Study on Antioxidant Enzyme Activity and Physiology and Biochemistry of Solanum nigrum L. under Glyphosate Stress
Si LIU Tingting ZOU Wenshuai ZENG Zihan MEI Chenzhong JIN Yihong HU

Abstract [Objectives] This study was conducted to investigate the scientific prevention and control of Solanum nigrum L.
[Methods] Through experiments on S. nigrum from different sources, it was found that glyphosate stress had significant effects on antioxidant enzyme activity and oxidative damage of sensitive S. nigrum plants.
[Results] Sensitive S. nigrum showed oxidative damage under glyphosate stress, while resistant S. nigrum responded to adversity damage by improving its antioxidant enzyme activity. The experimental results showed that the antioxidant enzymes and reduced glutathione of S. nigrum had certain metabolic detoxification effects under glyphosate stress.
[Conclusions] This study provides a theoretical basis for scientific prevention and control of S. nigrum, and has a certain reference value for revealing the glyphosate resistance mechanism of S. nigrum.
Key words Glyphosate; Solanum nigrum L.; Antioxidant enzyme; Malondialdehyde; Glutathione
DOI:10.19759/j.cnki.2164-4993.2024.02.001
Solanum nigrum L. is a perennial weed of Solanum L. in Solanaceae, which prefers to grow in slightly acidic to neutral soil, and is commonly found in wasteland, fields, and by villages and roads. S. nigrum is distributed in all provinces and cities in China, and has the characteristics of wide distribution, many seeds, strong abscission and strong resistance[1-3].
Glyphosate is a broad-spectrum herbicide with low toxicity, low residue and systemic conductivity, which is widely used to control weed S. nigrum in cultivation of cotton, soybean, rape and other crops[4]. In recent years, it was found that with the increase of glyphosate dosage year by year, S. nigrum showed resistance to glyphosate. Studies have shown that there are 28 kinds of weeds, including Lolium rigidum, Erigeron canadensis L. and Cirsium arvense var. integrifolium, which are resistant to glyphosate[5], and the problem of glyphosate-resistant weeds is becoming more and more serious.
The weeding mechanism of glyphosate is mainly to inhibit the activity of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), block the synthesis of aromatic amino acids in plants, and make shikimic acid accumulate in plants in large quantities, resulting in yellowing and withering of plants until death[6-8]. Glyphosate kills weeds by inhibiting the activity of target enzymes, and it has also been reported that weeds can develop drug resistance by enhancing their metabolic detoxification ability, such as glutathione S-transferase, cytochrome P450 monooxygenase and superoxide dismutase, which can metabolize herbicides and reduce the harm caused by herbicides to organisms[9]. The metabolic process of plants is closely related to the activity of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and reduced glutathione (GSH). The activity of SOD, POD and CAT can be increased and the content of MDA can be increased in the leaves of Microsorium pteropus suffered from glyphosate stress[10]. Stress on S. nigrum with cadmium can increase MDA content and SOD and POD activity[11], and treatment of S. nigrum leaves with glyphosate can increase MDA content[12], and reduced glutathione has metabolic detoxification effect[13].
Although the non-target resistance of weeds is very important to explaining the mechanism of weed resistance, there are few reports about the non-target resistance of S. nigrum to glyphosate at home and abroad. In this study, S. nigrum from different sources was used as experimental materials to study the effects of glyphosate stress on the non-target resistance of S. nigrum from the aspect of oxidation resistance, in order to provide a theoretical basis for scientific prevention and control of S. nigrum.
Materials and Methods
Experimental materials
The seeds of S. nigrum were collected from September to October in 2015 in Loudi City and Changde City, Hunan Province, and preserved after natural air drying. The soil with uniform particles was uniformly mixed with a substrate according to a ratio of 1:1, and the obtained mixture was added into flowerpots. N-(phosphonomethyl)glycine (41%) was purchased from Loudi Zhennong Technology. Methionine (Met), nitrotetrazolium blue chloride (NBT) and 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) were Sigma products. Other reagents were analytically pure in China.
