Th17/Treg balance and macrophage polarization ratio in lower extremity arteriosclerosis obliterans
Zhen-Zhen Li ,Min Liu ,Xiong-Hui He ,Zhen-Dong Liu ,Zhan-Xiang Xiao ,Hao Qian ,You-Fei Qi✉,Cun-Chuan Wang
1Department of Metabolic and Bariatric Surgery,The First Affiliated Hospital of Jinan University,Guangzhou,Guangdong Province,510632,China
2Department of Vascular Surgery,Hainan General Hospital,Hainan Affiliated Hospital of Hainan Medical University,Haikou,Hainan Province,570311,China
3Department of Physical Examination,Hainan General Hospital,Hainan Affiliated Hospital of Hainan Medical University,Haikou,Hainan Province,570311,China
4Department of Gastrointestinal Surgery,Hainan General Hospital,Hainan Affiliated Hospital of Hainan Medical University,Haikou,Hainan Province,570311,China
ABSTRACT Objective: To explore the balance of peripheral blood T helper 17 cells/regulatory T cell (Th17/Treg) ratio and the polarization ratio of M1 and M2 macrophages in lower extremity arteriosclerosis obliterans (ASO).Methods: A rat model of lower extremity ASO was established,and blood samples from patients with lower extremity ASO before and after surgery were obtained.ELISA was used to detect interleukin 6(IL-6),IL-10,and IL-17.Real-time RCR and Western blot analyses were used to detect Foxp3,IL-6,IL-10,and IL-17 expression.Moreover,flow cytometry was applied to detect the Th17/Treg ratio and M1/M2 ratio.Results: Compared with the control group,the iliac artery wall of ASO rats showed significant hyperplasia,and the concentrations of cholesterol and triglyceride were significantly increased (P<0.01),indicating the successful establishment of ASO.Moreover,the levels of IL-6 and IL-17 in ASO rats were pronouncedly increased (P<0.05),while the IL-10 level was significantly decreased (P<0.05).In addition to increased IL-6 and IL-17 levels,the mRNA and protein levels of Foxp3 and IL-10 in ASO rats were significantly decreased compared with the control group.The Th17/Treg and M1/M2 ratios in the ASO group were markedly increased (P<0.05).These alternations were also observed in ASO patients.After endovascular surgery (such as percutaneous transluminal angioplasty and arterial stenting),all these changes were significantly improved (P<0.05).Conclusions: The Th17/Treg and M1/M2 ratios were significantly increased in ASO,and surgery can effectively improve the balance of Th17/Treg,and reduce the ratio of M1/M2,and the expression of inflammatory factors.
KEYWORDS: Lower extremity arteriosclerosis;Regulatory T cells;Regulatory B cells;Inflammatory factors;M1 macrophages;M2 macrophages
Significance
The progression of arteriosclerosis obliterans is closely linked to the inflammatory response of immune cells.This study shows that arteriosclerosis obliterans caused the imbalance of immune cells and increased the expression of inflammatory factors.After endovascular surgery,the Th17/Treg and M1/M2 ratios as well as inflammation were significantly improved.
1.Introduction
Arteriosclerosis obliterans (ASO) is a major form of atherosclerosis disease,and its pathological feature is the narrowing or occlusion of the arterial lumen of the lower extremities,resulting in ischemic lesions of the lower extremities.At present,endovascular intervention and bypass surgery are used to slow down the progression of ASO;however,the postoperative restenosis rate is still high,seriously affecting the prognosis of ASO[1].
In the past few decades,experts have found that the immune response exists in the whole process of atherosclerotic plaque formation and progression and plays a very important role[2,3].By inducing the transformation of lymphocytes into different proinflammatory cytokines and anti-inflammatory chemokines and guiding the interaction of different immune cells[2],the immune tolerance regulation system decisively affects the plaque properties or the propensity to rupture[4].Relevant experiments have verified that the inflammatory response can induce the formation of a large number of atherosclerotic plaques and promote the progression of atherosclerosis[5].
The formation and progression of atherosclerosis involve many cells,such as vascular endothelial cells,vascular smooth muscle cells,macrophages,etc.[6,7].The prominent impact of macrophages in atherosclerosis is inextricably linked to macrophage polarization and the resulting phenotype[8].According to the surface markers of polarized macrophages,the secretion of cytokines and chemokines,and transcription factors,macrophages are divided into two different polarized types,namely classically activated macrophages (M1)and alternatively activated macrophages (M2)[9,10].The detrimental effect manifested in atherosclerotic plaques mainly depends on the polarization of macrophages resulting in a proinflammatoryassociated macrophage phenotype rather than the absolute number of infiltrates[8,11].In the treatment of vulnerable plaques of atherosclerosis,it was found that the stabilization of vulnerable plaques and the delay of atherosclerotic plaque progression were achieved by changing the polarization of macrophages in vulnerable plaques and increasing the anti-inflammatory macrophage phenotype[12].Therefore,changing the polarization of macrophages and regulating the proportion of anti-inflammatory macrophage phenotypes in vulnerable plaques are a new method for the treatment of atherosclerotic vulnerable plaques.
Evidence suggests that T cells are key drivers of atherosclerosis[13].Approximately 70% of T cells in atherosclerotic plaques are CD4+T cells[13].Activated CD4+T cells differentiate into effector cell subsets with different functions to regulate different immune responses,including helper T cell type 1 (Th1),Th2,Th17,T follicular helper cells (Tfh),and regulatory T cells (Treg),etc.[14].Among these subpopulations,increasing research has focused on the role of Th17 cells and Treg cells in autoimmunity.Th17 cells,through the production of cytokines such as IL-17,IL-22,and IL-23,play a role in mobilizing neutrophils and promoting the development of inflammatory responses at the infection sites[15].In contrast,Treg cells produce specific anti-inflammatory cytokines interleukin-10(IL-10) and transforming growth factor-β (TGF-β),thereby inhibiting the activity of immune cells and suppressing excessive immune responses[15].Therefore,attention should be paid to the pathogenic role of Th17/Treg cell imbalance in autoimmune diseases.In this article,we aimed to explore the balance of peripheral blood Th17/Treg ratio and the characteristics and relationship of M1 and M2 polarization ratio in ASO.
