NADPH氧化酶4在心血管损伤中的作用机制
石丹丹 宁梓淇 刘美霞 刘剑刚

【摘要】心血管结构和功能损伤是许多心血管疾病的重要病理基础,许多研究表明氧化应激在缺血性心脏病、动脉粥样硬化、高血压等诸多病理性心血管损伤中发挥重要作用。NADPH氧化酶(Nox)是调控氧化还原信号的关键酶,而血管内的活性氧主要来源于Nox4。随着研究的不断深入,发现Nox4在不同阶段或不同刺激下会发挥不同甚至截然相反的作用,如双向调节动脉粥样硬化的进展、双向作用影响血压等。现总结Nox4在不同心血管损伤中的不同影响及作用机制,为后续的研究提供一定的理论基础。
【关键词】NADPH氧化酶4;活性氧;心血管损伤
【DOI】10.16806/j.cnki.issn.1004-3934.2024.02.000
Mechanism of the Role of Reduced Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 in Cardiovascular Injury
SHI Dandan1,2,NING Ziqi1,2,LIU Meixia1,LIU Jiangang1
(1.Xiyuan Hospital,Chinese Academy of Chinese Medical Sciences,Institute of Geriatrics of Chinese Academy of Chinese Medical Sciences,Beijing 100091,China; 2. Graduate School of China Academy of Chinese Medicial Sciences,Beijing 100700,China)
【Abstract】The damage of cardiovascular structure and function is an important pathological basis of many cardiovascular diseases. Many studies have shown that oxidative stress plays an important role in many pathological cardiovascular injuries,such as ischemic heart disease,atherosclerosis,hypertension,etc. Reduced nicotinamide adenine dinucleotide phosphate oxidase (Nox) is a key enzyme in the regulation of redox signaling,and intravascular reactive oxygen species are mainly originated from Nox4.With the deepening of the research,it has been found that Nox4 plays different or even opposite roles at different stages or under different stimuli,such as bidirectional regulation of atherosclerosis progression,bidirectional effects affecting blood pressure,etc. This article summarizes the different roles and mechanisms of Nox4 in different cardiovascular injuries,providing a theoretical basis for subsequent research.
