Analyses of Chicken Tenderness Traits Based on Transcriptome Sequencing
Zengrong ZHANG Mohan QIU Chunlin YU Xia XIONG Xiaoyan SONG Bo XIA Shiliang ZHU Jialei CHEN Chaowu YANG
Abstract The calpain system is ubiquitous in cells, mainly comprising calpains and calpain inhibitors, and is a widespread calcium-dependent cysteine protease in organisms that is involved in many cellular processes such as muscle degradation in vivo and affects the tenderness of meat after animal slaughter. The study found 128 DEGs that probably regulated tenderness traits were selected from 16 significantly enriched GO terms by transcriptome sequencing analysis, and found that the developmental changes in the expression levels of the CAPN1 gene in the pectoral and leg muscles were significantly positively correlated (P<0.05) with the cumulative growth values of live weight and comb weight. The developmental changes in the expression levels of the CAST gene in the pectoral and leg muscles were not significantly correlated with the cumulative growth values of live weight and comb weight. Our results helped demonstrate the potential molecular mechanisms of tenderness in chickens and provide valuable information for chicken breeding.
Key words Chicken; Tenderness traits; Transcriptome sequencing
DOI:10.19759/j.cnki.2164-4993.2024.02.006
The calpain system is ubiquitous in cells, mainly comprising calpains and calpain inhibitors. Calpain (Calpain, CAPN) is a widespread calcium-dependent cysteine protease in organisms that is involved in many cellular processes such as muscle degradation in vivo and affects the tenderness of meat after animal slaughter. Calpastatin (Calpastatin, CAST) is an endogenous, Ca2+-activated calpain inhibitor that can inhibit the degradation of muscle proteins and inhibit the activity of calpains after slaughter, reducing protein hydrolysis[1-2]. Studies have shown that the genes of the calpain system are involved in the renewal of proteins during muscle growth and are closely related to the growth and tenderness of animal muscles[3-4].
Currently, the research on the calpain system in mammals mainly focuses on the correlation between calpain activity and the tenderness of postmortem muscle and the degradation amount of muscle protein. MicroRNAs (miRNAs) contain 20–22 nucleotides and are small non-coding RNAs responsible for post-translational regulation in plants and animals[5-6]. Several researchers have reported the association of miRNAs indicated in this study with chicken growth. The seed region of miR-1657 was found to be correlated with chicken growth and tenderness traits[7]. Transcriptome sequencing can provide comprehensive information about the chicken genome, including the structure and expression patterns of genes. Studying the expression of genes in different tissues and their association with tenderness traits helps to gain a deeper understanding of the molecular mechanisms that affect tenderness. In this study, global miRNA sequencing was used to investigate the differential expression of miRNA and its potential regulatory mechanisms related to tenderness and growth rates between the MC, ZJ and DH chicken.
Materials and Methods
Sample collection
MC, ZJ and DH chickens were obtained from the Sichuan Dahen Animal breeding company. All tissues were collected from 10-week-old chickens, which were then snap-frozen in liquid nitrogen and stored at -80 ℃. All experiments on chickens were carried out based on the guidelines of EU legislations on the ethical use and care of laboratory animals.
RNA isolation, Small RNA sequencing quality control and expression analysis
Total RNA was isolated from breast muscle using TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.) per the manufacturers instructions. The quantity and purity of RNA were determined using NanoDrop ND-1000 spectrophotometer at 260/280 nm (Nano Drop Technologies, Wilmington, Delaware). RNA integrity was measured via agarose gel electrophoresis. Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology). The differentially expressed miRNAs were identified by counts screening using the DESeq2[8] R package, with a threshold of Log2FC >1 or <-1 and P value <0.05. The miRNA target prediction was performed through the combination of Miranda (http://www.microrna.org/microrna/) with a score ≥150 and energy <-20 and RNAhybrid[9] with energy <-25. The results were obtained by the intersection of the two prediction tools. The heat map of differential expression was generated by Heml 1.0.