2.Materials and methods
2.1.Experimental animals
A total of 20 SD rats were purchased from Harbin Taikang Biology,China (SPF grade,male,6-8 weeks old,weighing 200-220 g).Throughout the experimentation,the rats were housed in a room that was properly ventilated,with a relative humidity of 40%-60%,and maintained at a controlled temperature range of 18-22 ℃,and a light/dark cycle of 12 h.The rats were provided with unrestricted access to feed pellets and clean water.
2.2.Chemicals,reagents and instruments
Vitamin D3 (YT63592,General Pharmaceutical Co.,Ltd,Shanghai),experimental rat food (XT310-1,Collaborative Biology Co.,Ltd,Nanjing),hematoxylin dye solution (H9627,Sigma Corporation,USA),eosin dye solution (AG1100~100 mL,Jizhi Co.,Ltd,Shanghai),human peripheral blood lymphocyte extraction kit(LTS1077,tbdscience,Tianjin),ELISA kit (Yunclone,Wuhan),RNA extraction kit (Invitrogen,USA),protein antibodies IL-6,IL-10,and IL-17 (A11115,A2171,A12454,ABclonal,Wuhan),flow antibodies CD4,CD86,CD206,Foxp3 (201505,305411,321103,320007,Biogene,Beijing),PTCA balloon dilation catheter (Diameter 2F,Metronic,USA),microscope (ZEISS,Germany),and optical microscope (CX-60,OLYMPUS,Japan) were used in the study.
2.3.Establishment of ASO rat models
A total of 20 healthy rats were randomly divided into two groups,with 10 rats in each group.The model group,which consisted of rats subjected to high-fat (plus VD3) and balloon injury,received intraperitoneal injections of vitamin D3 (VD3) at a total dose of 400 000 IU/kg over 3 d.Subsequently,they were given 20 g of highfat feed daily for 4 d.The balloon injury experiment was performed for 8 d.The rats were anesthetized,and the abdominal aorta and bilateral iliac arteries were separated.Heparin (300 U/kg) was subcutaneously injected.A longitudinal incision of approximately 2 mm was made on the anterior wall of the abdominal aorta between the two arterial clips using a sharp knife.The balloon rod was inserted into the arterial incision using forceps,guided into the left iliac artery through the iliac artery bifurcation using a pull wire,and pushed until it encountered resistance.Water was then injected into the balloon to expand it,maintaining the pressure at 1.5 atmospheres(1 standard atmosphere=101.325 kPa).The balloon was slowly pulled back into the abdominal aorta and drained.This process was repeated three times to complete the injury.After the operation,the rats were placed in separate cages and continued to receive 20 g of high-fat feed for 90 d[16].The control group was given 20 g of standard feed daily for 90 d.
2.4.Hematoxylin-eosin (H &E) staining
Samples were taken 90 d after balloon injury.The rats were anesthetized with isoflurane (3.0%-3.5% during the induction period,2.0%-2.5% during the maintenance period),and the iliac arteries on both sides were separated under a microscope (ZEISS,Germany) and placed in a plate containing 0.9% sodium chloride.The excess adventitial tissue was removed,and the rats were subsequently soaked in formaldehyde for 24 h.After fixation,the tissues were routinely embedded in paraffin and sectioned at 4 μm.Routine H &E staining was performed,after which the sections were photographed and analyzed under an optical microscope (×200,OLYMPUS,Japan).
2.5.Detection of blood lipids in rats
Rats were fasted for 12 h and anesthetized with isoflurane(3.0%-3.5% during the induction period,2.0%-2.5% during the maintenance period) 90 d after the injury.The blood samples were obtained from the abdominal aorta,stood at room temperature for 2 h,and centrifuged for 15 min.Subsequently,the supernatant was drawn and analyzed using an automatic biochemical analyzer.The content of cholesterol and triglyceride were detected in serum.
2.6.Research subjects
A total of 12 patients with arteriosclerotic occlusive disease of the lower extremities were diagnosed by clinical manifestations and auxiliary examinations from March 1,2023 to March 31,2023 in the Department of Vascular Surgery,Hainan General Hospital,Hainan Affiliated Hospital of Hainan Medical University.All the diagnoses were confirmed by color ultrasound and angiography.The clinical data of the subjects is shown in Supplementary Table 1.
2.7.Specimen collection
Ten mL of fasting venous blood was collected from all subjects before and after the operation,after which the patients were divided into 2 groups on average.One part was preserved for the flow cytometry analysis of peripheral blood,and the other part was used for separating the serum and cells.The separated serum was stored at -80 ℃ for further use.
2.8.ELISA detection
The ocular venous blood was collected from two groups of rats,and plasma was separated and stored at -80 ℃ for later use.The levels of IL-6,IL-10 and IL-17 in the peripheral blood supernatants of rats were determined by enzyme-linked immunosorbent assay (ELISA).The supernatants collected after centrifugation of peripheral blood were used to detect the expression levels of IL-6,IL-10 and IL-17 using a pre-coated ELISA kit (Yunclone,China),and the specific operation was performed according to the instruction manual.Finally,the absorbance values at 450 nm were read by an enzyme marker,and the concentration values of IL-6,IL-10 and IL-17 were calculated from the standard curve.