【Keywords】Reduced nicotinamide adenine dinucleotide phosphate oxidase 4; Reactive oxygen species; Cardiovascular injury
據世界卫生组织调查,心血管疾病每年导致近1 790万的死亡,是全球死亡的主要原因,严重威胁着人类的生命健康[1]。众多研究结果证实,氧化应激损伤在其中扮演关键角色。氧化应激是指体内氧化与抗氧化作用紊乱(更倾向于氧化),导致产生大量的活性氧(reactive oxygen species,ROS)在体内蓄积,进而损伤核酸、蛋白质和脂质等生物大分子。在心血管疾病中,氧化应激是重要的介导者。
ROS的来源众多,主要包括线粒体呼吸链以及NADPH氧化酶(reduced nicotinamide adenine dinucleotide phosphate oxidase,Nox),其中Nox4是血管内ROS的主要来源[2]。Nox4参与心血管的各种生理病理过程,对维持心血管结构和功能起着关键作用。多种研究表明,在心肌梗死、高血压、动脉粥样硬化等心血管疾病的不同阶段或不同刺激下,Nox4所发挥的作用也不同。现主要从以下几个方面阐明Nox4在心血管损伤中的双向作用。
1 Nox4的结构和功能
Nox是特殊的产生ROS的酶类家族,由胞膜成分、细胞色素b558[gp91phox和p22phox,其中gp91phox有NADPH、黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)潜在结合位点]、3种胞质蛋白(p67phox、p47phox和p40phox)及小G蛋白Rac组成,其主要生物学功能是产生ROS,以维持细胞正常的生理活动。但是在异常情况下,Nox家族蛋白出现异常表达或激活,不仅导致细胞内ROS过量产生和蓄积,还会作用于线粒体及其他ROS相关酶系统(如线粒体电子传递链)导致其功能异常,继而引发ROS的继发性蓄积,最终形成恶性循环,持续不断地对组织造成损伤[3]。目前已经鉴定出Nox的七个亚型,包括Nox1-5和双重氧化酶(dual oxidase,DUOX)1及DUOX2,其中Nox1、Nox2、Nox4和Nox5在血管系统中被发现,而在血管内皮细胞(endothelial cell,EC)中,Nox4的表达则高于其他Nox亚型[4],被认为是血管内ROS的主要来源[2]。
在Nox家族中,Nox4最为特殊:(1)Nox4为构成性活性,与稳定的p22phox胞膜亚基结合后具有活性,其活性被聚合酶-δ相互作用蛋白2(polymerase delta-interacting protein 2,Poldip2)调节,其激活无需额外的调控亚基;(2)Nox家族其他亚基生成超氧化物自由基O2·-,O2·-自由基依次发生歧化反应生成H2O2,而Nox4可直接产生H2O2[5-6]。这是由于其独特的内在激活的Nox4脱氢酶结构域。Nox4包括C端的脱氢酶结构域(包含FAD和NADPH结合位点)和N端的6个跨越膜的α-螺旋(包括环A-E),位于协调两个血红素在第三和第五跨膜螺旋上。正常情况下电子从NADPH通过FAD和两个血红素基传递给O2产生O2-,由于Nox4上的E环上结构,O2-被快速歧化为H2O2。见表1及图1。
