Applications of gastric peroral endoscopic myotomy in the treatment of upper gastrointestinal tract disease
Shi-Yu Chang,Guo-Hua Jin,Hai-Bo Sun,Dong Yang,Tong-Yu Tang
Abstract Gastric peroral endoscopic myotomy (G-POME) is an emerging minimally invasive endoscopic technique involving the establishment of a submucosal tunnel around the pyloric sphincter.In 2013,Khashab et al used G-POME for the first time in the treatment of gastroparesis with enhanced therapeutic efficacy,providing a new direction for the treatment of gastroparesis.With the recent and rapid development of G-POME therapy technology,progress has been made in the treatment of gastroparesis and other upper digestive tract diseases,such as congenital hypertrophic pyloric stenosis and gastric sleeve stricture,with GPOME.This article reviews the research progress and future prospects of GPOME for the treatment of upper digestive tract gastrointestinal diseases.
Key Words: Gastric peroral endoscopic myotomy;Upper digestive tract diseases;Gastroparesis;Congenital hypertrophic pyloric stenosis;Gastric sleeve stricture
lNTRODUCTlON
As a new endoscopic minimally invasive technique,gastric peroral endoscopic myotomy (G-POME) was first used to treat refractory gastroparesis by Khashabet al[1] in 2013.The technical basis of G-POME was established by Inoueet al[2] in 2010,who applied transoral endoscopic myotomy (POME) for the treatment of achalasia.As for POME,G-POME can be divided into four steps: Mucosal incision and tunnel entry,submucosal tunneling,pyloromyotomy,and closure of mucosal entry.Since G-POME is associated with less trauma and fewer postoperative adverse reactions and has better short-term or long-term effects than traditional surgical methods,G-POME has been popularized worldwide,providing a new paradigm for the treatment of some refractory upper gastrointestinal diseases[3].
PROCEDURE OF G-POME
Mucosal incision and tunnel entry
The initial mucosal incision is usually made on the great curvature/posterior wall of the stomach,which is considered the easiest approach.After the gastric mucosa is cut,the tunnel can be established easily and close to the pyloric area.The use of a lesser curvature for the stomach/anterior wall approach has also been reported[4].However,this method has the problem of difficult endoscopic localization.Although the approximate position can be determined by repeatedly withdrawing the endoscope,this approach adds many unnecessary steps compared with the conventional scheme.Similarly,Jovaniet al[5] reported a new surgical approach involving simultaneous incision of the mucosa at both the greater and lesser curvatures of the stomach and double-tunnel standard myotomy.This new method improves GPOME.
Before the incision of the mucosa is made,submucosal vesicles are formed by injecting stained saline into the submucosa.A volume expander can be added to the injection to assist in incision of the mucosa and maintain the opening of the tunnel.Since route deviation easily occurs during the establishment of a tunnel under endoscopy,we need to avoid creating a longer tunnel.A longitudinal incision of 2 cm approximately 4-5 cm is made at the proximal end of the pylorus,the submucosal fibers are carefully peeled off at the entrance of the tunnel,and if necessary,the incision is sprayed or injected with indigo carmine salt solution and a hardening needle to strengthen the boundary between the submucosa and the lamina propria.According to a study of adverse events with G-POME[6],longitudinal mucosal incisions are significantly associated with a lower incidence of adverse reactions,suggesting that transverse mucosal incisions should be avoided to reduce possible adverse events.After the separation is completed,the endoscope is slowly introduced into the submucosal space.
Submucosal tunneling
After entering the submucosal space,the proper muscle layer is kept facing 6:00,and the mucous layer was kept facing 12:00 to ensure the correct direction of the tunnel.The fibers on the surface of the lamina propria are continuously stripped to establish a submucosal tunnel.When establishing the tunnel,care should be taken to avoid damaging the integrity of the mucosa.According to a meta-analysis by Stojilkovicet al[7],62 of 835 patients who underwent G-POME experienced adverse events.In addition to the most common postoperative abdominal pain,bleeding and mucosal laceration often occur and may be related to intraoperative mucosal injury.Other complications associated with mucosal injury,such as capnoperitoneum[3,6,8] and delayed bleeding of gastric ulcers[9],have also been mentioned in a study of adverse events associated with G-POME.Other complications include delayed leakage and perforation,but the relationship between mucosal injury and these complications needs further study.Penetrating vessels are often observed in the proximal pylorus during endoscopy.Coagulation forceps should be used to prevent massive bleeding caused by vascular injury.