2.9.Real-time PCR
Trizol was used to extract RNA from peripheral blood lymphocytes(Invitrogen,Carlsbad,CA,USA),and a cDNA reverse transcription kit was subsequently used to reverse transcribe the RNA into cDNA (Suzhou Mona Biotechnology Co.,Ltd.,China).SYBR was purchased from Wuhan ABclonal Biotechnology Co.,Ltd.Primers were designed by Nachuan (Harbin,China) and synthesized by Suit Biosynthesis (Harbin,China).SYBR Green was used for real-time fluorescent PCR,and the reaction parameters were as follows: 95 ℃for 30 s,1 cycle;95 ℃ for 5 s,55 ℃ for 10 s;and 40 cycles.Specific primer sequences are listed in Supplementary Table 2.The relative levels of each gene after normalization were calculated using the 2-ΔΔCTmethod.
2.10.Western blot
Peripheral blood immune cells were fully lysed with protein lysis buffer on ice for 30 min.The cells were centrifuged at 1 000 g at 4 ℃ for 15 min,then the supernatant was collected for protein quantification with a BCA protein quantification kit.Forty mg of protein was added to each lane,and after SDS-PAGE,the protein was transferred from the gel to a PVDF membrane in a semidry transfer tank.The PVDF membrane was washed 3 times with TBST at room temperature.The membrane was blocked with TBST containing 5% skim milk powder for 2 h.The Foxp3 (abcam,ab253297),IL-6 (ABclona,A0286),IL-10 (ABclona,A12255),IL-17 (ABclona,A0688) and ACTB (ABclona,AC206) antibodies(1:1 000 dilution) were added and the samples were incubated overnight at 4 ℃.The next day,the sections were incubated with goat anti-rabbit IgG-HRP diluted 1:4 000 at 37 ℃ for 2 h.An enhanced chemiluminescence luminescence kit was used for luminescence.An enhanced chemiluminescence instrument was used for detection,the gray value of the strip was measured,and the target protein/internal reference protein × 100 was used for calculation to quantitatively analyze the protein expression.
2.11.Flow cytometric analysis
Ficoll separation medium was used to separate and isolate peripheral blood mononuclear cells,and the number of cells was adjusted to 2×106/mL.Then,1 000 ng/mL phorbol ester and 1 µg/mL ionomycin were added dropwise,mixed well,placed in a carbon dioxide incubator,and incubated for 60 min.Afterward,1 µL/mL monensin was added for 5 h.The cells were equally divided into two groups,and the corresponding monoclonal antibodies were added dropwise.Th17,Treg,M1,and M2 macrophages were detected via flow cytometry and on a FACSCalibur,after which the Th17/Treg and M1/M2 ratios were calculated.
2.12.Statistical analysis
The experimental data were analyzed statistically with SPSS 22.0 software.The data are expressed as mean ± SD,and the means are compared between the two groups by t-test.P<0.05 indicates that the difference is statistically significant.
2.13.Ethical statement
Experimental manipulation and sampling of rats follows the provisions of the Guidance on the Kindness of Laboratory Animals.All the subjects and all the animal use and care procedures were approved by the Ethics Committee of the Hainan General Hospital and Hainan Affiliated Hospital of Hainan Medical University (Ref.No.Med-Eth-Re [2023] 377/11/15/2023).
3.Results
3.1.Evaluation of ASO rats
In this experiment,there were 20 rats in total,and 3 rats died,all of which occurred in the ASO group.One rat died during surgery due to bleeding from proximal aortic rupture and two died after surgery,possibly due to excessive intraoperative blood loss and inability to recover.Compared with the control group,the arterial wall of the iliac arteries of rats in the ASO group had obvious hyperplasia(Figure 1A).Blood lipid results showed that the cholesterol concentration in the ASO group was (6.20±0.67) mmol/L,while in the control group,it was (4.18±1.20) mmol/L (P<0.01) (Figure 1B).Moreover,the concentration of triglyceride in the control group and the ASO group (Figure 1C) were (1.10±0.49) mmol/L and(3.36±1.68) mmol/L,respectively (P<0.01).
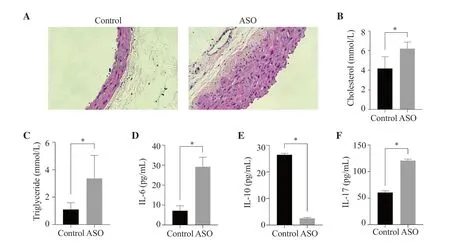
Figure 1.Establishment of an ASO rat model and serum IL-6,IL-10,IL-17,cholesterol and triglyceride concentrations.(A) H&E staining of rat iliac artery under light microscopy at ×200 magnification.Serum (B) cholesterol,(C) triglyceride,(D) IL-6,(E) IL-10,and (F) IL-17 concentrations in the ASO group and the control group.The results are expressed as mean ± SD.*P <0.01 compared with the control group.ASO: arteriosclerosis obliterans.
3.2.Changes of inflammatory factors in ASO rats
In order to detect the effect of arteriosclerosis occlusion on serum inflammatory factors,the levels of IL-6,IL-10,and IL-17 in rat serum were determined by ELISA.The ASO group showed significantly higher IL-6 and IL-17 levels and lowered IL-10 level compared with the control group (P<0.01) (Figure 1D-F).
3.3.Changes of Treg/Th17 in ASO rats
Compared with the control group,the percentage of Th17 cells in the ASO group was significantly increased (P<0.01),along with decreased Treg cell percentage (P<0.01).In addition,the ratio of Th17/Treg in the ASO group was significantly increased compared with the control (P<0.05) (Figure 2C).