2 Nox4对心血管损伤的影响及作用机制
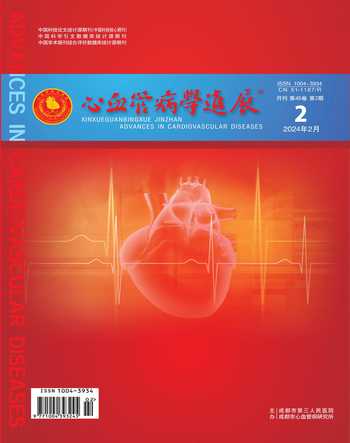
2.1 Nox4调控EC的定向迁移,促进血管生成
血管的生成是一个复杂的过程,与EC密切相关。在促血管生成的刺激下,EC进行增殖、迁移和分化,并招募血管平滑肌细胞(vascular smooth muscle cell,VSMC)或周细胞来覆盖以形成新的血管。Nox4介导的血管生成受多种因素如缺氧、缺血、血管内皮生长因子(vascular endothelial growth factor,VEGF)等的調控。当机体处于缺氧缺血状态时,缺氧诱导因子-1α(hypoxia inducible factor-1α,HIF-1α)随即生成。HIF-1α一方面刺激Nox4的表达,生成ROS,另一方面,HIF-1α激活下游靶标VEGF[11,12]。VEGF作为最有效的内皮特异性血管生成生长因子,与血管内皮生长因子受体-2(vascular endothelial growth factor receptor 2 ,VEGFR-2)相结合,激活酪氨酸激酶途径,促进EC的增殖、迁移,从而加速体内血管的形成。血管生成素2(angiopoietin2,Ang2)与VEGF相互协调促进血管生成。VEGF可促进Ang2的表达,破坏血管稳定性,从而促使血管生成增加[13]。实验[14,15]证明,Nox4可降低VEGF和HIF-1α的水平,Nox4缺失时VEGF、VEGFR-2、Ang2的表达显著降低。此外,Nox4的激活使得血管内生成大量H2O2,NO增多,高水平的H2O2减少核转录因子红系2相关因子2(nuclear factor-erythroid 2-related factor 2 ,Nrf2)的降解,促进血红素加氧酶-1(heme oxygenase 1,HO-1)的生成,发挥血管保护功能[16]。已有实验证明,股动脉结扎的全身Nox4敲除小鼠相较于对照组来说,其体内内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)、NO及HO-1的表达降低,Nrf2蛋白水平降低,且小鼠的血流恢复显著减弱[17]。转化生长因子-β1(transforming growth factor-β1,TGF-β1)是血管生成的另一个重要调节因子,在Nox4信号传导中发挥重要作用。实验[18]证明,经TGF-β1诱导的人脐静脉EC中Nox4蛋白的表达上调,而在Nox4敲除小鼠的心脏EC中,TGF-β1诱导EC细胞增殖、迁移和管形成被消除;在体内,TGF-β1诱导的血管生成在Nox4敲除的小鼠中显著降低。体外实验[19]中使用Nox4敲除的人脐静脉EC进行血管生成实验,结果显示血管生成显著减少。
综上,Nox4在血管生成中发挥重要的调控作用,特别是在缺血状态下的血运重建中起积极作用。Nox4参与调控多种与血管生成密切相关的细胞因子如VEGF、HIF-1α、TGF-β1等的信号传导,从而促进新血管的生成(见图2)。正因为Nox4在血管生成中的关键作用,它已成为治疗癌症、外周动脉疾病、脑梗死、心肌梗死等疾病的重要靶标之一。已有研究[20]表明Nox4与某些肿瘤,如纤维肉瘤、胶质母细胞瘤、星型胶质瘤等的血管生成相关。因此,Nox4可能成为肿瘤治疗中具有潜力的靶点之一,其抑制可能有助于抑制肿瘤的血管生成。

图2 Nox4调控血管生成的机制
2.2 Nox4双向调节参与动脉粥样硬化性血管损伤的发生发展
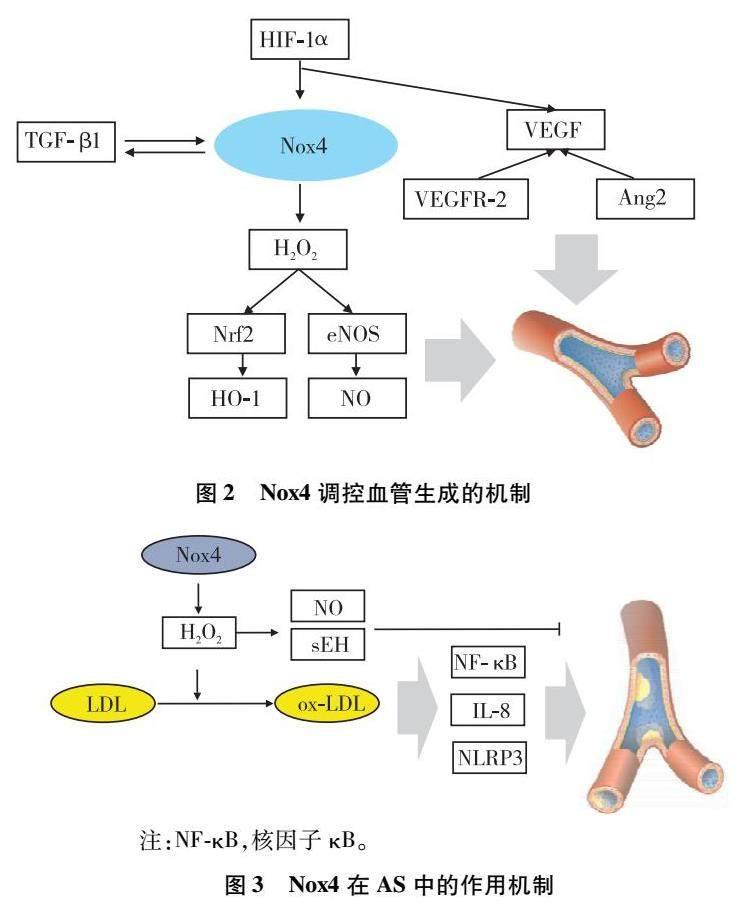