Experimental method
Seed germination
The seeds of S. nigrum were placed in a Petri dish with two layers of filter paper, and soaked in distilled water for 24 h. Subsequently, they were soaked in 13 g /L sodium hydroxide for 48 h after removing distilled water to break dormancy, and then washed and placed in a light incubator for germination at 25 ℃ and a relative humidity of 65%. After germination, the seeds were moved into flowerpots for pot culture, and one seedling was cultured in each pot. When the seedlings grew to six leaves, they were moved outdoor for culture.
Glyphosate treatment
When S. nigrum plants grew to 10-14 leaves, they were sprayed with different concentrations of glyphosate, and the effective components of glyphosate were 450, 900, 1 800 and 3 600 g/hm2, respectively, compared with clean water. Samples were taken in the morning of days 3, 6, 9 and 12 after glyphosate treatment, and the leaves of S. nigrum were cut and stored in a refrigerator at 4 ℃ for later use.
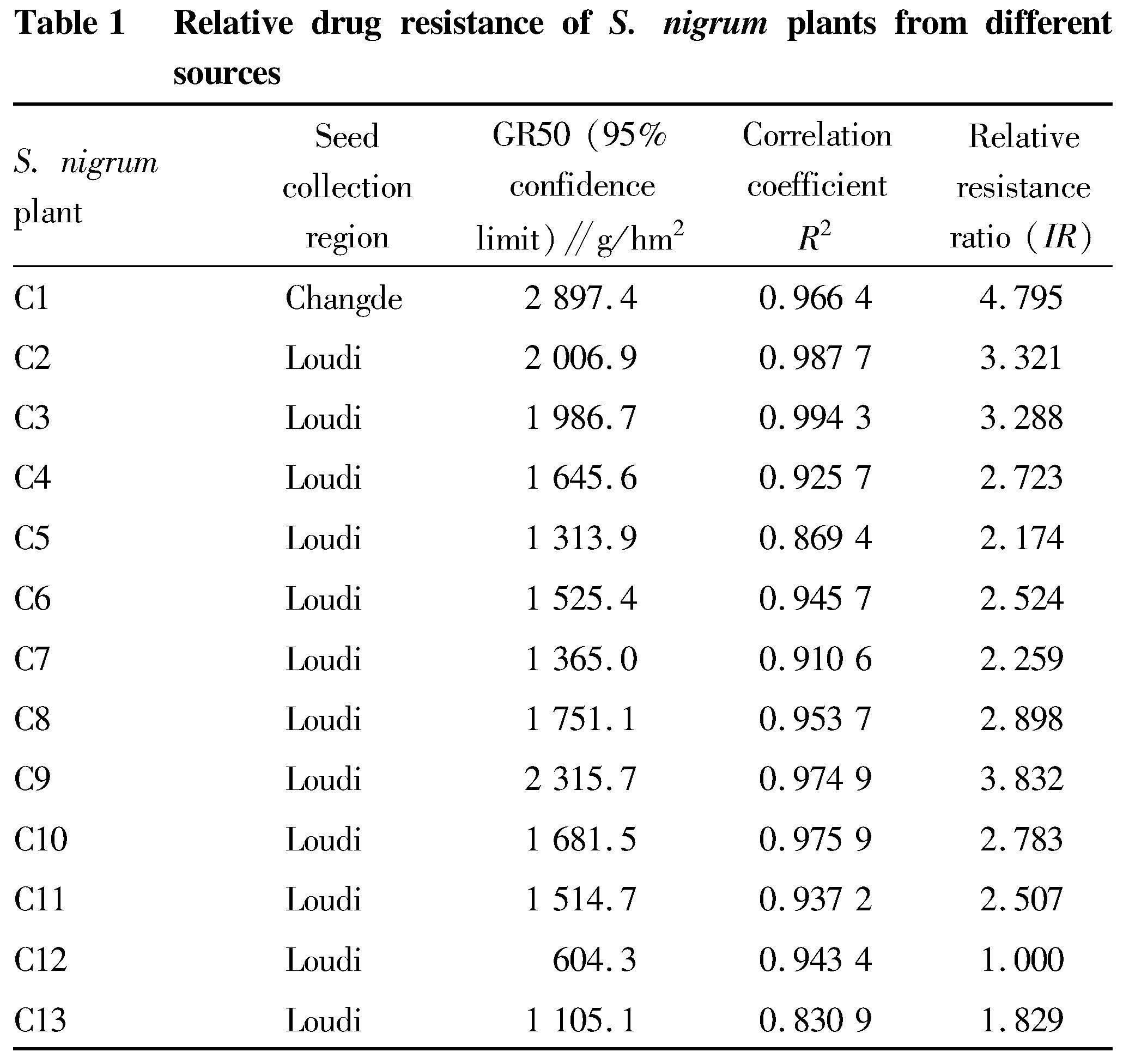
Determination of drug resistance level
On day 12 after glyphosate treatment, 10 plants were randomly selected from S. nigrum population to measure fresh weight, and the fresh weight inhibition rate was calculated, and the regression equation was obtained. According to the regression equation, the inhibition concentration GR50 and relative resistance ratio were obtained.
Fresh weight inhibition rate (%)=(Fresh weight of control-Fresh weight of treatment)×100/Fresh weight of treatment
Extraction and content determination of shikimic acid
According to the method of Lou et al.[14], 0.2 g of leaf sample was weighed and cut into a mortar, and added with 0.25 mol/L hydrochloric acid. The sample was ground and diluted to a constant volume of 1 ml. After centrifuging at 12 000 r/min for 20 min, the supernatant was collected and stored at 4 ℃, and each treatment was repeated for 3 times. Next, 100 μl of supernatant was added with 1 ml of 1% periodate solution, and 1 ml of 1mol/L NaOH was added after 3 h, and the liquid was mixed well. Next, 0.6 ml of 0.1 mol/L glycine was added, and after mixing well and standing for 5 min, the OD value was measured at 380 nm.