Bioinformatics analysis
Gene oncology analysis (http://geneontology.org) was performed to analyze the main function of the target genes based on Fishers exact test[10-12]. Pathway analysis was done to find out the significant pathway of miRNA targets based on the KEGG database (http://www.genome.jp/Kegg/).
Quantitative real-time PCR
Total RNA was extracted from tissues using Trizol reagent (Thermo Fisher Scientific) according to the manufacturers instructions. Two micrograms of RNA was used for cDNA synthesis through reverse transcription using PrimeScript RT Master Mix (Takara). Real-time PCR was performed in triplicate in a 96-well plate using 1 μl of cDNA and SYBR Green PCR mix (Bio-Rad) on a Real-Time PCR System (Thermo Fisher). The primer sequences of these miRNAs are listed. Expression of beta-actin was used to normalize gene expression. By convention, changes in expression were determined using 2-ΔΔCT method.
Results and Analysis
Analysis of DEGs between lines
To compare genetic difference among these three lines, the significantly expressed genes between lines MC and ZJ, lines MC and DH, and lines ZJ and DH under different development stages were mined separately. Initially, we compared the gene expression profile among lines MC, ZJ and DH, and found that 1953 DEGs were screened between ten-week-old line DH and line B, and 932 genes were more highly expressed in line ZJ than in line DH.
Gene-act-network and candidate genes for tenderness trait
After GO analysis and pathway analysis, 128 DEGs that probably regulated tenderness trait were selected from 16 significantly enriched GO terms (including terms related to muscle cell differentiation, muscle structure development, growth) and 13 significantly enriched pathways. To further explore the interactions between these DEGs, the gene-act-network was established based on the relationships between these DEGs in terms of expression and interaction. Several DEGs played a core role in the PPI network, including calcium-dependent cysteine protease, calpastatin, insulin like growth factor 2, apoptosis regulator, indicating that these genes may play key roles in regulating tenderness trait of chicken.
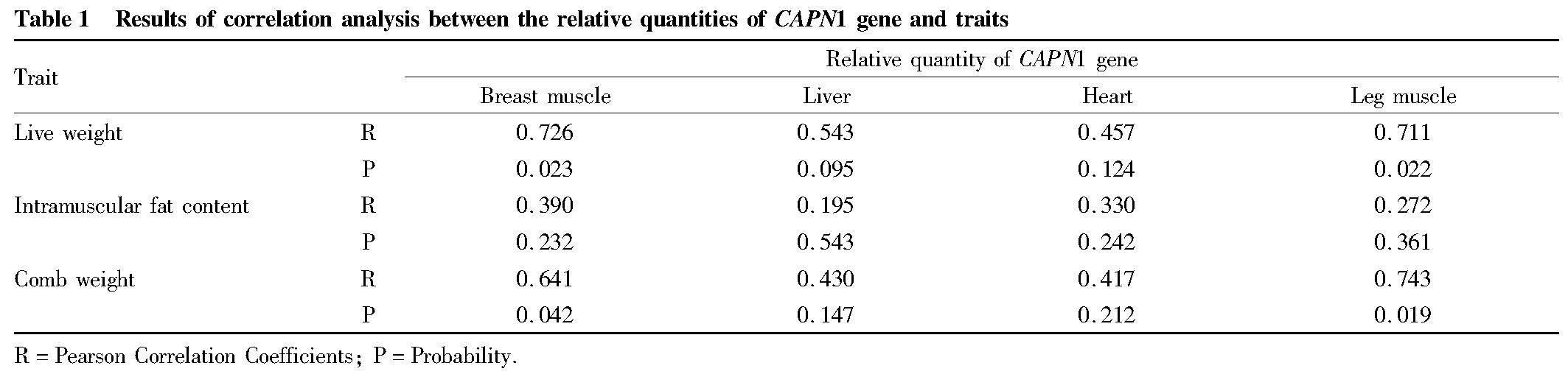
Correlation analysis of relative expression levels of CAPN1 gene with traits
The developmental changes in the expression levels of CAPN1 gene in pectoral muscle, leg muscle, heart, and liver tissues were respectively correlated with the cumulative growth values of the above traits by Pearson correlation analysis. As shown in Table 1, the developmental changes in the expression levels of CAPN1 gene in both pectoral muscle and leg muscle were significantly positively correlated with the cumulative growth values of live weight and comb weight (P<0.05); and the developmental changes in the expression levels of CAPN1 gene in heart and liver tissues had no significant correlation with the three traits under investigation (P>0.05).