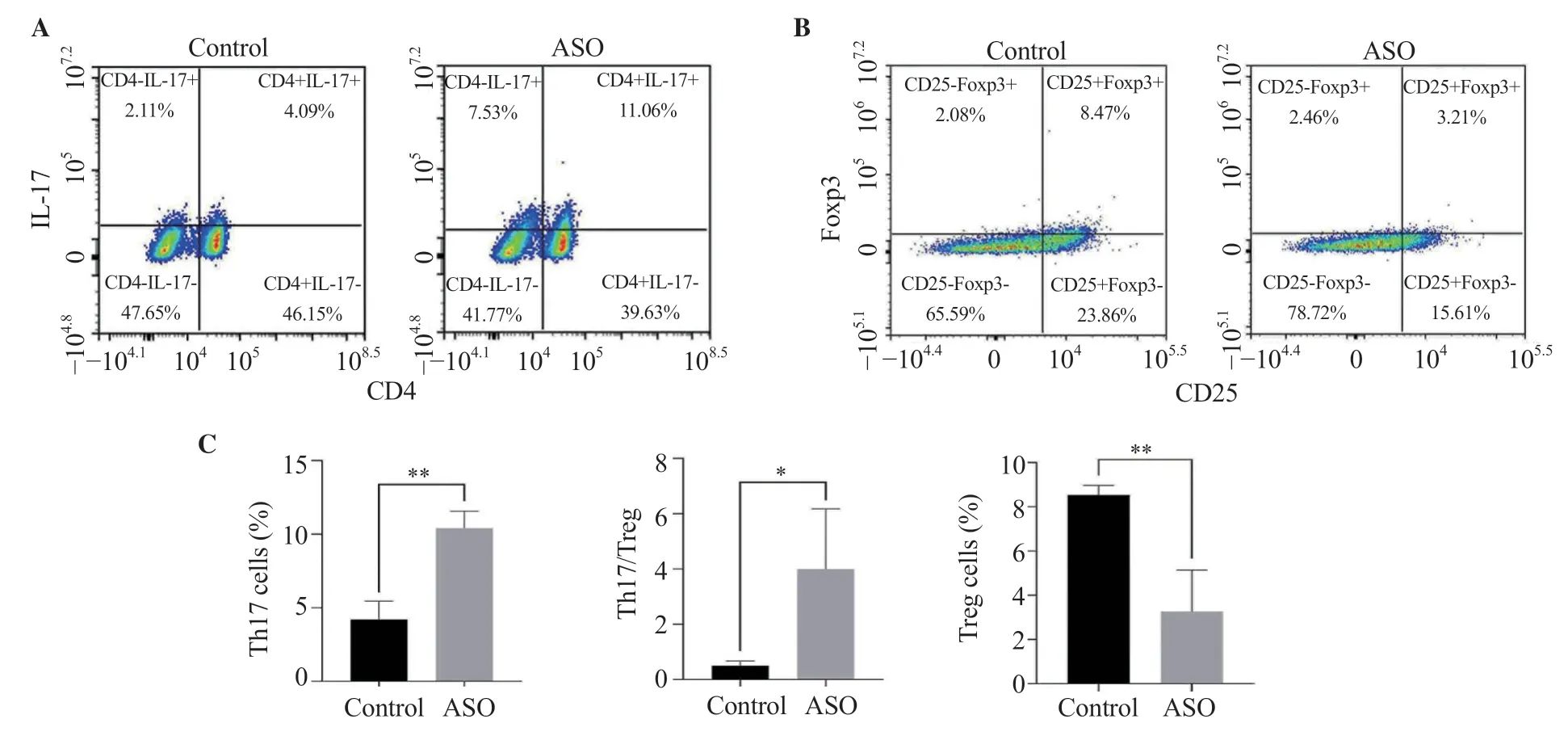
Figure 2.Comparison of Th17 and Treg as well as Th17/Treg ratio between the ASO group and the control group.The results are expressed as mean ± SD.*P<0.05,**P <0.01 compared with the control group.
3.4.Changes of M1/M2 macrophages in ASO rats
Compared with the control group,the percentage of M1 cells in the ASO group was significantly increased (P<0.05) while the percentage of M2 cells was significantly decreased (P<0.01) (Figure 3A-B).In addition,the ratio of M1/M2 in the ASO group was significantly elevated (P<0.01) (Figure 3C).
