一锅多组分反应合成3-(2-(芳基乙炔)苯基)喹唑啉酮
於兵 曾繁林
摘 要:以2-(芳基乙炔)苯胺、2-氨基芳基甲酸甲酯、原甲酸三乙酯为反应物,通过一锅多组分反应,在无需外加溶剂的条件下,以中等到良好的产率(30%~71%)实现了系列3-(2-(芳基乙炔)苯基)喹唑啉酮类化合物的合成.该类化合物可以作为新型的受体应用在自由基串联环化反应中,合成喹唑啉酮并喹啉化合物类含氮杂环.
关键词:3-(2-(芳基乙炔)苯基)喹唑啉酮;多组分反应;杂环;喹唑啉酮并喹啉
中图分类号:O612 文獻标志码:A文章编号:1000-2367(2024)03-0010-07
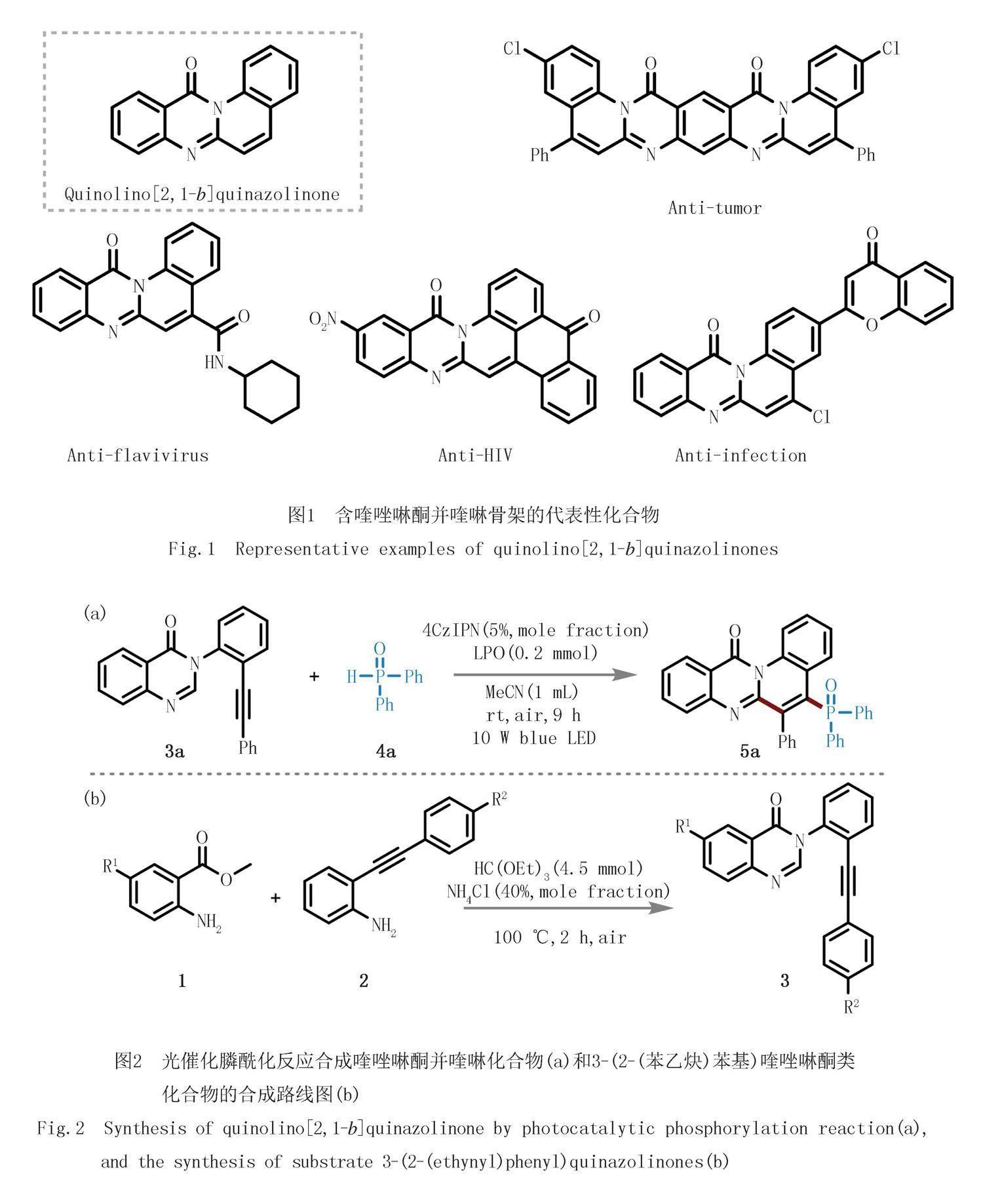
杂环化合物尤其是含氮杂环化合物的重要性不言而喻,约60%美国FDA(食品药品管理局)批准上市的药物都含氮杂环骨架[1].其中,喹唑啉酮并喹啉作为喹啉融合的稠杂环骨架,因其具有良好的生物活性和药用价值而受到合成化学家的极大关注[2-4].如图1所示的含有喹唑啉酮并喹啉骨架的代表性化合物具有抗肿瘤、抗黄病毒、抗HIV、抗感染等重要的生物活性[5-7].因此,合成结构新颖的喹唑啉酮并喹啉衍生物可以为活性分子的发现提供物质基础.