Endoscopic injection of blue saline (a mixture of methylene blue and saline) is repeatedly used to stain submucosal fibers during tunnel extension.This approach not only provides a protective pad for the mucosa but also outlines the lamina propria and helps to identify the pyloric muscle ring (PMR).Indeed,identification of the PMR is a very important step in G-POME,as it directly affects the efficacy of surgical treatment and the possibility of postoperative complications.The conventional way to locate the PMR is to pull the endoscope out of the tunnel,push it toward the pylorus and look for blue saline injected into the submucosa.However,Xueet al[10] determined the location of the PMR by placing an inner clip at the pylorus,providing an ingenious method for locating the PMR.This approach avoids the trauma that may be caused by the application of traditional methods and effectively shortens the time needed for surgery.In POME,in which a submucosal tunnel is established,Khashabet al[11] used a double endoscope to locate the gastroesophageal junction without withdrawing from the tunnel;this finding suggests that double endoscopy can also be used to locate the PMR in G-POME.The extension of the tunnel should be exposed behind the pyloric ring (a "crescent-shaped" thick muscle bundle under endoscopy).Since the duodenal mucosal layer is perpendicular to the pyloric ring,perforation of the mucosa can easily occur when crossing the pyloric ring and continuing to establish a submucosal tunnel;thus,extra care should be taken to avoid excessive extension of the tunnel.
Pyloromyotomy
After the pylorus is exposed,a full-thickness myotomy of the inner circular and oblique muscle bundles is performed starting 2 cm from the proximal end of the pylorus.Myoptosis is gradually performed during the operation to avoid damaging the serosal layer and abdominal viscera.The outer longitudinal muscle layer is often preserved to ensure separation from important surrounding structures,especially the larger vascular system around the duodenum.The myotomy is performed from the pylorus to the gastric antrum,with a length of 2 cm ± 1 cm.During the resection process,it is necessary to avoid making long incisions in the gastric antral circular muscle,as this may weaken the contraction function of the gastric antrum and reduce the pushing of food particles toward the pylorus,thus exacerbating the symptoms of gastroparesis[12].
In a 12-month follow-up study,Kia Vosoughiet al[13] tracked 80 patients who underwent G-POME surgery.The clinical success rate was 79% at 1 month and 56% at 12 months (clinical success was defined as a decrease in the average GCSI score of 1 point and a decrease in at least two subscale scores on the GCSI of 25%)[14].A prospective study by Gonzalezet al[15] also showed that the clinical success rate of G-POME for treating gastroparesis decreases over time.These studies suggest that some patients have recurrent symptoms after G-POME surgery.This may be related to the relatively short distance between muscle fibers caused by standard myotomy,which increases the risk of muscle tissue remodeling during healing.In response to this situation,Jovaniet al[5] reported two cases in which the risk of recurrence was reduced.In one patient,a pyloric myotomy was performed again on the right side of the first myotomy.The direction of the incision was parallel to the direction of the first myotomy,and the muscle fibers between the two pyloric myotomies were removed using a 10 mm cold ring cutter,thereby enlarging the distance between the edges of the muscle layers of the two incisions.The other patient had recurrent symptoms after undergoing standard G-POME,and doubletunnel G-POME was performed to establish a submucosal tunnel along both the greater curvature and lesser curvature of the stomach for myotomy.Although both improved G-POME procedures employed more extensive myotomy to circumvent potential muscle tissue remodeling and symptom recurrence,a comparison with the improvement in symptoms observed after conventional G-POME should be conducted to assess efficacy.
Closure of mucosal entry
After the pyloric muscle has been resected,the endoscope is carefully withdrawn from the tunnel.Subsequently,the mucosal incision is meticulously closed.Complete and secure closure of the mucosal entrance is a crucial step in this procedure,as it plays a pivotal role in preventing complications such as peritonitis and postoperative leakage.The commonly used closure techniques include endoscopic clips,endoscopic suture out-of-range ligation clips,and snares[16-19].In most clinical centers where G-POME is performed,the most commonly employed closure method involves the use of endoscopic clips and sutures.Specifically,in POME,the mucosal incision is typically closed using endoscopic clips[2,12,20].In G-POME,the thick mucosal layer can make it challenging to approach and grasp the mucosal cutting edge stably.This may cause difficulties in closing it with a clamp.Specifically,when the incision is wide,a larger clip is often needed to achieve closure.