Nox4在动脉粥样硬化(atherosclerosis,AS)中具有复杂作用。在AS进展过程中,Nox4生成的ROS促进氧化型低密度脂蛋白(oxidized low-density lipoprotein,ox-LDL)的生成和泡沫细胞的形成。同时,具有细胞毒性的ox-LDL还通过信号转导激活NF-κB信号通路,降低eNOS活性,减少NO生成,同时增加炎症因子如白细胞介素-8(interleukin-8,IL-8)、Nod样受体蛋白3(nod-like receptor protein 3,NLRP3)等释放,引起EC功能障碍,从而进一步促进AS的形成。实验[21]证明,在AS中可观察到Nox4表达上调,而在全身性Nox4敲除小鼠中AS进展缓慢。
值得注意的是,有其他学者提出Nox4对内皮功能也有一定的保护作用。Nox4产生的H2O2不与NO相互作用,不会导致过氧亚硝酸盐的生成,从而保护血管内皮。在低密度脂蛋白受体缺失的小鼠中,Nox4的表达可抑制EC功能障碍和AS的发展[22]。最新研究[23]发现,EC的Nox4功能紊乱,可能通过增加内质网应激、上调水溶性环氧化合物水解酶(soluble epoxide hydrolase,sEH)水平,从而诱导血管炎症,加速AS进程(见图3)。

图3 Nox4在AS中的作用机制
此外,关于Nox4在AS中的双向作用,还可能与细胞类型有关。有研究者发现,VSMC的Nox4与EC的Nox4在AS中发挥相反的作用:VSMC的Nox4可能对AS起保护作用,EC的Nox4可能促进AS的进展[23]。AS是一个复杂的疾病过程,多个因素参与其中,对于Nox4在AS中的作用机制和具体调控方式仍需要更深入的研究和验证。
2.3 Nox4影响血管舒缩功能从而影响血压
Nox4在高血压的发生发展中发挥重要作用。VSMC异常增殖和向内膜迁移是高血压发生和发展的关键阶段,而基质金属蛋白酶(matrix metalloproteinase,MMP)降解VSMC的细胞外基质是其迁移的必要步骤。Nox4可介导胰岛素样生长因子1诱导的MMP-2和MMP-9的激活,从而启动VSMC的迁移、增殖[2]。动物实验[24]表明,高线粒体Nox4表达的小鼠主动脉僵硬度显著增加。在盐敏感型高血压大鼠中敲除Nox4基因,盐敏感型大鼠模型对高盐的血压反应减弱[25]。
关于Nox4在高血压中的作用,目前还存在一些争议。其他研究[26-27]发现高血压患者中Nox4水平较低,过表达的Nox4可增强血管舒张作用,降低血压,对心血管系统发挥保护作用。这些结果之间的矛盾可能与不同的实验条件、样本来源和研究方法有关。总体而言,Nox4在高血压中的作用仍处于早期阶段,进一步的研究将有助于更好地理解Nox4与高血压之间的关系,以及发掘其在治疗高血压方面的潜在作用。
2.4 Nox4加重心肌缺血再灌注损伤
缺血性心肌病是心血管损伤的基础和重要因素之一。Hearse[28]等发现,当恢复血流时心肌损伤反而加重,被称为“再灌注损伤”。ROS是心肌缺血再灌注损伤的潜在介质,通过介导线粒体功能障碍和内质网应激,从而损伤心肌细胞。Nox4作为心肌细胞中主要的Nox亚型,抑制Nox4衍生的ROS可防止线粒体功能障碍和内质网应激,从而减轻心肌缺血再灌注损伤。研究[29-30]发现,心脏特异性Nox4过表达小鼠在心肌缺血再灌注期间表现出ROS产生与梗死面积的增加,而心脏特异性Nox4敲除小鼠心肌损伤显著减少。另一项研究[31]发现,下调Nox4的表達后,小鼠心肌缺血再灌注后梗死面积减小。这些发现表明,Nox4在心肌缺血再灌注损伤中起有害作用。
2.5 Nox4可促进心血管细胞的衰老与凋亡