Determination of antioxidant enzyme activity
According to the method of Zhang et al.[15], 0.1 g of leaf sample was ground with 0.8 ml of 0.2 mol/L phosphate buffer (pH = 7.8) and diluted to a constant volume of 1 ml, and the obtained slurry was centrifuged at 10 000× g for 10 min. The supernatant was determined for the activity of SOD, POD and CAT, among which the SOD activity was taken as 1 U based on photochemical reduction of 50% of NBT, the POD activity was taken as 1 U based on the increase of A480 by 0.1 per minute, and the CAT activity was taken as 1 U based on the decrease of A240 by 0.1 per minute.
Determination of MDA content
According to the method of Lin et al.[15], 0.1 g of leaf sample was ground with 10% trichloroacetic acid (TCA) and diluted to a constant volume of 6 ml, and the obtained slurry was centrifuged at 12 000×g for 20 min. Next, 3 ml of supernatant was added with 3 ml of 0.6% thiobarbituric acid, and the obtained liquid was mixed well, and heated in a boiling water bath for 15 min. After cooling, the liquid was centrifuged at 12 000×g for 5 min, and measured for OD values at 450 and 532 nm.
Determination of GSH content
According to the method of Piao et al.[17], 0.1 g of leaf sample was ground with 5 % TCA and diluted to a constant volume of 2 ml, and the obtained slurry was centrifuged at 12 000×g for 15 min. Next, 0.5 ml of supernatant was added with 1.8 ml of 0.2 mol/L phosphate buffer with a pH of 7.7, 0.5 ml of distilled water and 0.2 ml of 0.001 mol/L DTNB, and the OD value was determined at 412 nm.
Data processing
Each determination was repeated for 3 times. The differences were compared by SPSS 19.0 (LSD method) (P<0.05), and significant differences were indicated by lowercase letters.