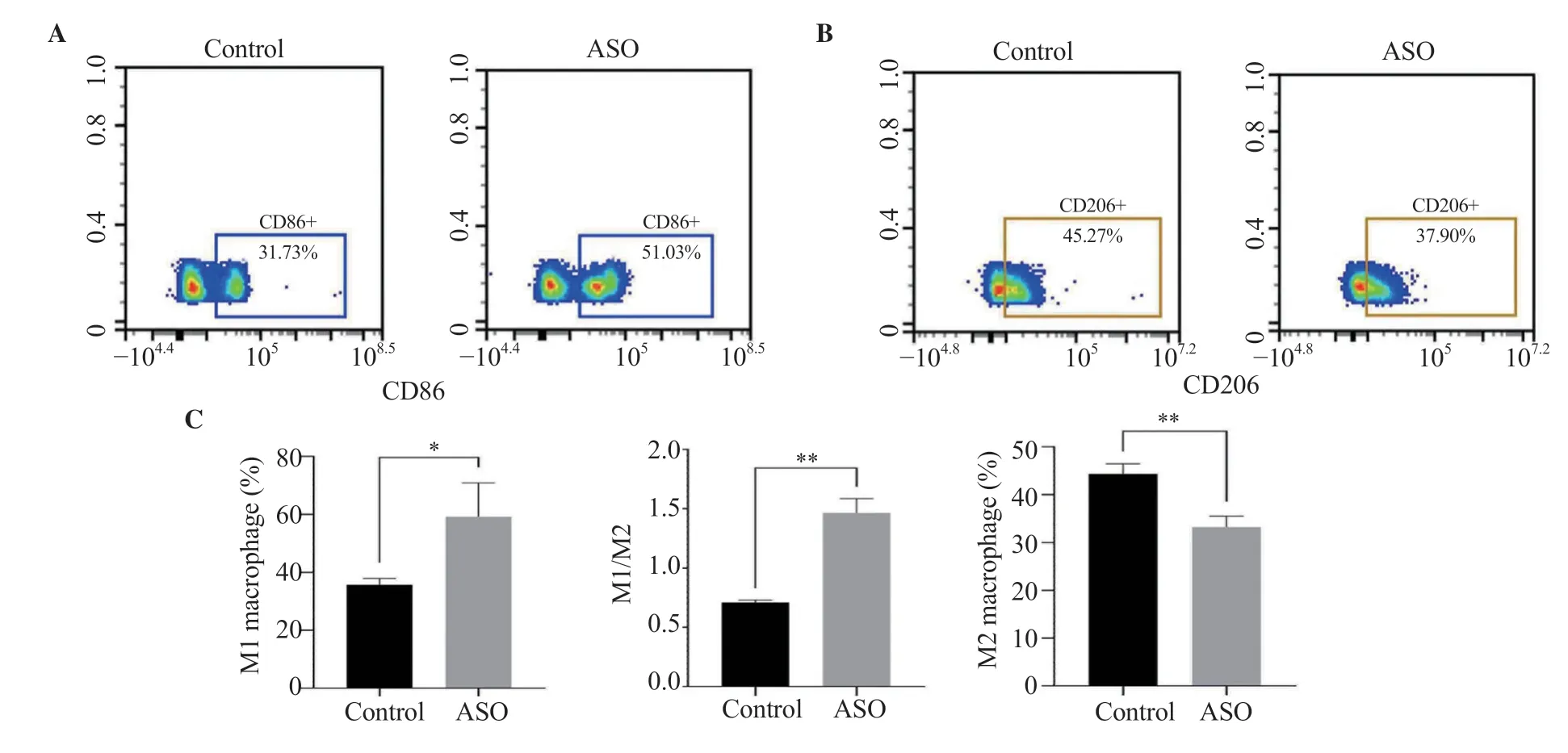
Figure 3.Comparison of M1 and M2 between the ASO group and the control group.The results are expressed as mean ± SD.*P <0.05,**P <0.01 compared with the control group.
3.5.Inflammatory gene expression in peripheral blood lymphocytes of ASO rats
The mRNA levels of IL-6 and IL-17 in the immune cells of rats in the ASO group were significantly higher than those in the control group (P<0.05),while the mRNA levels of Foxp3 and IL-10 were significantly lower than those in the control group (Figure 4).The results of protein detection were consistent with the results of RNA(Figure 4E).Consistent with the PCR results,the protein expressions of Foxp3 and IL-10 in ASO rats were significantly lower than those of the control group (P<0.01),while the protein expressions of IL-6 and IL-17 were significantly higher than those of the control group (P<0.01).
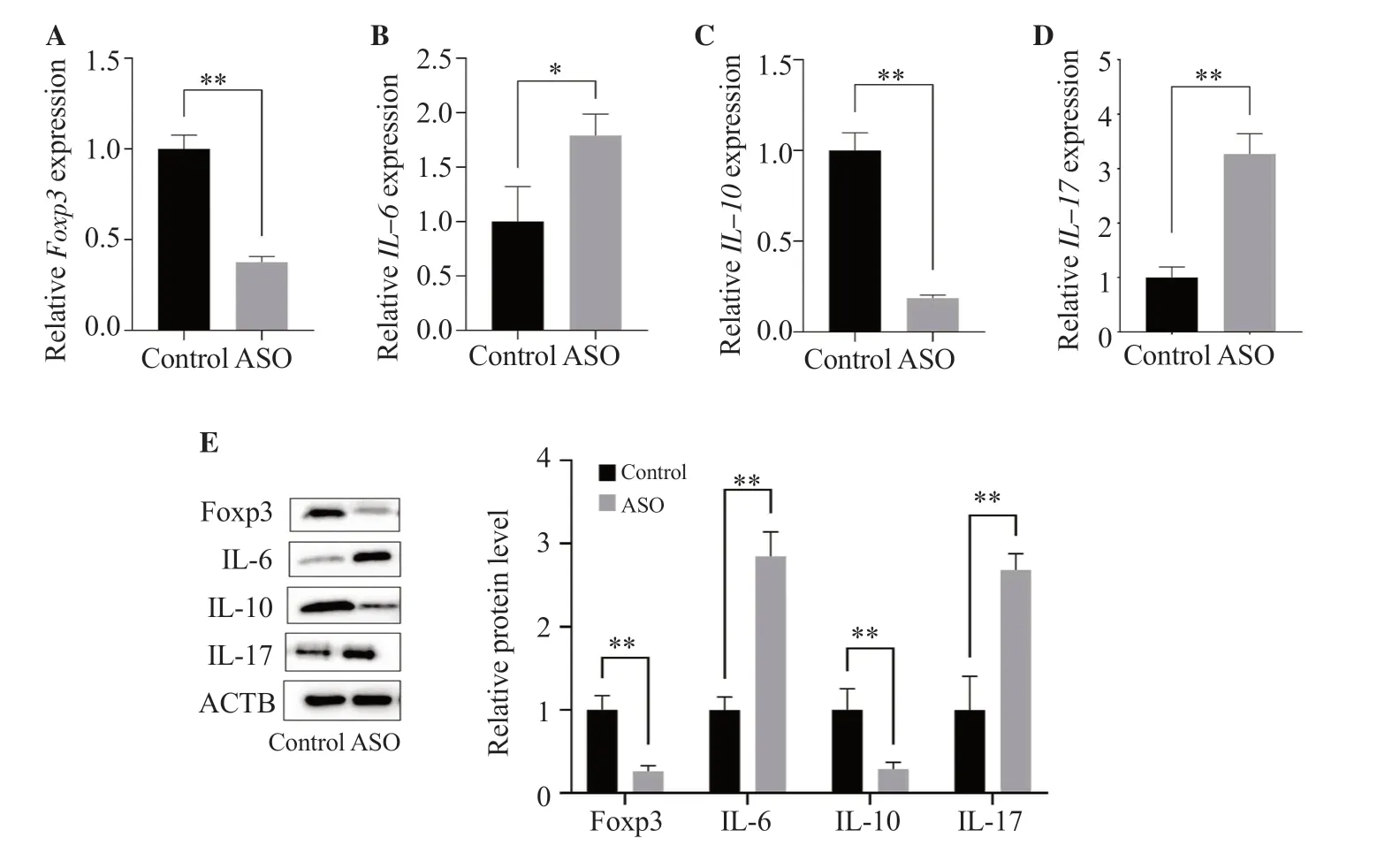
Figure 4.Foxp3,IL-6,IL-10,and IL-17 mRNA and protein expressions in rat lymphocytes.Foxp3,IL-6,IL-10,and IL-17 (A-D) mRNA and (E) protein expressions in rat lymphocytes were measured by real-time PCR and Western blot analyses,respectively.The results are expressed as mean ± SD.*P <0.05,**P <0.01 compared with the control group.