In general,the method used to close the mucosal incision depends on the direction of the incision[21].If the operator is more skilled at applying endoscopic clips and it is simpler to align the clip with longitudinal incisions,endoscopic clamp closure is the preferred method for closing longitudinal mucosal incisions.Nonetheless,Kahalehet al[17],Xuet al[22],and others have also reported instances in which endoscopic clamp closure was unsuccessful,rendering endoscopic suturing an alternative option.Endoscopic suturing is considered safer and more effective than endoscopic clipping,but it places a heavier economic burden on patients.Additionally,endoscopic suturing takes longer (14.1 minutesvs9.8 minutes).Although the total operation time for patients who undergo endoscopic clip placement tends to be shorter than that for patients who do not,there is no significant difference in the total operation time between the two methods.Furthermore,the shorter closing time of endoscopic clips does not translate into any economic benefits[16].For the center of the incision to be closed with an endoscopic clip,remedial closure methods such as endoscopic suturing should be used.To avoid postoperative complications such as leakage or delayed bleeding of gastric ulcers due to poor closure of the mucosal entrance,combined endoscopic clamping and endoscopic suturing to close mucosal incisions have been used in some research centers,and good results have been achieved[23].For centers in which G-POME is performed,the mucosal closure methods should be selected based on the actual situation of the patient during surgery.For patients for whom closure is difficult or poor when using a single method,the combination of endoscopic clips and endoscopic sutures is necessary (Table 1).

Table 1 lnnovation in gastric peroral endoscopic myotomy
G-POME FOR GASTROPARESlS
Introduction to gastroparesis
Gastroparesis is defined as delayed gastric emptying in the absence of mechanical obstruction.The most common causes of gastroparesis are diabetes,surgery,or infection,but it may also present as idiopathic gastroparesis.The pathogenesis of gastroparesis is not fully understood and may involve impaired gastric regulation,autonomic neuropathy,gastric contraction incoordination,pyloric dysfunction,degeneration of Cajal interstitial cells (ICCs),and neurohormone disorders[24-28].Gastroparesis often presents with vague symptoms such as nausea,vomiting,early satiety,postprandial abdominal distension,and abdominal pain.In severe cases,patients may experience weight loss and malnutrition.
The diagnosis of gastroparesis requires three main criteria: (1) Symptoms of gastroparesis,such as nausea,vomiting,early satiety,and postprandial abdominal distension;(2) absence of mechanical obstruction;and (3) obvious delayed gastric emptying[29] (Figure 1).Patients with symptoms of gastroparesis should undergo endoscopy to rule out obstruction and a gastric emptying examination to confirm the diagnosis of gastroparesis.The main symptom index (GCSI) of gastroparesis is often used to evaluate the clinical response;the quality-of-life assessment scale and gastrointestinal life index score are used to evaluate quality of life in patients with upper digestive tract diseases[30].The gold standard method for evaluating delayed gastric emptying is based on the Tougas regimen,which involves a radiographic study of a 4-hour T99-labeled solid diet[29,31],with gastric emptying objectively measured according to the results of gastric emptying scintigraphy (GES).A clear diagnosis of gastroparesis requires certain equipment and technical support,and the gold standard for diagnosing this disease involves a radiological examination,which may cause concern for some female patients and cause them to reject diagnostic examination.Gastroparesis is more common in women,with a prevalence rate of approximately 37.8/100000 person-years,compared with 9.8/100000 person-years in men[32].For these reasons,the true prevalence of gastroparesis is difficult to accurately assess,and many patients with gastroparesis are diagnosed with functional dyspepsia.Thus,the actual number of patients may be much greater than previously believed.
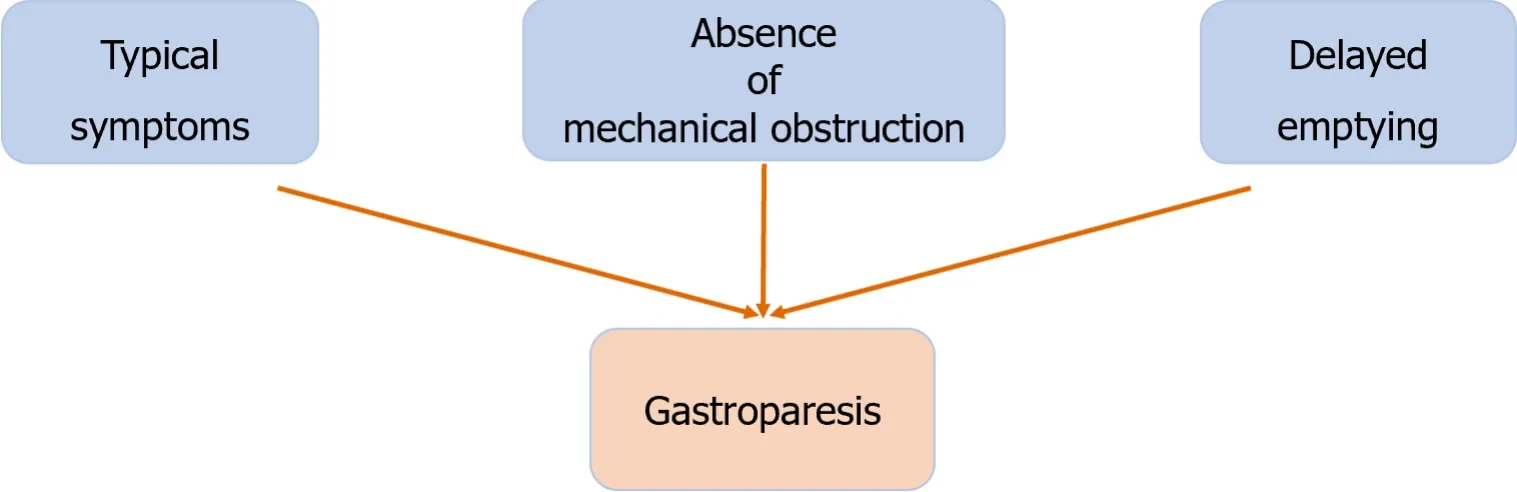
Figure 1 Diagnosis of gastroparesis.