Results and Analysis
Screening of resistant plants
As can be seen from Fig. 1, except for the shikimic acid content of C12, which increased first and then tended to be stable, the shikimic acid contents of other treatments showed a trend of first increasing and decreasing then, and the shikimic acid contents of C1, C2, C3 and C9 decreased greatly.
The resistance level test also showed that the relative resistance ratios of C1, C2, C3 and C9 to glyphosate were all more than 3 times, showing a moderate resistance level (Table 1). Accordingly, C1, C2, C3 and C9 were resistant plants.
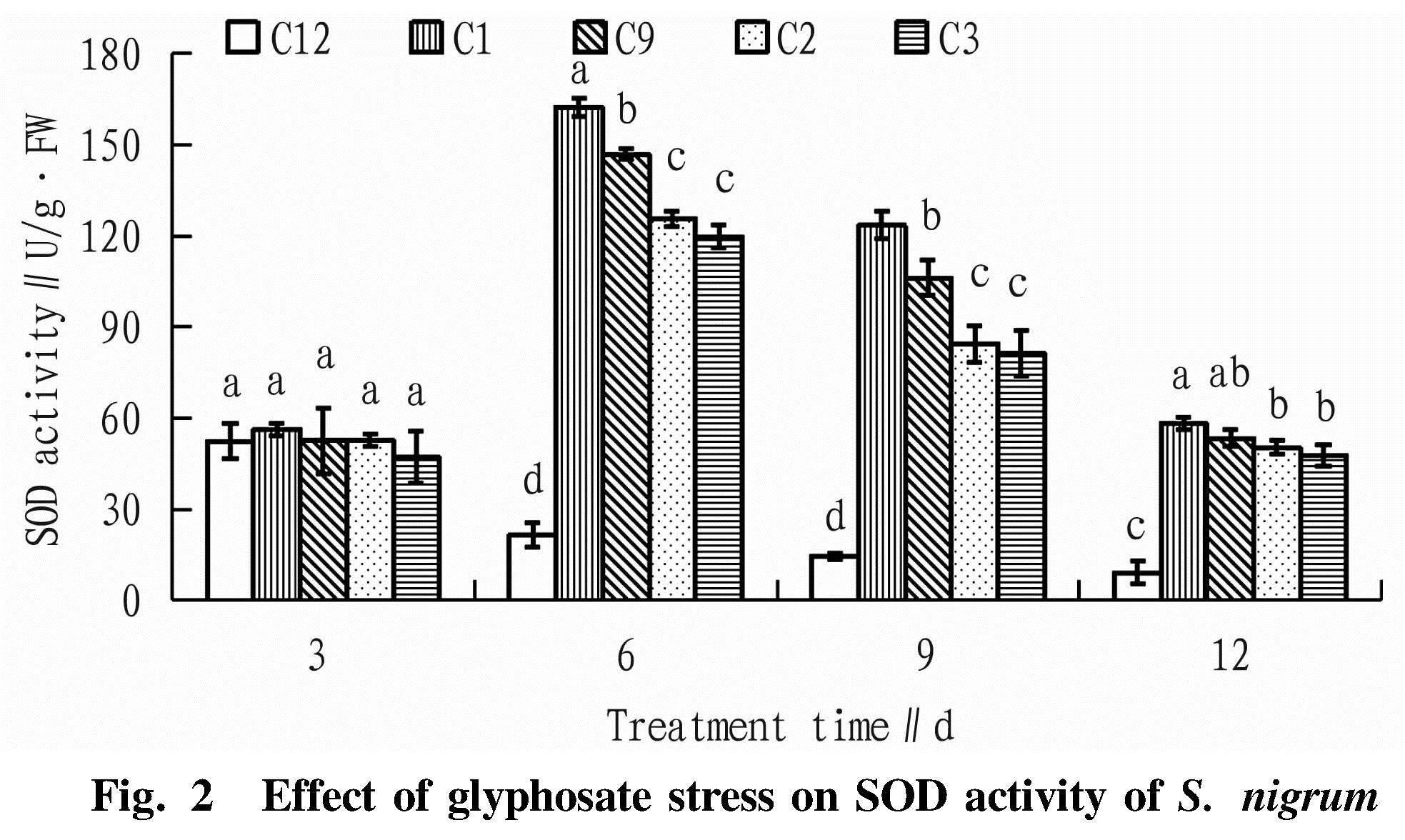
Effect of glyphosate stress on SOD activity of S. nigrum
As can be seen from Fig. 2, when S. nigrum plants were treated with glyphosate at 1 800 g/hm2, with the extension of time, the SOD activity of C12 decreased gradually, and the SOD activity of C1, C2, C3 and C9 generally increased first and then decreased. On day 3 after glyphosate treatment, there was no significant difference in SOD activity of S. nigrum. On days 6 and 9 after glyphosate treatment, there were significant differences in SOD activity among C1, C2, C3, C9 and C12. On day 12 after treatment, the SOD activity levels of C1, C2, C3 and C9 basically decreased to the initial level, but were still significantly higher than that of C12. The decrease of SOD activity in C12 might be caused by oxidative damage caused by glyphosate stress, while C1, C2, C3 and C9 might resist oxidative damage caused by glyphosate stress by increasing SOD activity in their bodies.
Effect of glyphosate stress on POD activity of S. nigrum
It can be seen from Fig. 3 that when the plants of S. nigrum were treated with glyphosate at the same dose concentration, except for the POD activity of C12, which showed a decreasing trend, the levels of other S. nigrum increased first and then decreased, and were all the highest at day 6 after treatment. On day 3 after treatment, there were no significant differences in POD activity between C12 and other S. nigrum. On day 6 and 9 after treatment, except for the POD activity of C2 and C3 showing no significant difference, there were significant differences in POD activity among other S. nigrum plants. On day 12 after treatment, the POD activity of C1, C2, C3 and C9 basically decreased to the initial level, but