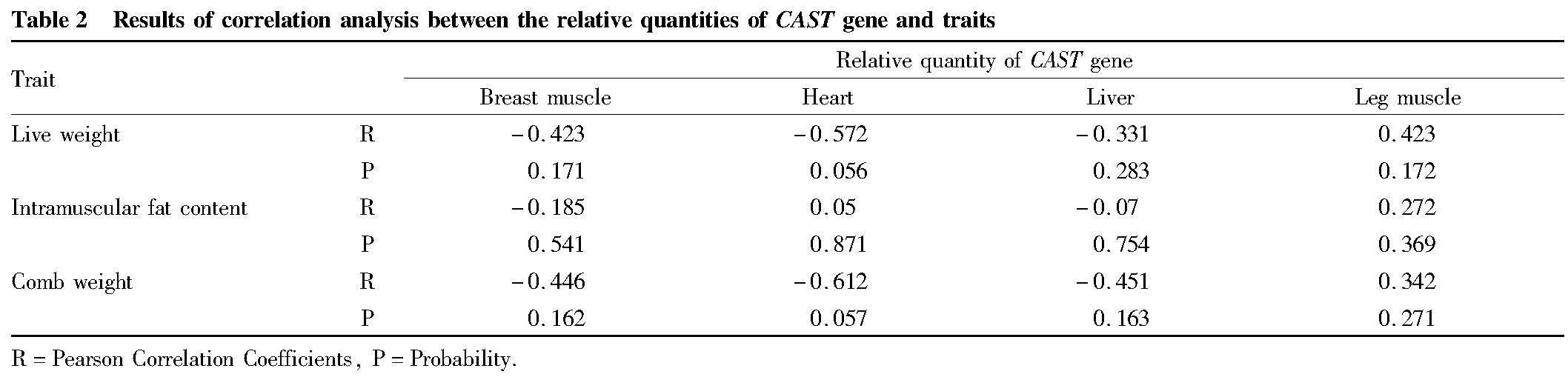
Correlation analysis between the relative expression of CAST gene and traits
The developmental changes of CAST gene expression in pectoral muscle, leg muscle, heart and liver tissues were respectively correlated with the cumulative growth values of the above traits by Pearson correlation analysis. As shown in Table 2, there was no significant correlation between the developmental changes of CAST gene expression in pectoral muscle, leg muscle, heart and liver tissues and the three traits under investigation (P>0.05).
Conclusions
Chicken muscle growth is important traits to evaluate the production of poultry meat. miRNAs play a vital role in the growth and development[13-14].
The calpain system controls the degradation of myofibrillar proteins. Myofibers are the basic units that make up muscles, and the state of myofibers will directly affect the quality of chicken meat. In this study, we found 128 DEGs that probably regulated tenderness trait were selected from 16 significantly enriched GO terms and 13 significantly enriched pathways.
The expression changes of the CAPN1 and CAST genes in the pectoral and leg muscle tissues mainly manifest in the degradation of muscle proteins and the growth and development changes of myofibers. It is known that the development of the comb can directly reflect the sexual maturity of chickens. The early-maturing MC chicken has a small body weight. Therefore, comb weight can also reflect the growth and development of chickens to a certain extent. Meanwhile, this study found that the developmental changes in the expression levels of the CAPN1 gene in the pectoral and leg muscles were significantly positively correlated (P<0.05) with the cumulative growth values of live weight and comb weight. The developmental changes in the expression levels of the CAST gene in the pectoral and leg muscles were not significantly correlated with the cumulative growth values of live weight and comb weight.