自由基串联环化反应具有效率高、操作简单、步骤经济等优点,它能“一锅”实现多个化学键、多个环系的构建,近年来已经成为一种合成杂环化合物的重要策略[8-11].自由基串联环化反应的关键之一是设计合理的反应底物,底物在反应中接受自由基的加成,并经过分子内环化、脱质子等步骤实现官能团化杂环的合成[12-14].因此,开发新型的自由基串联环化反应底物具有重要意义,可为新型杂环骨架的高效构筑提供全新的平台.
最近,本课题组首次通过光催化的自由基串联环化反应策略实现了喹唑啉酮并喹啉衍生物的构筑[15].该研究中,以3-(2-(芳基乙炔)苯基)喹唑啉酮类化合物(3a)作为自由基串联环化反应底物,以二苯基氧化膦(4a)为自由基前体,在可见光诱导下实现了膦酰化喹唑啉酮并喹啉化合物(5a)的合成(图2(a)).
由于3-(2-(芳基乙炔)苯基)喹唑啉酮类化合物作为一类新型的平台化合物,在自由基串联环化反应中表现出了广阔的应用前景,因此,本文详细报道了该类化合物的合成方法(图2(b)),即:以2-氨基芳基甲酸甲酯(1),2-(芳基乙炔)苯胺(2)、原甲酸三乙酯为反应物,通过一锅多组分反应,在无需外加溶剂的条件下,100 ℃油浴中反应2 h,以中等到良好的产率(30%~71%)实现了系列3-(2-(芳基乙炔)苯基)喹唑啉酮类化
收稿日期:2023-11-05;修回日期:2023-12-04.
基金项目:国家自然科学基金(21971224;22171249);河南省高校科技创新人才支持计划(23HASTIT003);河南工业大学高层次人才基金(31401601).
作者简介:於兵(1987-),男,湖北黄梅人,郑州大学教授,博士,研究方向为有机化学,E-mail:bingyu@zzu.edu.cn.
通信作者:曾繁林(1992-),男,河南信阳人,河南工业大学讲师,博士,研究方向为有机化学,E-mail:caist_zfl@haut.edu.cn.
引用本文:於兵,曾繁林.一锅多组分反应合成3-(2-(芳基乙炔)苯基)喹唑啉酮[J].河南师范大学学报(自然科学版),2024,52(3):10-16.(Yu Bing,Zeng Fanlin.Synthesis of 3-(2-(arylethynyl)phenyl)quinazolinones via one-pot multi-component reaction[J].Journal of Henan Normal University(Natural Science Edition),2024,52(3):10-16.DOI:10.16366/j.cnki.1000-2367.2023.11.05.0002.)
合物的合成.
1 实验部分
1.1 仪器与试剂
旋转蒸发仪(上海爱朗仪器有限公司N-1300型),八位平行光化学反应系统(北京诺植科技有限公司RLH-18型),加热磁力搅拌器(北京大龙兴创实验仪器股份公司),高分辨质谱仪(Waters Micromass Q-Tof Micro instrument),核磁共振仪(Bruker Avance III-400 MHz NMR,CDCl3(氘代氯仿)为溶剂,TMS(四甲基硅烷)为内标).
2-氨基芳基甲酸甲酯、2-(芳基乙炔)苯胺和原甲酸三乙酯(HC(OEt)3)等商品化试剂均购买于安徽泽升科技有限公司.石油醚、二氯甲烷等常见溶剂购买于成都市科隆化学品有限公司.薄层色谱板(TLC,GF254)和柱层析硅胶(200~300目)购买于山西诺泰生物科技有限公司.所有商品化试剂均未经纯化直接使用.