3.6.Serum inflammatory factors in blood samples after operation
In order to detect the changes of inflammatory factors in the blood of patients with ASO before and after endovascular surgery (such as percutaneous transluminal angioplasty and arterial stenting),the concentrations of IL-6,IL-10,and IL-17 in serum were measured by ELISA.The concentrations of IL-6 and IL-17 after operation were (25.640±1.186) pg/mL and (187.600±4.200) pg/mL,which were significantly lower (P<0.01) than those before operation[(60.310±1.328) pg/mL and (221.300±0.781) pg/mL] (Figure 5A and C).The concentration of IL-10 after operation was significantly higher than that before operation (P<0.01) (Figure 5B).
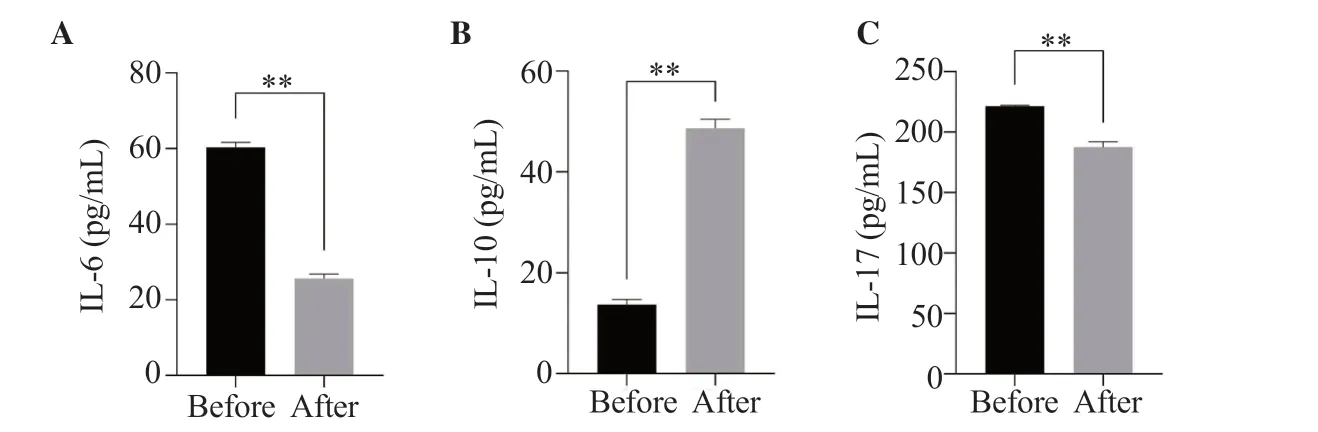
Figure 5.Changes of serum IL-6 (A),IL-10 (B),and IL-17 (C) concentrations before and after surgery in ASO patients.The results are expressed as mean ±SD.**P <0.01 compared with before surgery.
3.7.Improvement of Treg/Th17 cell balance in peripheral blood of patients with ASO after surgery
The percentage of Th17 cells in the peripheral blood of patients after surgery (Figure 6A) was significantly lower than that before surgery (P<0.05).The proportion of Treg cell subsets (Figure 6B)was significantly increased (P<0.05).In addition,the balance of Th17/Treg in the peripheral blood of patients was significantly improved after operation (P<0.05) (Figure 6C).
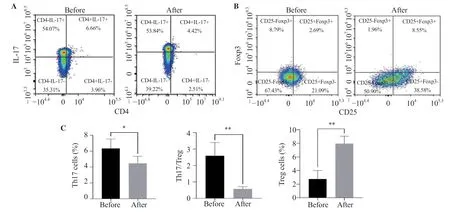
Figure 6.Changes of Th17 and Treg cells as well as Th17/Treg ratio in ASO patients before and after surgery.The results are expressed as mean ± SD.*P <0.05,**P <0.01 compared with before surgery.
3.8.M1/M2 ratio in peripheral blood of ASO patients after surgery
In order to clarify the effect of surgery on the ratio of peripheral blood M1/M2 macrophages in patients with ASO,flow cytometry was used to detect the M1/M2 ratio before and after surgery.The proportion of M1 macrophages was decreased after surgery (P<0.01)(Figure 7A).Figure 7B shows the changes of M2 macrophages in the blood of patients before and after surgery.M2 cell percentage was increased after surgery (P<0.01).The M1/M2 ratio was also pronouncedly improved (P<0.05,Figure 7C).