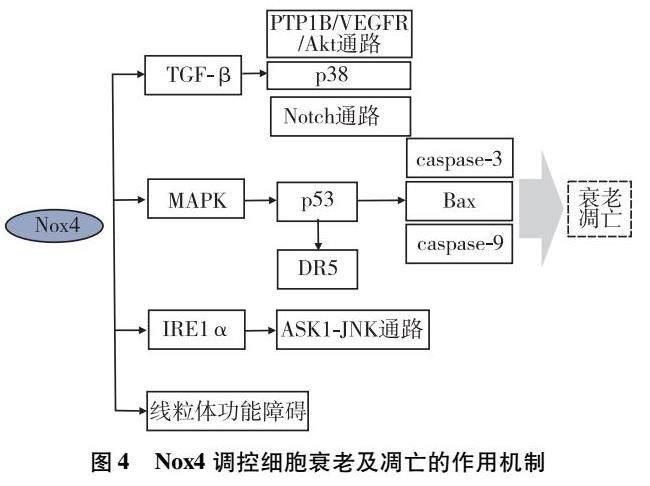
年龄被认为是心血管疾病的主要危险因素,随着年龄的增长,机体抗氧化能力减弱,使得氧化系统和抗氧化系统之间的平衡向氧化应激倾斜,最终导致心血管损伤。在主动脉VSMC和线粒体中,Nox4的表达和ROS水平随年龄增加而增加,老年主动脉中Nox4 mRNA和蛋白的表达明显高于年轻主动脉。体外实验[32]同样表明,内质网ROS及其相关Nox4的表达和活性都随着衰老而增加。血管Nox4水平与年龄呈正相关,这提示Nox4可能成为治疗衰老性心血管疾病的潜在治疗靶点。
此外,Nox4可通过多种机制触发细胞凋亡。过表达的Nox4可活化促分裂原活化的蛋白激酶(mitogen-activated protein kinase,MAPK)途径,进而激活p53。这个过程伴随着促凋亡蛋白B细胞淋巴瘤-2相关X蛋白(B-cell lymphoma-2 related X protein,Bax)和caspace信号通路中caspace-9、caspace-3的上调,增加心肌细胞的凋亡[33]。TGF-β依赖于Nox4的上调和ROS的产生,通过调节p38、PTP1B/VEGFR/Akt和Notch信号通路影响EC功能[34],诱导EC凋亡。此外,Nox4还可以通过引发内质网功能障碍从而促进细胞凋亡[35],其具体机制为Nox4与内质网膜的肌醇需求酶1α(inositol requiring enzyme 1 alpha,IRE1α)作用,募集肿瘤坏死因子受体相关因子2(tumor necrosis factor receptor-associated factor 2,TRAF2),并与凋亡信号调节激酶1(apoptosis signal regulating kinase-1,ASK1)形成IRE1α-TRAF2-ASK1复合体,进而激活c-Jun氨基端蛋白激酶(c-Jun N-terminal protein kinase,JNK)的磷酸化,激活凋亡信号,诱导细胞凋亡。死亡受体5(death receptor 5,DR5)是外源性凋亡通路的主要组成部分,由p53依赖的转录激活介导。Nox4下调阻止了ROS/p53/DR5轴的激活,从而抑制细胞凋亡[36]。此外,Nox4还会通过破坏线粒体功能导致细胞衰老和死亡。Nox4直接产生H2O2会破坏线粒体,从而导致线粒体功能障碍,促进细胞的衰老和凋亡[37](见图4)。

图4 Nox4调控细胞衰老及凋亡的作用机制
3 总结与展望
Nox4作为血管中ROS的主要来源,常常发挥双向调控作用,在不同的心血管疾病作用不同。随着众多学者对Nox4研究的不断深入,关于Nox4的几个关键问题仍待解答。(1)Nox4功能的细胞类型特异性:目前尚不清楚Nox4在不同细胞类型中的具体功能和调控机制,还有待进一步研究;(2)Nox4的亚细胞定位和作用:Nox4被发现存在于多个亚细胞位置如内质网、线粒体、核内等,目前对于Nox4亚细胞定位的调控机制了解还比较有限,需要进一步的研究来揭示其详细信息;(3)如何调控Nox4的双向作用,有赖于确定不同状态下ROS的不同水平。了解不同状态下ROS水平的变化以及其对细胞功能的影响,是调控Nox4双向作用的关键。新的特异性Nox4抑制剂可能为AS、高血压、冠心病等心血管性疾病的治疗带来新的前景。
參考文献
[1] Jung YS. Natural antioxidant in cardiovascular and cerebrovascular diseases[J]. Antioxidants (Basel),2022,11(6):1159-1161.