1.2 3-(2-(苯乙炔)苯基)喹唑啉酮类化合物的合成通法
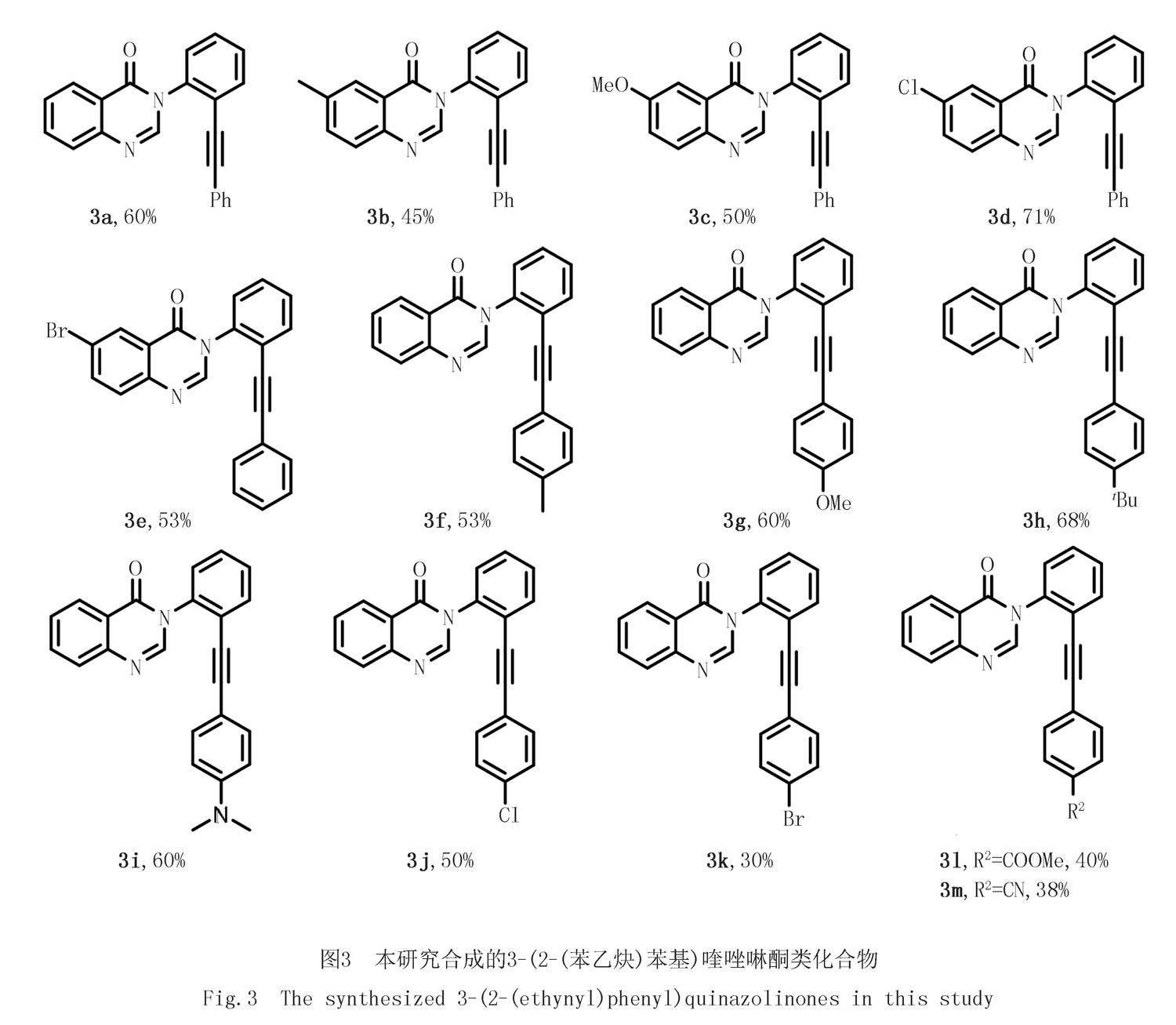
在50 mL圆底烧瓶中,先加入磁子,再依次加入2-(芳基乙炔)苯胺(3.6 mmol)、2-氨基芳基甲酸甲酯(3 mmol)、原甲酸三乙酯(4.5 mmol)和NH4Cl(1.2 mmol),无需外加溶剂,在100 ℃的油浴中加热搅拌反应2 h.反应结束后,取出反应瓶冷却至室温,加入15 mL水,然后用二氯甲烷(3×15 mL)萃取.萃取完后,合并有机相,然后用适量无水硫酸钠干燥,再通过旋转蒸发仪除去溶剂,残留物利用柱层析分离纯化,得到目标产物.利用上述方法合成以下产物(图3).
3-(2-(苯乙炔基)苯基)喹唑啉-4(3H)-酮(3a):白色固体,580 mg,产率60%,Rf=0.3(石油醚/乙酸乙酯,V/V=5/1),m.p.130~132 ℃.1H NMR(400 MHz,CDCl3)δ:8.46~8.37(m,1H),8.17(s,1H),7.88~7.81(m,2H),7.79~7.73(m,1H),7.65~7.47(m,4H),7.28~7.17(m,5H).13C NMR(101 MHz,CDCl3)δ:160.7,148.0,146.6,138.5,134.6,133.1,131.6,129.5,129.3,128.8,128.5,128.3,127.6,127.5,127.3,122.6,122.3,122.1,95.7,84.4.HRMS(ESI-TOF)m/z:计算值C22H15N2O[M+H]+323.117 9,实测值323.118 6.
6-甲基-3-(2-(苯乙炔基)苯基)喹唑啉-4(3H)-酮(3b):白色固体,454 mg,产率45%,Rf=0.3(石油醚/乙酸乙酯,V/V=5/1),m.p.152~154 ℃.1H NMR(400 MHz,CDCl3)δ:8.23~8.17(m,1H),8.11(s,1H),7.82~7.68(m,2H),7.64(dd,J=8.3,2.1 Hz,1H),7.59~7.45(m,3H),7.33~7.15(m,5H),2.53(s,3H).13C NMR(101 MHz,CDCl3)δ:160.7,146.0,145.8,138.7,137.8,136.0,133.1,131.6,129.4,129.3,128.8,128.5,128.3,127.5,126.7,122.3(3),122.2(8),122.2,95.6,84.5,21.4.HRMS(ESI-TOF)m/z:计算值C23H17N2O 337.133 5,实测值337.134 0.