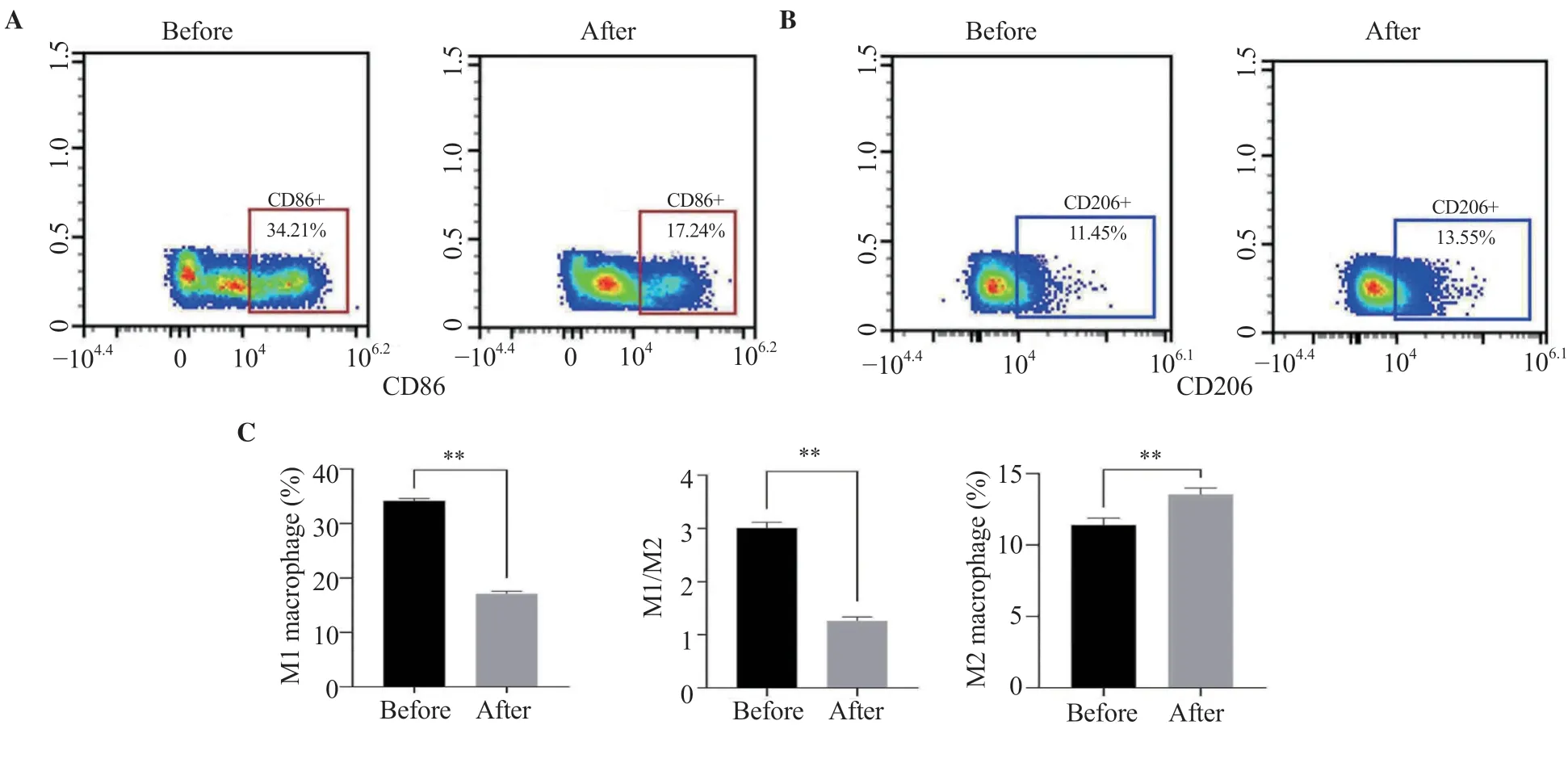
Figure 7.Change of M1 and M2 cell percentage as well as M1/M2 ratio in ASO patients before and after surgery.The results are expressed as mean ± SD.**P<0.01 compared with before surgery.
3.9.Gene expression of blood monocytes in ASO patients after surgery
The Foxp3 and IL-10 mRNA levels of lymphocytes after surgery were significantly higher than those before surgery,while the IL-6 and IL-17 mRNA levels were significantly lower than those before surgery (P<0.05) (Figure 8).Similarly,the results of Western blot(Figure 8E) showed that the expression levels of Foxp3 and IL-10 proteins in lymphocytes were significantly upregulated after surgery (P<0.05),while those of IL-6 and IL-17 were downregulated(P<0.05).
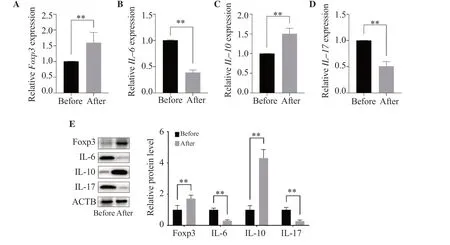
Figure 8.Foxp3,IL-6,IL-10,and IL-17 mRNA and protein expressions in the lymphocytes of ASO patients.Foxp3,IL-6,IL-10,and IL-17 (A-D) mRNA and(E) protein expressions were measured by real-time PCR and Western blot analyses,respectively.The results are expressed as mean ± SD.**P <0.01 compared with before surgery.