there were still significant differences between them and C12. Glyphosate stress will cause oxidative damage to plants. When sensitive S. nigrum plants are damaged, their metabolic system is also damaged, which makes it impossible to metabolize and detoxify, while resistant S. nigrum plants may improve the activity of metabolic enzymes through the antioxidant mechanism and eliminate the oxidative damage caused by stress.
Effect of glyphosate stress on CAT activity of S. nigrum
As can be seen from Fig. 4, under the treatment of glyphosate with the same dose concentration, with the extension of time, the CAT activity of C12 gradually decreased, and the CAT activity of the rest S. nigrum generally showed a trend of increasing first and then decreasing. On day 3 after treatment, there were no significant differences in CAT activity among the plants of S. nigrum. On day 6 and 9 after treatment, the CAT activity levels of C1, C2, C3 and C9 were significantly higher than that of C12. On day 12 after treatment, there were no differences in CAT activity among C1, C2, C3 and C9, and the values decreased to the initial level, but there were significant differences between them and C12. It showed that the antioxidant enzyme system of S. nigrum may be an important mechanism to resist glyphosate stress.
Si LIU et al. Study on Antioxidant Enzyme Activity and Physiology and Biochemistry of Solanum nigrum L. under Glyphosate Stress
Effect of glyphosate stress on MDA content in S. nigrum
As can be seen from Fig. 5, the MDA content of C12 increased first and then tended to be stable, while the MDA content of other S. nigrum plants generally increased first and then decreased. On day 3 after treatment, there were no significant differences in MDA content among all S. nigrum plants. On day 6 after treatment, the MDA content of C12 was significantly higher than those of other S. nigrum plants. On day 9 after treatment, the MDA content of C12 still increased slightly, while the MDA contents of C1, C2, C3 and C9 decreased significantly. On day 12 after treatment, the MDA content of C12 was significantly higher than those of C1, C2, C3 and C9, and tended to be stable, while the MDA contents of C1, C2, C3 and C9 decreased to the initial level. It indicated that glyphosate stress caused serious oxidative damage to C12 cell membrane.