6-甲氧基-3-(2-(苯乙炔基)苯基)喹唑啉-4(3H)-酮(3c):黄色固体,528 mg,产率50%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.121~123 ℃.1H NMR(400 MHz,CDCl3)δ:8.06(s,1H),7.76(dd,J=8.2,2.7 Hz,3H),7.52~7.47(m,3H),7.43(dd,J=9.1,3.0 Hz,1H),7.32~7.12(m,5H),3.93(s,3H).13C NMR(101 MHz,CDCl3)δ:160.6,158.9,144.5,142.6,138.8,133.1,131.6,129.4,129.3,129.2,128.8,128.5,128.3,124.7,123.4,122.4,122.2,106.8,95.6,84.5,55.9.HRMS(ESI-TOF)m/z:计算值C23H16N2NaO2[M+Na]+375.110 4,实测值375.111 2.
6-氯-3-(2-(苯乙炔基)苯基)喹唑啉-4(3H)-酮(3d):白色固体,760 mg,产率71%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.156~158 ℃.1H NMR(400 MHz,CDCl3)δ:8.37(t,J=1.5 Hz,1H),8.15(s,1H),7.81~7.67(m,3H),7.63~7.44(m,3H),7.35~7.11(m,5H).13C NMR(101 MHz,CDCl3)δ:159.6,146.8,146.5,138.2,135.0,133.4,133.2,131.5,129.6,129.4,129.3,128.9,128.4,128.3,126.6,123.6,122.2,122.0,95.8,84.2.HRMS(ESI-TOF)m/z:計算值C22H14ClN2O[M+H]+357.078 9,实测值357.079 0.
6-溴-3-(2-(苯乙炔基)苯基)喹唑啉-4(3H)-酮(3e):白色固体,630 mg,产率53%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.162~164 ℃.1H NMR(400 MHz,CDCl3)δ:8.54(d,J=2.3 Hz,1H),8.16(s,1H),7.92(dd,J=8.6,2.4 Hz,1H),7.79~7.74(m,1H),7.70(d,J=8.6 Hz,1H),7.58~7.46(m,3H),7.35~7.16(m,5H).13C NMR(101 MHz,CDCl3)δ:159.5,146.9,146.8(6),138.1,137.8,133.2,131.5,129.8,129.6,129.5,129.4,128.9,128.4,128.3,123.9,122.2,122.0,121.2,95.8,84.2.HRMS(ESI-TOF)m/z:计算值C22H14BrN2O[M+H]+401.028 4,实测值401.029 3.
3-(2-(对甲苯乙炔基)苯基)喹唑啉-4(3H)-酮(3f):白色固体,534 mg,产率53%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.144~146 ℃.1H NMR(400 MHz,CDCl3)δ:8.42(d,J=7.9 Hz,1H),8.16(s,1H),7.83(dd,J=3.6,1.0 Hz,2H),7.77~7.69(m,1H),7.60~7.53(m,1H),7.54~7.46(m,3H),7.09(d,J=8.2 Hz,2H),7.01(d,J=7.8 Hz,2H),2.29(s,3H).13C NMR(101 MHz,CDCl3)δ:160.7,148.1,146.6,139.1,138.5,134.6,133.0,131.5,129.4,129.1,129.0(5),128.5,127.6,127.5,127.3,122.6,122.5,119.1,96.0,83.9,21.5.HRMS(ESI-TOF)m/z:计算值C23H17N2O[M+H]+337.133 5,实测值337.134 3.
3-(2-((4-甲氧基苯基)乙炔基)苯基)喹唑啉-4(3H)-酮(3g):白色固体,634 mg,产率60%,Rf=0.3(石油醚/乙酸乙酯,V/V=2/1),m.p.120~122 ℃.1H NMR(400 MHz,CDCl3)δ:8.43~8.38(m,1H),8.16(s,1H),7.83(dd,J=4.4,2.0 Hz,2H),7.77~7.67(m,1H),7.54~7.58(m,1H),7.50(d,J=2.7 Hz,3H),7.12(d,J=8.8 Hz,2H),6.72(d,J=8.8 Hz,2H),3.75(s,3H).13C NMR(101 MHz,CDCl3)δ:160.7,150.0,148.1,146.7,138.3,134.6,133.1,132.8,129.4,128.9,128.4,127.6,127.5,127.3,122.6(3),122.5(9),114.2,113.9,95.9,83.3,55.3.HRMS(ESI-TOF)m/z:计算值C23H17N2O2[M+H]+353.128 5,实测值353.129 8.