[2] Gola L,Bierhansl L,Csatári J,et al. NOX4-derived ROS are neuroprotective by balancing intracellular calcium stores[J]. Cell Mol Life Sci,2023,80(5):127-145.
[3] Vermot A,Petit-H?rtlein I,Smith S,et al. NADPH oxidases(NOX):an overview from discovery,molecular mechanisms to physiology and pathology[J]. Antioxidants (Basel),2021,10(6):890-945.
[4] Tang X,Wang J,Abboud HE,et al. Sustained upregulation of endothelial Nox4 mediates retinal vascular pathology in type 1 diabetes[J]. Diabetes,2023,72(1):112-125.
[5] Lee H,Jose P. Coordinated contribution of NADPH oxidase- and mitochondria-derived reactive oxygen species in metabolic syndrome and its implication in renal dysfunction[J]. Front Pharmacol,2021,12:670076-600094.
[6] Moghadam ZM,Henneke P,Kolter J. From flies to men:ROS and the NADPH oxidase in phagocytes[J]. Front Cell Dev Biol,2021,9:628991-629007.
[7] Gimenez M,Schickling BM,Lopes LR,et al. Nox1 in cardiovascular diseases:regulation and pathophysiology[J]. Clin Sci (Lond),2016,130(3):151-165.
[8] Bode K,Hauri M,Jaquet V,et al. Unlocking the power of NOX2:a comprehensive review on its role in immune regulation[J]. Redox Biol,2023,64:102795-102812.
[9] García JG,Ansorena E,Izal I,et al. Structure,regulation,and physiological functions of NADPH oxidase 5 (NOX5)[J]. J Physiol Biochem,2023,79(2):383-395.
[10] Ashtiwi NM,Sarr D,Rada B. DUOX1 in mammalian disease pathophysiology[J]. J Mol Med (Berl),2021,99(6):743-754.
[11] Islam R,Dash D,Singh R. An antioxidant ameliorates allergic airway inflammation by inhibiting HDAC 1 via HIF-1α/VEGF axis suppression in mice[J]. Sci Rep,2023,13(1):9637-9651.
[12] Ding H,Tang C,Wang W,et al. Polydatin ameliorates high fructose-induced podocyte oxidative stress via suppressing HIF-1α/NOX4 pathway[J]. Pharmaceutics,2022,14(10):2202-2230.
[13] Niapour A,Miran M,Seyedasli N,et al. Anti-angiogenic effects of aqueous extract from Agrostemma githago L. seed in human umbilical vein endothelial cells via regulating Notch/VEGF,MMP2/9,ANG2,and VEGFR2[J]. Environ Sci Pollut Res Int,2023,30(9):22413-22429.
[14] Chai D,Zhang L,Xi S,et al. Nrf2 activation induced by Sirt1 ameliorates acute lung injury after intestinal ischemia/reperfusion through NOX4-mediated gene regulation[J]. Cell Physiol Biochem,2018,46(2):781-792.
[15] Miyano K,Okamoto S,Yamauchi A,et al. The NADPH oxidase NOX4 promotes the directed migration of endothelial cells by stabilizing vascular endothelial growth factor receptor 2 protein[J]. J Biol Chem,2020,295(33):11877-11890.
[16] Wang Y,Wang W,Zhou S,et al. Poldip2 knockdown protects against lipopolysaccharide-induced acute lung injury via Nox4/Nrf2/NF-κB signaling pathway[J]. Front Pharmacol,2022,13:958916.
[17] Schr?der K,Zhang M,Benkhoff S,et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase[J]. Circulation research,2012,110(9):1217-1225.
[18] Shah MH,Chan EC,van Bergen NJ,et al. Nox4 facilitates TGFβ1-induced fibrotic response in human Tenons fibroblasts and promotes wound collagen accumulation in murine model of glaucoma filtration surgery[J]. Antioxidants (Basel),2020,9(11):1126-1140.