Effect of glyphosate stress on GSH content in S. nigrum population
It can be seen from Fig. 6 that the GSH content of C12 gradually decreased with time, while the GSH contents of C1, C2, C3 and C9 generally increased first and then decreased, and reached a maximum value on day 6 after treatment, which was consistent with the changes of SOD, POD and CAT activity. On day 3 after treatment, there were no significant differences in GSH content among all S. nigrum plants. On days 6 and 9 after treatment, except for the GSH contents of C2 and C3 which showed no significant difference, there were significant differences among other S. nigrum plants. On day 12 after treatment, the GSH contents of C1, C2, C3 and C9 basically decreased to the initial level, but they were still significantly higher than that of C12. It showed that reduced glutathione in S. nigrum had certain detoxification ability to glyphosate.
Conclusions and Discussion
The process of plant metabolism is often accompanied by the generation of reactive oxygen species, and antioxidant enzymes can scavenge reactive oxygen species, so that the generation and elimination of reactive oxygen species in plants are in a dynamic balance. When stressed by adversity, because the metabolic balance of free radicals in plants is broken, a large number of superoxide anion free radicals will be produced. Too many free radicals will damage the cell membrane system, make the membrane lipid peroxidate and increase the MDA content. In severe cases, they will have adverse effects on the metabolic system of plants and eventually lead to plant death. Plant cells are damaged by oxidation due to adversity stress, and SOD, POD and CAT cooperate to play an antioxidant role to eliminate oxidative damage. GSH molecule also contains an active sulfhydryl group, which is easy to be oxidized and dehydrogenated. Therefore, plants can resist oxidative damage and reduce stress damage by increasing the contents of SOD, POD, CAT and GSH. This acquired resistance is not caused by glyphosate target mutation, but by non-target resistance caused by oxidative respiration processes such as cell fluid, chloroplasts and mitochondria. Through the synergistic effect of SOD, POD, CAT and GSH, the activity of antioxidant enzymes is improved. The changing trend of MDA can reflect the elimination effect of toxic effect.
This study also showed that the MDA content of sensitive S. nigrum C12 was the highest under glyphosate stress of 1 800 g/hm2, while the activity of SOD, POD, CAT and GSH decreased gradually with time, which indicated that C12 was the most stressed under glyphosate, and the antioxidant enzyme activity of sensitive S. nigrum was decreased under adversity stress, so it could not effectively cope with the stress. The MDA contents of resistant S. nigrum plants C1, C2, C3 and C9 increased first and then decreased with time, and reached a maximum value on day 6 after treatment, which indicated that glyphosate stress also caused oxidative damage to resistant S. nigrum plants, but the activity of SOD, POD, CAT and GSH also increased first and then decreased with time, which indicated that resistant S. nigrum plants could cope with stress injury by improving their antioxidant enzyme activity. On day 12 after treatment, the MDA content of resistant S. nigrum plants dropped below the initial level, indicating that the resistant S. nigrum population effectively responded to adversity damage. The results showed that the resistant S. nigrum plants could repair themselves by increasing their antioxidant enzyme activity after a certain concentration of glyphosate stress for a period of time, thus alleviating free radical toxicity and membrane lipid peroxidation damage.
References
[1] CAO XM, FAN CL. Research progress on development and utilization of wild Solanum nigrum[J]. Guangdong Agricultural Sciences, 2011(3): 40-42. (in Chinese).
[2] LU RM, TAN XW, ZHOU YY. Research progress of Solanum nigrum[J]. Lishizhen Medicine and Materia Medica Research, 2009, 20(7): 1820-1822. (in Chinese).
[3] JIANG HL, WANG JG, DENG XX, et al. Effects of glyphosate on the physiological character of Solanum nigrum[J]. Acta Agriculturae Boreali-occidentalis Sinica, 2011, 20(6): 186-189. (in Chinese).