3-(2-((4-(叔丁基)苯基)乙炔基)苯基)喹唑啉-4(3H)-酮(3h):白色固体,772 mg,产率68%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.129~131 ℃.1H NMR(400 MHz,CDCl3)δ:8.43(d,J=8.0 Hz,1H),8.17(s,1H),7.84(d,J=4.1 Hz,2H),7.75(dd,J=7.0,2.9 Hz,1H),7.63~7.45(m,4H),7.24(d,J=8.2 Hz,2H),7.15(d,J=8.1 Hz,2H),1.27(s,9H).13C NMR(101 MHz,CDCl3)δ:160.6,152.2,148.1,146.6,138.4,134.6,133.0,131.3,129.4,129.1,128.5,127.7,127.5,127.3,125.3,122.6,122.5,119.1,96.0,83.9,34.8,31.1.HRMS(ESI-TOF)m/z:计算值C26H23N2O[M+H]+379.180 5,实测值379.180 5.
3-(2-((4-(二甲基氨基)苯基)乙炔基)苯基)喹唑啉-4(3H)-酮(3i):黄色固体,657 mg,产率60%,Rf=0.3(石油醚/乙酸乙酯,V/V=4/1),m.p.161~163 ℃.1H NMR(400 MHz,CDCl3)δ:8.39(d,J=7.9 Hz,1H),8.14(s,1H),7.80(d,J=4.2 Hz,2H),7.73~7.62(m,1H),7.53(dd,J=8.2,4.1 Hz,1H),7.48~7.39(m,3H),7.03(d,J=8.4 Hz,2H),6.45(d,J=8.4 Hz,2H),2.89(s,6H).13C NMR(101 MHz,CDCl3)δ:160.7,150.3,148.1,146.8,138.0,134.5,132.7,132.6,129.3,128.3,128.2,127.6,127.4,127.3,123.2,122.7,111.6,108.7,97.5,82.7,40.1.HRMS(ESI-TOF)m/z:计算值C24H20N3O[M+H]+366.160 1,实测值366.160 9.
3-(2-((4-氯苯基)乙炔基)苯基)喹唑啉-4(3H)-酮(3j):黄色固体,531 mg,产率50%,Rf=0.3(石油醚/乙酸乙酯,V/V=3/1),m.p.163~165 ℃.1H NMR(400 MHz,CDCl3)δ:8.47~8.36(m,1H),8.14(s,1H),7.91~7.80(m,2H),7.78~7.71(m,1H),7.61~7.48(m,4H),7.23~7.15(m,2H),7.15~7.05(m,2H).13C NMR(101 MHz,CDCl3)δ:160.6,148.0,146.5,138.6,134.9,134.7,133.0,132.7,129.6,129.5,128.7,128.5,127.7,127.6,127.3,122.5,122.0,120.6,94.5,85.4.HRMS(ESI-TOF)m/z:计算值C22H14ClN2O[M+H]+357.078 9,实测值357.079 3.
3-(2-((4-溴苯基)乙炔基)苯基)喹唑啉-4(3H)-酮(3k):黄色固体,360 mg,产率30%,Rf=0.3(石油醚/乙酸乙酯,V/V=3/1),m.p.170~172 ℃.1H NMR(400 MHz,CDCl3)δ:8.41(d,J=7.9 Hz,1H),8.14(s,1H),7.84(d,J=7.4 Hz,2H),7.74(d,J=6.9 Hz,1H),7.59~7.54(m,4H),7.33(d,J=8.1 Hz,2H),7.03(d,J=8.0 Hz,2H).13C NMR(101 MHz,CDCl3)δ:160.6,148.0,146.5,138.6,134.7,133.0,132.9,131.6,129.6,129.5,128.5,127.7,127.6,127.3,123.2,122.5,122.0,121.1,94.5,85.6.HRMS(ESI-TOF)m/z:计算值C22H14BrN2O[M+H]+401.028 4,实测值401.028 4.
4-((2-(4-氧代喹唑啉-3(4H)-基)苯基)乙炔基)苯甲酸甲酯(3l):黄色固体,456 mg,产率40%,Rf=0.3(石油醚/乙酸乙酯,V/V=3/1),m.p.140~142 ℃.1H NMR(400 MHz,CDCl3)δ:8.44~8.33(m,1H),8.13(s,1H),7.91~7.79(m,4H),7.77~7.69(m,1H),7.62~7.46(m,4H),7.25~7.08(m,2H),3.87(s,3H).13C NMR(101 MHz,CDCl3)δ:166.3,160.6,148.0,146.4,138.8,134.7,133.1,131.4,129.9(3),129.8(6),129.5,129.4,128.5,127.7,127.6,127.2,126.7,122.5,121.8,94.7,87.2,52.2.HRMS(ESI-TOF)m/z:計算值C24H16N2NaO3[M+Na]+403.105 3,实测值403.105 7.