[19] Song IK,Kim HJ,Magesh V,et al. Ubiquitin C-terminal hydrolase-L1 plays a key role in angiogenesis by regulating hydrogen peroxide generated by NADPH oxidase 4[J]. Biochem Biophys Res Commun,2018,495(1):1567-1572.
[20] Barlin M,Clements J,Held J. Nox4 regulates cancer cell plasticity influencing autophagy state of cells[J]. Free Radic Biol Med,2022,192:97-98.
[21] Zhou T,Li S,Yang L,et al. microRNA-363-3p reduces endothelial cell inflammatory responses in coronary heart disease via inactivation of the NOX4-dependent p38 MAPK axis[J]. Aging (Albany NY),2021,13(8):11061-11082.
[22] Langbein H,Brunssen C,Hofmann A,et al. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice[J]. Eur Heart J,2016,37(22):1753-1761.
[23] Kim SA,Lee AS,Lee HB,et al. Soluble epoxide hydrolase inhibitor,TPPU,attenuates progression of atherosclerotic lesions and vascular smooth muscle cell phenotypic switching[J]. Vascul Pharmacol,2022,145:107086-107098.
[24] Canugovi C,Stevenson MD,Vendrov AE,et al. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening[J]. Redox Biol,2019,26:101288.
[25] Cowley AW Jr,Yang C,Zheleznova NN,et al. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats[J]. Hypertension,2016,67(2):440-450.
[26] Meister ML,Najjar RS,Danh JP,et al. Berry consumption mitigates the hypertensive effects of a high-fat,high-sucrose diet via attenuation of renal and aortic AT1R expression resulting in improved endothelium-derived NO bioavailability[J]. J Nutr Biochem,2023,112:109225.
[27] Zhang K,Kan H,Mao A,et al. Single-cell analysis of salt-induced hypertensive mouse aortae reveals cellular heterogeneity and state changes[J]. Exp Mol Med,2021,53(12):1866-1876.
[28] Hearse DJ,Humphrey SM,Chain EB. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart:a study of myocardial enzyme release[J]. J Mol Cell Cardiol,1973,5(4):395-407.
[29] Yu Q,Lee CF,Wang W,et al. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury[J]. J Am Heart Assoc,2014,3(1):e000555.
[30] Matsushima S,Kuroda J,Ago T,et al. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1α and upregulation of peroxisome proliferator–activated receptor-α[J]. Circ Res,2013,112(8):1135-1149.
[31] Olejnik A,Banaszkiewicz M,Krzywonos-Zawadzka A,et al. The Klotho protein supports redox balance and metabolic functions of cardiomyocytes during ischemia/reperfusion injury[J]. Cardiol J,2022,29(5):836-849.
[32] Lee HY,Kim HK,Hoang TH,et al. The correlation of IRE1α oxidation with Nox4 activation in aging-associated vascular dysfunction[J]. Redox Biol,2020,37:101727.
[33] Wang Y,Zhong L,Liu X,et al. ZYZ-772 prevents cardiomyocyte injury by suppressing Nox4-derived ROS production and apoptosis[J]. Molecules,2017,22(2):331-343.
[34] Yan F,Wang Y,Wu X,et al. Nox4 and redox signaling mediate TGF-β-induced endothelial cell apoptosis and phenotypic switch[J]. Cell Death Dis,2014,5(1):e1010.
[35] Riaz TA,Junjappa RP,Handigund M,et al. Role of endoplasmic reticulum stress sensor IRE1α in cellular physiology,calcium,ROS signaling,and metaflammation[J]. Cells,2020,9(5):1160.
[36] Song C,Shi D,Chang K,et al. Sodium fluoride activates the extrinsic apoptosis via regulating NOX4/ROS-mediated p53/DR5 signaling pathway in lung cells both in vitro and in vivo[J]. Free Radic Biol Med,2021,169:137-148.
[37] Zhong Y,Wang L,Jin R,et al. Diosgenin inhibits ROS generation by modulating NOX4 and mitochondrial respiratory chain and suppresses apoptosis in diabetic nephropathy[J]. Nutrients,2023,15(9):2164.
收稿日期:2023-07-26