[4] BAERSON SR, RODRIGUEZ DJ, TRAN M, et al. Glyphosate-resistant goosegrass identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase[J]. Plant Physiology, 2002, 129(3): 1265-1275.
[5] GONG YY, GUO SQ, SHU HM, et al. Advances on resistance mechanism of glyphosate-resistant weeds[J]. Weed Science, 2012, 30(3): 9-13. (in Chinese).
[6] SCHONBRUNN E, ESCHENBURG S, SHUTTLEWORTH WA, et al. Interaction of the herbicide glyphosate with its target enzyme 5-enlpyruvylshikimate-3-phosphate synthase in atomic detail[J]. PNAS, 2001, 98: 1376 -1380.
[7] HERMANN KM, WEAVER LM. The shikimate pathway[J]. Annual Review of Plant Biology, 1999, 50: 473-503.
[8] WILLIAMS GM, KROES R, MUNRO IC. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans[J].Regulatory Toxicology and Pharmacology, 2000, 31(2): 117-165.
[9] SONG CQ, LIAO HF, ZHU B, et al. The role of plant glutathione S-transferase in phytoremediation[J]. Anhui Agricultural Science Bulletin, 2010, 16(7): 56-57. (in Chinese).
[10] ZHANG JC, YANG DH. Activity of SOD, CAT, POD and content of MDA in Microsorium pteropus caused by glyphosate stress at normal and low temperature[J]. Natural Science Journal of Harbin Normal University, 2010, 26(4): 90-95. (in Chinese).
[11] LIU SL, YANG RJ, MA MD, et al. Effects of soil cadmium on growth and physiological characteristics of Solanum nigrum L. plants[J]. Journal of Agro-Environment Science, 2015, 34(2): 240-247. (in Chinese).
[12] JIANG HL, WANG JG, DENG XX, et al. Effects of glyphosate on the physiological character of Solanum nigrum[J]. Acta Agriculturae Boreali-occidentalis Sinica, 2011, 20(6): 186-189. (in Chinese).
[13] WEI LL, CHEN Y, ZHENG SJ, et al. Effect of reduced glutathione on detoxification and metabolic function of stem cells in vitro[J]. Chronic Pathematology Journal, 2007(3): 97-99. (in Chinese).
[14] LOU YL, ZHENG YY, SHEN JL, et al. Effects of metsulfuron-methyl and glyphosate on acetolactate synthase activities and shikimate levels of Alternanthera philoxeroides[J]. Journal of Plant Protection, 2005(32): 185-188. (in Chinese).
[15] ZHANG LX, ZHANG TF, KONG LY. Biochemical experimental methods and techniques[M]. Beijing: Higher Education Press, 1997. (in Chinese).
[16] LIN Y, GUO WZ, XU ZH, et al. Cold resistance and changes on MDA and soluble sugar of leaves of Ligustrun lucidum Ait in winter[J]. Chinese Agricultural Science Bulletin, 2012, 28(25): 68-72. (in Chinese).
[17] PIAO YL, SHEN L, HAN L, et al. Reduced glutathione (GSH) and total thiol detection in some fruits by derivatizing methods[J]. Science and Technology of Food Industry, 2012, 33(20): 60-64. (in Chinese).
- 农业生物技术(英文版)的其它文章
- Research Progress on Effects of Continuous Cropping on Soil Microbial Florae and Its Restoration
- Slaughter Performance, Muscle Quality and Nutritional Composition of Duoluo Goats in Sichuan Province
- Analyses of Chicken Tenderness Traits Based on Transcriptome Sequencing
- Effects of Different Grinding Methods on the Quality of Soybean Bean Milk
- A method for Improving the Efficiency of Pear Tree Crossbreeding
- Analysis of the Effect and Influencing Factors of Rural Domestic Sewage Treatment Based on A2O-MBBR Integrated Process