Traditional treatment options for gastroparesis
Dietary modifications and pharmacological interventions are the first-line treatments for gastroparesis,with approximately 70% of patients demonstrating adequate responses[33].Gastric motility is a complex process involving the coordinated interaction of motor,secretory,and neuroregulatory activities[34],and single interventions are unlikely to effectively target the entire gastric emptying mechanism.Metoclopramide is currently the only drug approved by the U.S.Food and Drug Administration for the treatment of gastroparesis,but its severe extrapyramidal side effects limit its longterm use in patients with gastroparesis[35].Domperidone,another D2 receptor antagonist similar to metoclopramide,can promote gastric emptying but may also lead to cardiovascular-related adverse effects[36].Erythromycin is an antibiotic with prokinetic properties,but due to rapid tolerance,it does not improve symptoms well over a long period[37].Other drugs,including 5-HT3 receptor antagonists such as ondansetron,5-HT4 agonists such as prucalopride and cisapride,and growth hormone-releasing peptide receptor agonists such as phenothiazines and muscarinic receptor antagonists,have been used in the management of gastroparesis symptoms.Although these drugs can improve nausea and vomiting symptoms in patients,they have no significant effect on gastric emptying due to the association between abdominal bloating and early satiety with impaired gastric fundus regulation[38].These limitations highlight the need for alternative treatment options for gastroparesis (Table 2).

Table 2 Traditional medication treatment methods
For patients who do not respond to the conventional regimen,treatment strategies include peripyloric botulinum toxin injection,gastric electrical stimulation,surgery,and endoscopic intervention to disrupt the pyloric outlet,thereby improving gastric emptying and alleviating gastroparesis symptoms.Peripyloric botulinum toxin injection is highly anticipated to be useful for the treatment of gastroparesis[39];however,although some studies seem to demonstrate the effectiveness of this treatment,randomized controlled trials have failed to show any improvement in symptoms[40].According to a meta-analysis of gastric electrical stimulation for the treatment of gastroparesis,the clinical response rate at 24 months was 53.7%,and 56.5% of gastric electrical stimulation patients experienced clinical relapse within 2 years[41].Furthermore,implantation of the device necessitates surgery,and only a limited number of centers possess expertise in both inserting and managing the device,presenting certain practical challenges for its widespread adoption.Surgical options include laparoscopic pyloroplasty and surgical pyloromyotomy.The clinical efficacy of laparoscopic pyloric ring myotomy for the treatment of gastroparesis is approximately 83%-86%,with approximately 60%-90% of patients reporting normalized gastric emptying[42].However,laparoscopic pyloric ring myotomy is associated with adverse effects such as leakage,bleeding,and wound infection,which increase patient risk.Endoscopic methods include endoscopic pyloromyotomy and perpendicular surgical stent placement.Pyloric stent placement has been shown to effectively improve gastric emptying in patients[43],but it is associated with the risk of stent migration,which greatly increases the risk of requiring another surgery.Therefore,this approach is not considered a feasible long-term solution.Several studies have also applied temporary pyloric stents for the treatment of postoperative gastroparesis patients,with stent removal after symptom relief[44].However,this approach may not be useful for long-term treatment in patients with refractory gastroparesis.