In summary, it is hypothesized that the expression level of the CAPN1 gene in muscle tissues may be closely related to the growth and development of myofibers and plays an important regulatory role in the metabolism of myofibrillar proteins. The research on CAST genes as candidate genes for meat quality has just begun. In general, the specific regulatory mechanism of the calpain system is not very clear and requires further in-depth research.
Zengrong ZHANG et al. Analyses of Chicken Tenderness Traits Based on Transcriptome Sequencing
References
[1] HUANG J, FORSBERG NE. Role of calpain in skeletal-muscle protein degradation[J]. Proc Natl Acad Sci USA, 1998, 95(21): 12100-12105.
[2] GOLL DE, THOMPSON VF, TAYLOR RG, et al. Role of the calpain system in muscle growth[J]. Biochimie., 1992, 74(3): 225-237.
[3] MURPHY RM. Calpains, skeletal muscle function and exercise[J]. Clin Exp Pharmacol Physiol., 2010, 37(3): 385-391.
[4] ZHAO L, JIANG N, LI M, et al. Partial autolysis of μ/m-calpain during post mortem aging of chicken muscle[J]. Anim Sci J. 2016, 87(12): 1528-1535.
[5] AMBROS V. The functions of animal microRNAs[J]. Nature, 2004(431): 350-355.
[6] BARTEL DP. MicroRNAs: Genomics, biogenesis, mechanism, and function[J]. Cell, 2004(116): 281-297.
[7] LI H, SUN GR, LV SJ, et al. Association study of polymorphisms inside the miR-1657 seed region with chicken growth and meat traits[J]. British Poultry Science, 2012(53): 770-776.
[8] LOVE MI, HUBER W, ANDERS S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2[J]. Genome Biology, 2014(15): 550.
[9] KRUGER J, REHMSMEIER M. RNAhybrid: microRNA target prediction easy, fast and flexible[J]. Nucleic Acids Research, 2006(34): W451-W454.
[10] AL-SHAHROUR F, DIAZ-URIARTE R, DOPAZO J. FatiGO: A web tool for finding significant associations of gene ontology terms with groups of genes[J]. Bioinformatics, 2004(20): 578-580.
[11] DRAGHICI S, KHATRI P, BHAVSAR P, et al. Onto-tools, the toolkit of the modern biologist: Onto express, onto-compare, onto-design and onto-translate[J]. Nucleic Acids Research, 2013(31): 3775-3781.
[12] ZEEBERG BR, FENG W, WANG G, et al. GoMiner: A resource for biological interpretation of genomic and proteomic data[J]. Genome Biology, 2003(4): R28.
[13] BHASKARAN M, MOHAN M. MicroRNAs: History, biogenesis,and their evolving role in animal development and disease[J]. Veterinary Pathology, 2014(51): 759-774.
[14] LUI JC. Regulation of body growth by microRNAs[J]. Molecular and Cellular Endocrinology, 2017(456): 2-8.
- 农业生物技术(英文版)的其它文章
- Research Progress on Effects of Continuous Cropping on Soil Microbial Florae and Its Restoration
- Slaughter Performance, Muscle Quality and Nutritional Composition of Duoluo Goats in Sichuan Province
- Effects of Different Grinding Methods on the Quality of Soybean Bean Milk
- A method for Improving the Efficiency of Pear Tree Crossbreeding
- Analysis of the Effect and Influencing Factors of Rural Domestic Sewage Treatment Based on A2O-MBBR Integrated Process
- Study on Detection of Antibiotic Contents in Water around Landfill Sites

