受体、生长激素受体mRNA相对表达量的影响[J].动物营养学报,2014,26(9):2902-2910.
CAO W N,REN ZH J,LI Y L,et al.Effects of lactic acid bacteria on growth performance,meat quality and mRNA relative expression of insulin-like growth factor-1,insulin-like growth factor-1 receptor and growth hormone receptor in muscle of rex rabbits[J].Chinese Journal of Animal Nutrition,2014,26(9):2902-2910.
[24] 孔庆娟,郭丹丹.益生菌补充剂对断奶仔兔饲料效率、生长性能和微生物种群的影响[J].中国饲料,2020(2):33-38.
KONG Q J,GUO D D.Effects of probiotic supplements on feed efficiency,growth performance and microbial population of weaned rabbit[J].China Feed,2020(2):33-38.
[25] SHAH A A,YUAN X,KHAN R U,et al.Effect of lactic acid bacteria-treated King grass silage on the performance traits and serum metabolites in New Zealand white rabbits (Oryctolagus cuniculus)[J].Journal of Animal Physiology and Animal Nutrition (Berl),2018,102(2):e902-e908.
[26] DAHIYA D,NIGAM P S.The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer[J].Microorganisms,2022,10(3):665.
[27] 付 祥,吴钰宸,苏建青,等.乳酸菌在畜牧生产中的应用进展[J].饲料研究,2021,44(16):137-140.
FU X,WU Y CH,SU J Q,et al.Application progress on application of lactic acid bacteria in animal production[J].Feed Research,2021,44(16):137-140.
[28] 靳胜男,舒慧萍,张冬星,等.猪源生物被膜乳酸菌的筛选、鉴定与体外益生特性评价[J].河南农业科学,2019,48(12):121-127.
JIN SH N,SHU H P,ZHANG D X,et al.Screening,identification and probiotic properties assessment of swine lactic acid bacteria with biofilm[J].Journal of Henan Agricultural Sciences,2019,48(12):121-127.
[29] 姜 芹,孙冰清,顾 欣,等.不同粪肠球菌饲料添加剂质量分析[J].饲料研究,2020,43(2):74-77.
JIANG Q,SUN B Q,GU X,et al.Quality analysis of marketed Enterococcus faecalis feed additive[J].Feed Research,2020,43(2):74-77.
[30] 刘 辉,季海峰,张董燕,等.饲粮添加短乳杆菌对生长猪生长性能和血清生化指标的影响[J].动物营养学报,2013,25(1):182-189.
LIU H,JI H F,ZHANG D Y,et al.Effects of Lactobacillus brevis supplementation on growth performance,serum biochemical indices of growing pigs[J].Chinese Journal of Animal Nutrition,2013,25(1):182-189.
[31] 郑 越,段 涛,宋 丹,等.植物乳杆菌在动物饲料中应用的研究进展[J].中国饲料,2022(3):1-7.
ZHEGN Y,DUAN T,SONG D,et al.Review on the application of Lactobacillus plantarum in animal feed[J].China Feed,2022(3):1-7.
Isolation and Identification of Lactic Acid Bacteria in Gastrointestinal Tract of Rex Rabbits
ZHANG Feifei1,XUE Haitao2,WU Jie2 and REN Zhanjun2
(1.Urumqi Customs,Urumqi 836500,China; 2.College of Animal Science and Technology,Northwest A&F University,Yangling Shaanxi 712100,China)
Abstract In order to explore the inherent lactic acid bacteria in the gastrointestinal tract of rex rabbits and enrich the application of lactic acid bacteria,the neutral or acidic MRS medium was used to isolate and culture the lactic acid bacteria from the gastrointestinal tract of healthy rex rabbits.The species of the strains were identified by 16SrDNA sequencing,and the relative content of the strains in the gastrointestinal tract of 40-and 80-day-old rex rabbits was detected by genomic DNA fluorescence quantitative PCR.The results were as follows: Enterococcus faecalis strains were isolated from the stomach,jejunum,cecum,colon and rectum; Lactobacillus brevis strains were isolated from stomach,duodenum,jejunum,ileum and cecum; Lactobacillus plantarum strains were isolated from stomach,duodenum,ileum,cecum,colon and rectum respectively; Enterococcus hirae strains were isolated from duodenum,jejunum,cecum and colon.The strains obtained in this experiment are widely distributed in the gastrointestinal tract of rex rabbits,and their relative level increases with the age of rex rabbits,which suggests that these bacteria can be an alternative choice of endogenous lactic acid bacteria feed additives for rex rabbits.
Key words Lactic acid bacteria;Rex;16S rDNA
Received 2022-09-13 Returned 2022-10-09
Foundation item Innovation and Drive Project of Agricultural Science and Technology of Shaanxi Province[No.NYKJ-2021-YL(XN)28].
First author ZHANG Feifei,female,bachelor.Research area: animal and plant inspection and quarantine.E-mail: 1181953766@qq.com
Corresponding author REN Zhanjun,male,Ph.D,professor.Research area: healthy breeding and industrialization of economic animals.E-mail:renzhanjun@nwsuaf.edu.cn
(责任编辑:顾玉兰 Responsible editor:GU Yulan)