G-POME for gastroparesis
In 2013,Khashabet al[1] first used G-POME for the treatment of gastroparesis and achieved good results.Traditionalpyloroplasty has been proven to effectively improve the clinical symptoms of nausea,vomiting,etc.,in patients with gastroparesis.Compared with other conventional surgical protocols,G-POME has a safer profile and relatively less invasiveness[42].Thus,this approach has been affirmed and applied by many centers.The surgical success and postoperative adverse reaction rates of G-POME are similar to those of pyloroplasty[45],and the clinical success rates in terms of the GCSI score and quality of life are also similar[46].However,as a new endoscopic surgery,G-POPE is minimally invasive and more acceptable to patients.In terms of surgical duration,the average G-POME surgery duration ranges from 33 to 120 min,while the average pyloroplasty duration ranges from 99 to 175 min[45].A study on the learning curve of G-POME showed that as the number of operations performed increased,the time needed for the endoscopist to perform G-POME gradually decreased.After performing 18 operations,the surgical duration reached 60 minutes[47].For endoscopists with experience in POME,the time needed to master G-POME decreased.Hence,it is beneficial for centers with endoscopic operating conditions to carry out and promote G-POME such that more gastroparesis patients for whom drug and dietary management have failed can receive safe and effective treatment,reducing the problems caused by gastroparesis.
The safety and technical success rate of G-POME in the treatment of gastroparesis have been confirmed[14,39,48],with an overall incidence of adverse events ranging from 0% to 6.7%[4,18,49,50].Serious adverse events include gastrointestinal bleeding,pyloric ulcers,and capnoperitoneum.One study reported an unusually high perforation rate (20%).This may have been due to the use of full-thickness pyloromyotomy[51,52],which is extremely rare in selective myotomy.With the promotion of G-POME and the follow-up studies on this procedure,its effectiveness has also been confirmed.During a 36-month follow-up period,significant improvements in symptoms and quality of life were observed in 73% to 85.7% of patients[53,54].However,in some studies,a high overall effectiveness in refractory gastroparesis has not been shown for G-POME[13],which is mainly related to differences in the definition of clinical success.Many previous studies have defined clinical success as an improvement in the GCSI score,SF-36 score,or GES after GPOME surgery[14,18].This study[13] defined clinical success as an average GCSI score reduction of 1 point and > 25%.
On the other hand,one study[13] suggested a guideline for the selection of gastroparesis patients who undergo GPOME surgery.G-POME is a novel procedure for targeting the pyloric sphincter and is expected to achieve good clinical results in patients with gastroparesis caused by pyloric spasm.Compliance and distension of the pylorus have been proven to be predictive of the clinical response to pyloric-directed therapy[51,55,56].In addition,the severity of clinical symptoms at baseline and a gastric retention > 20% for 4 h before G-POME are independent predictive factors for clinical success[57].Hence,for patients with severe symptoms and great GES retention,G-POEM should be considered a priority treatment for gastroparesis after conservative treatment has been proven to be ineffective.In addition,current research on the pathogenesis of gastroparesis has focused on ICCs.G-POME can be used to sample pyloric muscles during the procedure,thereby facilitating pathological studies of gastroparesis.
One of the latest advances in the endoscopic evaluation of gastroparesis is the use of intraluminal functional probe imaging (EndoFLIP®) to measure impedance planes.EndoFLIP®is being applied in G-POME procedures for evaluation and has achieved good results[58-61].By using the data feedback from EndoFLIP®,physicians can make intuitive evaluations of the compliance and dilation of the pylorus,providing a new and objective scheme for research on the effectiveness of G-POME.
USE OF G-POME FOR CONGENlTAL HYPERTROPHlC PYLORlC STENOSlS
Background
Congenital hypertrophic pyloric stenosis (CHPS) is a serious disease that occurs in children younger than 1 year and is the third most common gastric abnormality in newborns and infants.CHPS is caused by pyloric muscle hypertrophy,which leads to pyloric obstruction,and usually presents as severe projectile vomiting after eating[62].Early and accurate diagnosis and treatment are crucial for children with CHPS.A delay in treatment may result in severe malnutrition,multiple organ dysfunction,and even death.
Since Ramstedt first described CHPS in 1912,surgical pyloromyotomy has been the standard treatment for CHPS.This involves a longitudinal pyloric muscle incision under open abdominal conditions[63].Open surgery can provide good intraoperative pyloric exposure and good treatment results.However,due to the large external incision and the fact that the abdominal scar grows with the growth of the child,this procedure can significantly impact the child's appearance[64] and may even have negative social and psychological effects.With the development of laparoscopic technology,Alainet al[65] attempted laparoscopic treatment of CHPS,namely,laparoscopic pyloromyotomy.From a therapeutic perspective,open surgery and laparoscopic surgery have similar safety and efficacy.However,laparoscopic surgery is increasingly used for the treatment of CHPS due to its better cosmetic results and faster postoperative recovery[66,67].These findings are also in line with the request of parents for good cosmetic results and complete oral feeding recovery after surgery.However,compared with conventional open surgery,laparoscopic pyloromyotomy slightly increases the risk of mucosal perforation and incomplete pyloric muscle incision[64,68],which may require additional surgery,increasing the risk of secondary surgery or even open surgery.