4-((2-(4-氧代喹唑啉-3(4H)-基)苯基)乙炔基)苯腈(3m):黄色固体,396 mg,产率38%,Rf=0.3(石油醚/乙酸乙酯,V/V=3/1),m.p.99~101 ℃.1H NMR(400 MHz,CDCl3)δ:8.39(dd,J=8.0,1.4 Hz,1H),8.12(s,1H),7.91~7.79(m,2H),7.76(dd,J=7.4,1.8 Hz,1H),7.63~7.50(m,4H),7.51~7.42(m,2H),7.30~7.19(m,2H).13C NMR(101 MHz,CDCl3)δ:160.6,147.9,146.3,138.9,134.9,133.2,132.0,130.3,129.6,128.6,127.8,127.7,127.2,126.9,122.4,121.4,118.3,112.0,93.6,88.6.HRMS(ESI-TOF)m/z:计算值C23H14N3O[M+H]+348.113 1,实测值348.113 1.
2 结果与讨论
2.1 合成分析
在合成3-(2-(苯乙炔)苯基)喹唑啉酮类化合物(3)的过程中,考察了底物芳环上不同取代基对反应产率的影响,发现不同芳环上取代基的电子效应对产率的影响不同.
(1)当底物1芳环上取代基R1为给电子基团(例如甲基、甲氧基)时,所得对应产物3b、3c的产率分别为45%和50%.而当R1为吸电子基团(例如氯、溴)时,产率分别为71%(3d)和53%(3e).该结果表明底物1芳环上取代基的电子效应没有明显的规律.
(2)当底物2芳环上取代基R2为给电子基团(例如甲基、甲氧基等)时,产率高于为吸电子基团(例如氯、溴、氰基等)的产率.该结果表明电子效应对该反应有一定的影响,R2为给电子基团时产率下降.
2.2 反应机理研究
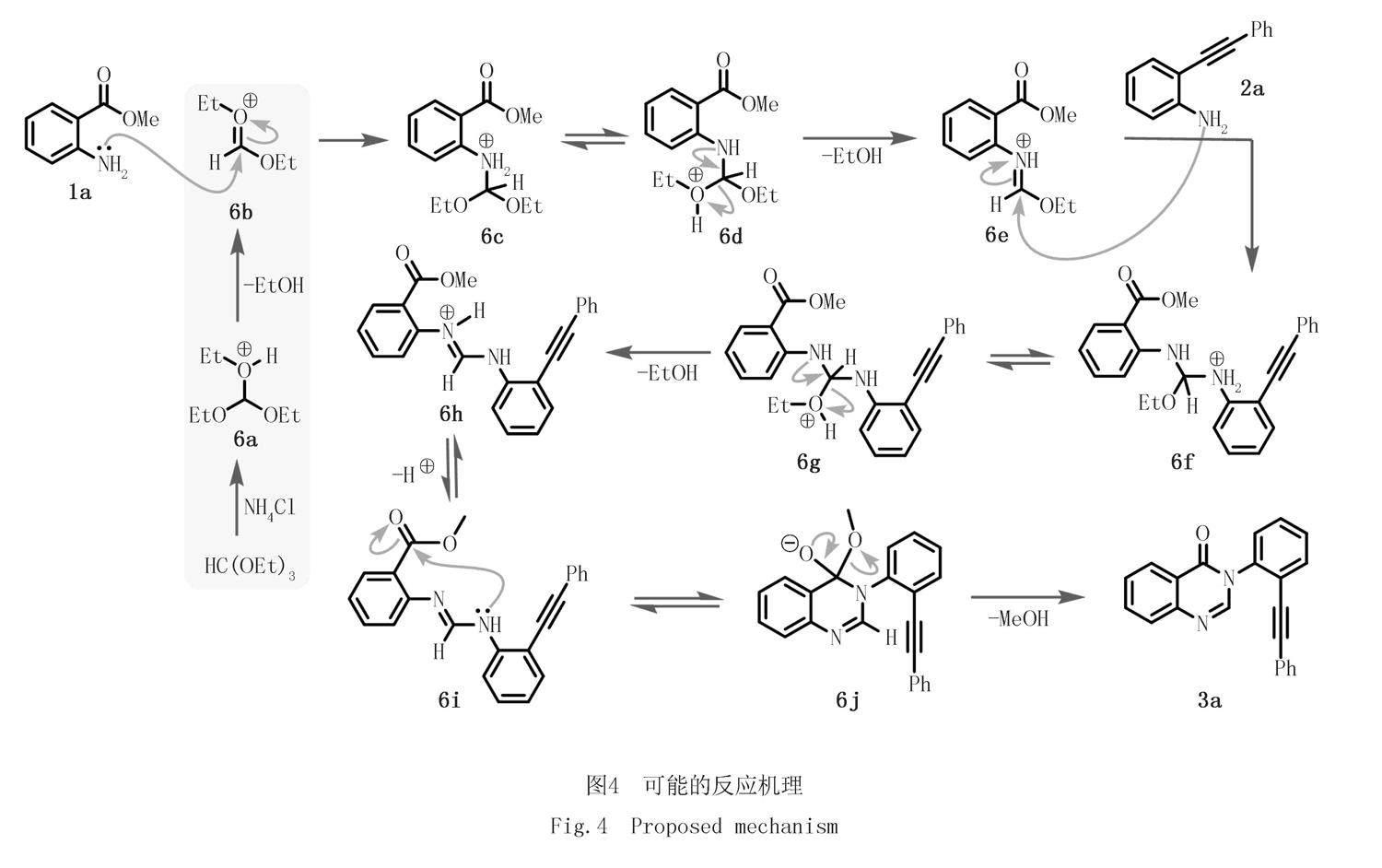
结合相关研究报道[16-17],以模型反应为例,提出了该反应的可行性机理(图4).首先,NH4Cl对原甲酸三乙酯进行质子化生成中间体6a,进一步失去一分子乙醇后生成中间体6b.随后,反应物1a的氨基对6b亲核进攻生成中间体6c;6c互变异构为中间体6d,随后失去一分子乙醇产生亚胺正离子中间体6e.6e与反应物2a发生亲核加成生成中间体6f.类似地,6f通过先后经历互变异构、失去乙醇分子、去质子化生成6i.6i的胺基对分子内羰基的亲核进攻,产生关环中间体6j;中间体6j失去一分子甲醇,生成目标产物3-(2-(苯乙炔)苯基)喹唑啉酮3a.