Endoscopic pyloromyotomy
In this context,CHPS patients urgently need safe,effective and cosmetically pleasing treatment.In 2005,Ibarguen-Secchia[69] first reported the use of endoscopic pyloromyotomy in CHPS patients.This procedure involves direct sectioning of the muscle layer through a mucosal incision.Although this procedure is theoretically and technically feasible,a high level of skill and experience is needed for physicians to accurately identify the circular muscle layer and control the length and depth of the endoscopic incision during the operation.The risk of postoperative bleeding and perforation is also greater.In addition,after myotomy,the exposed mucosa and muscle layer are exposed to acidic gastric juice,which may induce ulcers and potentially lead to further obstruction[70,71],ultimately resulting in surgical failure.
Application of G-POME
With the emergence and promotion of G-POME,which can also effectively improve pyloric muscle spasm,Liuet al[72] applied G-POME to treat CHPS for the first time and achieved good results.Thus,G-POME is a technically feasible,safe,and successful procedure for treating CHPS,but the data available for evaluating the safety and efficacy of G-POME in infants with CHPS are quite limited.Zhanget al[73] analyzed 21 patients with CHPS treated with G-POME.All patients successfully underwent G-POME,and on the third day after surgery,oral meglumine diatrizoate was used for upper gastrointestinal radiography,with the contrast agent smoothly passing through the pylorus.During the median followup period of 25.5 months,no patients developed vomiting,fever,or gastrointestinal bleeding.Due to its endoscopic operation characteristics,G-POME leaves almost no scar on the skin of the child.Compared with traditional surgical methods (open surgery and laparoscopic surgery),G-POME not only has the advantage of being minimally invasive but can also rapidly improve the child's feeding condition while ensuring efficacy,which is consistent with the expectations of parents.
Compared with those of endoscopic pyloromyotomy performed by Ibarguen-Secchia[69],the risk of full-thickness perforation and bleeding after G-POME is greatly reduced.As the main operation of G-POME is carried out in a submucosal tunnel,after wound closure,full-thickness perforation can be avoided,reducing the risk of postoperative perforation and bleeding.During G-POME,the submucosal and circular muscle layers can be clearly and directly identified by submucosal injection[70],facilitating and increasing the accuracy of myotomy and greatly preventing adverse events.In G-POME,after the myotomy operation is completed,the mucosal incision is closed,thus avoiding direct contact between the gastric acid and the muscle incision and mucosa.The incidence of postoperative adverse reactions (such as ulcers) and failure is lower than that of traditional endoscopic pyloromyotomy.This method not only ensures effective treatment but also is associated with decreased risk.
Unlike the G-POME procedure routinely used for gastroparesis,in the treatment of CHPS,the pyloric muscle is not selectively incised,but a full-thickness pyloric muscle incision is made.An incomplete pyloric muscle incision may lead to uncertain results and clinical recurrence[71].After G-POME,a nasal jejunal tube is placed in one nostril of the child,with the distal end located approximately 10-15 cm behind the pylorus.Enteral nutritional support is given 6 h after the operation.Another nasal gastric tube is placed in the other nostril,with the distal end located in the stomach,and is connected to an external drainage device to fully discharge gastric juice.If there is no leakage of contrast agent during the 3-day follow-up,the mucosal incision is allowed to recover,and oral enteral nutrition,breast milk,or high-energy milk powder is provided[73].
Compared with those in G-POME routinely used in adults,the gastric wall mucosa and submucosa in infants and young children are immature,which may pose difficulties in establishing a submucosal tunnel.However,due to the limited number of patients currently receiving G-POME for CHPS,the safety of this novel treatment approach in patients of different weights requires further study.In addition,because the gastric cavity of infants is narrow,there is less space for surgery,requiring greater technical skills and experience from the physician performing G-POME.It is recommended that surgeons be prepared for surgical rescue treatment during G-POME.
The application of G-POME in the treatment of CHPS has received relatively little attention.Although satisfactory clinical responses were shown in short-term follow-up,further research is needed to compare the long-term effects of GPOME with those of traditional regimens.Nevertheless,the use of G-POME provides a novel treatment option for CHPS patients.
G-POME FOR GASTRlC SLEEVE STRlCTURES
Background
Obesity is a global epidemic whose incidence is increasing with economic development,and the popularity of weight loss surgery is also increasing steadily.Among these methods,laparoscopic sleeve gastrectomy (LSG) has become the most common weight loss surgery[74].Compared to other weight loss surgeries,such as Roux-en-Y gastric bypass (RYGB),LSG can greatly maintain the continuity of the gastrointestinal tract.As a result,patients who undergo LSG rarely exhibit signs of malabsorption and do not require vitamin supplementation for possible deficiencies after surgery[75].
Although the safety of LSG has been confirmed[76],adverse events such as suture leakage,fistula,and bleeding may still occur after surgery,especially for patients with gastric sleeve stenosis (GSS)[77-79].GSS is a common complication after LSG,with an incidence rate of approximately 4%.GSS can present with various clinical symptoms,such as dyspepsia,reflux,early satiety,abdominal pain,nausea,and vomiting[75].These symptoms seriously affect the quality of life and physical health of patients.Therefore,timely and effective treatment is needed for patients with GSS.
Traditional treatment regimen
For patients with GSS,the existing treatment options include pneumatic balloon dilation,placement of a full-coverage self-expanding metal stent (FCSEMS),and conversion to a gastric bypass procedure such as RYGB.
Balloon dilation is generally a safe and effective treatment option for GSS after LSG,and it is also the preferred treatment option after GSS occurs.According to the report by Dhorepatilet al[80],67.7% of GSS patients experienced symptomatic relief after a single dilation procedure.After multiple dilations,93.9% of patients showed improvement in their symptoms.However,it is undeniable that a small proportion of patients do not experience symptomatic relief after balloon dilation.
Fayadet al[81] reported the use of FCSEMS for treating GSS after LSG and gastric-intestinal anastomotic stenosis after RYGB.This approach is theoretically feasible,but the clinical effect is not satisfactory.However,there is an unavoidable problem in the application of stents,namely,stent migration.Stent migration may lead to serious adverse events that require further intervention or surgical treatment[82].Even in patients without stent migration,53.3% of patients experienced symptom recurrence after stent removal,which is similar to what has been observed when using stents to treat gastroparesis patients.
Patients who develop GSS after LSG are usually reluctant to undergo further invasive surgeries,such as gastroplasty by RYGB.In addition,in a study by Sillénet al[75],among 21 GSS patients who underwent surgical conversion to RYGB,nearly 50% reported residual GSS symptoms.Moreover,RYGB patients may experience adverse events such as gastricintestinal anastomotic stenosis,incisional hernia,and bleeding.Therefore,a minimally invasive,safe and effective method for treating GSS after LSG is urgently needed.
Application of G-POME
In 2019,Farhaet al[83] reported for the first time the application of G-POME in patients who developed GSS after LSG.One patient underwent LSG for two months and subsequently developed symptoms of GSS.Endoscopic examination revealed spiral stenosis at the level of the angular notch.After endoscopic balloon dilation,the patient’s symptoms did not improve.After refusing to undergo RYGB for revision surgery,the patient ultimately underwent G-POME to relieve the stenosis.
During G-POME,the physician established an 8-cm submucosal tunnel and performed a 6-cm myotomy.Before and after myotomy,an EndoFLIP®was used to evaluate the diameter,cross-sectional area,and compliance with the stenosis.The results showed that after myotomy,the diameter,cross-sectional area,and compliance with the stenosis significantly improved.At the follow-up visits,the patient's symptoms had almost completely disappeared.Zhanget al[74] reported the results of G-POME in 13 patients with GSS.The authors divided the patients into spiral (n=11) and nonspiral (n=2) GSS groups and evaluated their symptoms using the Gastroparesis Symptom Control Index (GSCI).After a follow-up period of approximately 4-9 months,clinical success was achieved in 10 patients (improvement in symptoms after sufficient intake was restored).
From a technical perspective,G-POME for GSS patients is challenging.G-POME for GSS patients requires the establishment of a tunnel from the cardia to the pylorus and full-thickness myotomy within this tunnel.Typically,the length of the tunnel established in G-POME for gastroparesis patients is approximately 4 cm,while that needed for GSS patients reaches 8 cm[83];GSS patients require a longer length of myotomy.Technically,G-POME for GSS patients is more accurately described as tunneling or narrow-tube incisions than traditional pyloromyotomy.In addition,the presence of a tortuous gastric sleeve and larger gastric vessels further complicate the procedure,requiring greater technical skills from the endoscopic physician.