2.3 3-(2-(苯乙炔)苯基)喹唑啉酮作为受体的可见光诱导自由基串联环化反应
上述合成的3-(2-(苯乙炔)苯基)喹唑啉酮类化合物,可以作为新型底物骨架应用于自由基串联环化反应中.例如,3-(2-(苯乙炔)苯基)喹唑啉酮骨架与二苯基氧化膦的反应(图2(a)),经历可见光驱动的自由基串联环化反应,高效合成了新型膦酰化喹唑啉酮并喹啉化合物.具体操作方法如下:依次将3-(2-(苯乙炔)苯基)喹唑啉酮(3a,0.1 mmol)、二苯基氧化膦(4a,0.2 mmol)、2,4,5,6-四(9-咔唑基)-间苯二腈(4CzIPN,5%(摩尔分数))、过氧化十二酰(LPO,0.2 mmol)以及乙腈(MeCN 1 mL)加入到装有磁子的25 mL Schlenk反应管中,随后冷冻脱气并置换氮气3次,保持氮气氛围,将反应管置于蓝色LED(10 W,460 nm)照射、室温下搅拌反应9 h.反应结束后,将反应液转移到圆底烧瓶中,再通过旋转蒸发仪除去溶剂,残留物通过制备薄层色谱法分离纯化,得到目标产物5a.
5-(二苯基膦酰)-6-苯基-12H-喹啉并[2,1-b]喹唑啉-12-酮(5a):黄色固体,45.4 mg,产率87%,Rf=0.3(石油醚/乙酸乙酯,V/V=2/1),m.p.278~280 ℃.1H NMR(400 MHz,CDCl3)δ:8.97(d,J=8.7 Hz,1H),8.47(d,J=7.9 Hz,1H),8.05(d,J=8.1 Hz,1H),7.73(t,J=7.7 Hz,1H),7.59~7.51(m,2H),7.43(dd,J=12.3,7.4 Hz,5H),7.29~7.23(m,2H),7.21~7.11(m,8H),7.02(t,J=7.6 Hz,2H).13C NMR(101 MHz,CDCl3)δ:162.5,147.0(d,J=15.3 Hz),146.6(d,J=8.3 Hz),146.1(d,J=1.7 Hz),136.1(d,J=98.3 Hz),135.2(d,J=6.1 Hz),134.5(d,J=106.2 Hz),133.5(d,J=8.5 Hz),132.8,130.9(d,J=9.5 Hz),130.7(d,J=2.9 Hz),128.9(d,J=6.0 Hz),128.8,128.3,128.2,128.1(5),128.1,127.3,127.2(3),127.1(9),125.7,124.8(d,J=9.2 Hz),120.6,120.3.31P NMR(162 MHz,CDCl3)δ:24.29.HRMS(ESI-TOF)m/z:计算值C34H24N2O2P[M+H]+523.157 0,实测值523.156 9.
3 结 论
通过一锅多组分反应,在无需外加溶剂的条件下,以中等到良好的产率(30%~71%)合成了13个新型3-(2-(芳基乙炔)苯基)喹唑啉酮类化合物,并通过1H NMR,13C NMR和HRMS相关表征手段确证了其结构.同时,研究了不同取代基对产率的影响.所合成的3-(2-(苯乙炔)苯基)喹唑啉酮类化合物可以作为新型的底物骨架,应用在自由基串联环化反应中,构建喹唑啉酮并喹啉类化合物.这类新型的自由基串联环化反应新骨架可以为新型杂环骨架的高效构筑提供全新的平臺.
参 考 文 献
[1] VITAKU E,SMITH D T,NJARDARSON J T.Analysis of the structural diversity,substitution patterns,and frequency of nitrogen heterocycles among U.S.FDA approved pharmaceuticals[J].Journal of Medicinal Chemistry,2014,57(24):10257-10274.
[2]SUN J,TAN Q,YANG W,et al.Copper-catalyzed aerobic oxidative annulation and carbon-carbon bond cleavage of arylacetamides:domino synthesis of fused quinazolinones[J].Advanced Synthesis & Catalysis,2014,356(2/3):388-394.
[3]GUPTA P K,YADAV N,JAISWAL S,et al.Palladium-catalyzed synthesis of phenanthridine/benzoxazine-fused quinazolinones by intramolecular C-H bond activation[J].Chemistry A European Journal,2015,21(38):13210-13215.
[4]BIN LEE J,KANG M E,KIM J,et al.Direct diversification of unmasked quinazolin-4(3H)-ones through orthogonal reactivity modulation[J].Chemical Communications,2017,53(75):10394-10397.
[5]SHARMA K,KHANDELWAL S,SAMARTH R M,et al.Natural product-mimetic scaffolds with privileged heterocyclic systems:design,synthesis,and evaluation of antioxidant activity of quinazoquinobenzothiazinones[J].Journal of Heterocyclic Chemistry,2016,53(1):220-228.
[6]BHALEKAR S M,PARAB H M.Synthesis of new heterocyclic compounds derived from 2-(2,4-dichloroquinolin-6-yl)-4H-1-benzopyran-4-one and their biological evaluation[J].Indian Journal of Heterocyclic Chemistry,2011,20(4):301-304.
[7]COVELL D G,HUANG R L,WALLQVIST A.Anticancer medicines in development:assessment of bioactivity profiles within the National Cancer Institute anticancer screening data[J].Molecular Cancer Therapeutics,2007,6(8):2261-2270.
[8]LU L Q,CHEN J R,XIAO W J.Development of cascade reactions for the concise construction of diverse heterocyclic architectures[J].Accounts of Chemical Research,2012,45(8):1278-1293.
[9]高凡,呂琪妍,於兵.2-甲硫基芳炔化合物的自由基串联环化反应[J].聊城大学学报(自然科学版),2020,33(6):66-75.
GAO F,LYU Q Y,YU B.Radical cascade cyclization reaction of methyl(2-alkynylaryl)sulfanes[J].Journal of Liaocheng University(Natural Science Edition),2020,33(6):66-75.
[10]LIU H,WANG L,YU J T.Radical cascade cyclization of alkene-tethered compounds:versatile approach towards ring-fused polycyclic structures[J].Asian Journal of Organic Chemistry,2023,12(5):e202300101.
[11]LIAO J H,YANG X,OUYANG L,et al.Recent advances in cascade radical cyclization of radical acceptors for the synthesis of carbo-and heterocycles[J].Organic Chemistry Frontiers,2021,8(6):1345-1363.
[12]HUANG M H,HAO W J,LI G G,et al.Recent advances in radical transformations of internal alkynes[J].Chemical Communications,2018,54(77):10791-10811.
[13]WEI W,CUI H H,YANG D S,et al.Visible-light-enabled spirocyclization of alkynes leading to 3-sulfonyl and 3-sulfenyl azaspiro[4,5]trienones[J].Green Chemistry,2017,19(23):5608-5613.
[14]ZENG F L,CHEN X L,SUN K,et al.Visible-light-induced metal-free cascade cyclization of N-arylpropiolamides to 3-phosphorylated,trifluoromethylated and thiocyanated azaspiro[4.5]trienones[J].Organic Chemistry Frontiers,2021,8(4):760-766.
[15]ZENG F L,ZHANG Z Y,YIN P C,et al.Visible-light-induced cascade cyclization of 3-(2-(ethynyl)phenyl)quinazolinones to phosphorylated quinolino[2,1-b]quinazolinones[J].Organic Letters,2022,24(43):7912-7917.
[16]JALANI H B,PANDYA A N,PANDYA D H,et al.An efficient,greener,and solvent-free one-pot multicomponent synthesis of 3-substituted quinazolin-4(3H)ones and thienopyrimidin-4(3H)ones[J].Tetrahedron Letters,2012,53(32):4062-4064.
[17]HUANG G L,LIU B,TENG M Y,et al.Ammonium chloride-catalyzed one-pot synthesis of 4(3H)-quinazolinones under solvent-free conditions[J].Synthetic Communications,2014,44(12):1786-1794.
Synthesis of 3-(2-(arylethynyl)phenyl)quinazolinones via one-pot multi-component reaction
Yu Bing1, Zeng Fanlin2
(1. College of Chemistry, Zhengzhou University, Zhengzhou 450001, China; 2. College of Advanced Interdisciplinary Science and Technology, Henan University of Technology, Zhengzhou 450001, China)
Abstract: Using 2-(arylethynyl)aniline, 2-aminobenzyl formate, and ethyl trifluoromethylthioacetate as reactants, a one-pot multicomponent reaction was carried out to achieve a series of 3-(2-(arylethynyl)phenyl)quinazolinones in moderate to good yields(30%-71%) without the need for additional solvents. These compounds can be utilized as novel receptors in radical cascade cyclization reactions, leading to the synthesis of quinolino[2,1-b]quinazolinones.
Keywords: 3-(2-(arylethynyl)phenyl)quinazolinones; multi-component reaction; heterocycle; quinolino[2,1-b]quinazolinone
[责任编校 赵晓华 陳留院]