Currently,there is no effective evaluation tool for quantifying the response to GSS treatment.In the study by Zhanget al[74],the GSCI was used for scoring.Similar to the report by Farhaet al[83] on the use of G-POME in GSS patients,Janeset al[84] also used EndoFLIP®to evaluate the therapeutic effect of airbag dilation in GSS patients.Volumetric threedimensional computerized tomographic reconstruction of the stomach may also be useful for assessing symptoms in GSS patients[85].In the future,a more effective protocol for assessing the condition of GSS patients is needed to better evaluate the effectiveness of G-POME and other treatment options for GSS patients.
Based on the limited available research on G-POME for GSS,G-POME appears to be a safe,effective and minimally invasive treatment option for refractory GSS.For patients who do not respond well to balloon dilation,G-POME-guided GSS correction may be a good choice.Due to the potential risks and postoperative adverse events associated with GPOME,we do not recommend it as a first-line treatment for all GSS patients.Additionally,the effectiveness of G-POME may vary depending on the type of GSS,but this requires further objective evaluation through larger studies.
CONCLUSlON
In the future,further development of G-POME should focus mainly on the following key points: (1) The surgical technique should be optimized for gastroparesis patients to increase clinical efficacy and reduce potential adverse reactions and symptom recurrence after surgery;(2) For more extensive application of G-POME in the treatment of CHPS and GSS,larger sample sizes are needed to further evaluate its effectiveness;and (3) Innovative use of G-POME in the treatment of more upper gastrointestinal system diseases should be carried out.Although identifying more suitable diseases for G-POME may be challenging due to its specificity for the pylorus muscle,as a promising novel approach,GPOME may lead to unexpected changes in the treatment of some upper gastrointestinal diseases.Overall,G-POME is a promising endoscopic treatment technique that is safe and minimally invasive and surpasses surgical procedures,meeting the needs of many patients.While ensuring safety and minimal invasiveness,G-POME has considerable effectiveness in treating gastroparesis,CHPS,and GSS.Currently,G-POME,which is recognized and promoted by many centers,is mainly used to treat refractory gastroparesis and has achieved good clinical results.Several centers are pioneering the use of G-POME in the treatment of CHPS/CHPS and GSS/GSS.Although these studies have shown some effectiveness,due to the small sample sizes reported thus far,we cannot yet make a definitive conclusion about the efficacy of G-POME.Nevertheless,these exciting attempts provide a new perspective for the treatment of CHPS and GSS,and additional centers should invest in related clinical research.
FOOTNOTES
Co-first authors:Shi-Yu Chang and Guo-Hua Jin.
Author contributions:Chang SY and Jin GH wrote the paper,provided equal contributions to the article;Sun HB and Yang D collected relevant data and conducted analysis;Tang TY provided research directions and reviewed the paper;all the authors report no relevant conflicts of interest for this article.The completion of this paper is the result of the joint efforts and significant contributions of both authors,Chang SY and Jin GH.They have played an indispensable role at every stage of this research project,from the initial conceptualization and experimental design to data analysis and interpretation of results,as well as the final drafting and revision of the paper.Both authors have invested equal amounts of time and energy into the work.Their collaboration has been equal and close-knit,and thus,in this paper,Chang SY and Jin GH should be recognized as co-first authors.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Shi-Yu Chang 0009-0006-2943-375X;Guo-Hua Jin 0000-0002-0034-7819;Hai-Bo Sun 0000-0001-6474-1762;Dong Yang 0000-0002-3148-4197;Tong-Yu Tang 0000-0001-8259-919X.
S-Editor:Lin C
L-Editor:A
P-Editor:Cai YX
 World Journal of Gastrointestinal Surgery2024年3期
World Journal of Gastrointestinal Surgery2024年3期
- World Journal of Gastrointestinal Surgery的其它文章
- Alcohol associated liver disease and bariatric surgery: Current perspectives and future directions
- Ex vivo liver resection and auto-transplantation and special systemic therapy in perihilar cholangiocarcinoma treatment
- Evaluation of bacterial contamination and medium-term oncological outcomes of intracorporeal anastomosis for colon cancer: A propensity score matching analysis
- Rescue from complications after pancreaticoduodenectomies at a low-volume Caribbean center: Value of tailored peri-pancreatectomy protocols
- Comparison of prognosis and postoperative morbidities between standard pancreaticoduodenectomy and the TRlANGLE technique for resectable pancreatic ductal adenocarcinoma
- Analysis of the impact of immunotherapy efficacy and safety in patients with gastric cancer and liver metastasis