4.Discussion
Atherosclerosis is the main cause of most cardiovascular and cerebrovascular diseases.The pathogenesis of atherosclerosis includes lipid infiltration,injury response,monocyte invasion,and inflammatory response[17].Atherosclerosis is currently considered a chronic inflammatory disease of the vessel wall and involves a humoral immune response.The balance between pro-inflammatory and anti-inflammatory cytokines has emerged as a major determinant of atherosclerosis.This study explores the relationship between inflammatory factors and immune cells and lower extremity arteriosclerosis obliterans using animal models and clinical samples.In this study,a rat model of lower limb ASO was established,and blood samples from patients with lower limb ASO before and after surgery were obtained.We analyzed three important inflammatory factors: IL-6,IL-10,and IL-17 in peripheral blood of animal models and clinical blood samples.IL-6 is a pro-inflammatory factor that can induce the expression of low-density lipoprotein receptors on the surface of macrophages and promote the uptake of lowdensity lipoprotein by macrophages,accelerate lipid deposition,and promote foam cell formation[18].IL-6 can cause high expression of cell adhesion molecule CD44 in macrophages,positive feedback promotes the secretion of IL-6 from macrophages and then aggravates the progression of atherosclerosis[19].Studies have shown that IL-6 can also induce inflammatory responses in vascular endothelial cells[20].IL-10,an inhibitory cytokine produced by activated lymphocytes and monocytes,is thought to be protective against the development and progression of atherosclerosis.IL-10 inhibits antigen-presenting ability,dendritic cell activity,and T-cell proliferation,and negatively regulates the production of proinflammatory cytokines[21,22].It is an anti-inflammatory cytokine whose deficiency increases the incidence of atherosclerosis[23].Data have shown that higher IL-10 levels in acute coronary syndrome can reduce the risk of future cardiovascular events[24,25].IL-17 is involved in the occurrence and development of atherosclerosis and is one of the important injury factors.In immune,endothelial,and stromal cells,IL-17 induces the secretion of the pro-inflammatory cytokines IL-6,granulocyte-colony-stimulating factor,and macrophage-colony-stimulating factor,as well as chemokines,all of which can be proatherogenic like sclerosis factor[26].But some studies have shown that IL-17 can help stabilize atherosclerotic plaques[27],and has a certain protective effect.Our study found that compared with the control group,IL-6,and IL-17 in the peripheral blood of rats in the ASO model group were significantly increased,while IL-10 was significantly decreased,which indicates that the inflammatory response plays a role in ASO.Inhibition of inflammation may play a key role in the treatment of ASO.In clinical samples,we found that the levels of IL-6 and IL-17 in the blood of patients with lower extremity ASO were significantly decreased and the level of IL-10 was increased significantly after surgery,indicating that surgery improved the inflammatory environment in peripheral blood of ASO patients.
In each stage of atherosclerosis,T lymphocytes are involved.During the entire process of atherosclerosis,T lymphocytes can secrete factors that induce or inhibit immune responses.Most T cells in atherosclerosis are CD4+T cells that can interact with major histocompatibility complex molecules.T lymphocytes are divided into multiple subtypes,the common ones are Th17,Treg,etc.Th17 secretes IL-6,IL-7,IL-17,etc.,which can promote immune response,and the increase of IL-17 level makes it easier for atherosclerotic plaque to fall off[28,29].Treg releases factors such as IL-10 and TGF-β to play an anti-inflammatory and immune-suppressing role[30].In patients with various cardiovascular diseases associated with atherosclerosis,the number and function of Treg cells are reduced[31].The normal ratio of Treg to Th17 plays an important role in maintaining immune balance,suppressing immune disorders,and suppressing autoimmune responses.Studies have suggested that the balance of Th17/Treg can control inflammation and play an important role in plaque stability.Therefore,the balance between Th17 and Treg may be of great significance for the occurrence and prevention of atherosclerosis.The plasticity between regulatory T cells and helper T cells is influenced by various signals,factors,epigenetic modifications,metabolic pathways,and microbiota[32].This study found that in the ASO rat model,compared with the healthy control group,the proportion of Th17 cells was significantly increased,while the proportion of Treg cells was significantly decreased.Moreover,after surgery,the proportion of Th17 cells in the blood of ASO patients was significantly decreased while the proportion of Treg cells was significantly increased.The Th17/Treg balance was significantly improved.
The pathway by which adaptive lymphocytes affect atherosclerosis is still unclear,how T cells with mixed phenotypes are triggered by specific proteins,and the specific mechanism for preferentially exerting a protective effect on atherosclerosis is also poorly understood.Macrophage polarization is considered one of the possible reasons[14].Classically activated macrophages (M1) are able to secrete a large number of pro-inflammatory molecules,so they are one of the phenotypes of pro-inflammatory macrophages[33,34].High expression of M1-associated pro-inflammatory proteins can promote plaque growth and plaque instability in humans and mice[35].Alternately activated macrophages (M2) play a powerful protective role in the progression of atherosclerotic plaques in humans and mice by secreting a large number of anti-inflammatory cytokines[35,36].M2 possesses powerful functions in atherosclerosis,such as reducing plaque size and enhancing plaque stability[35,36].Macrophage polarization can transform macrophages into macrophages expressing different phenotypes,and these macrophages expressing different phenotypes have different effects on the pathophysiology and development of atherosclerosis.A thorough analysis of plaques in atherosclerotic lesions of ApoE-/-mice at 10 and 20 weeks found that M1 and M2 phenotypes were found in both early and late atherosclerotic plaque lesions[12].As atherosclerosis progresses,the proportion of M2 decreases in advanced stages[12].The results of our study are consistent with those of previous studies.We found that in the ASO rat model,the proportion of M1 was significantly increased,while that of M2 was significantly decreased,along with increased M1/M2 ratio,indicating that macrophage polarization also plays an important role in promoting ASO.In contrast,in clinical samples,the ratio of M1/M2 decreased significantly after surgery,which reduced the intensity of the immune response.
Furthermore,we detected the mRNA and protein expression of Fxop3,IL-6,IL-10,and IL-17 in blood lymphocytes.The Foxp3 gene is one of the family members of the transcription factor forkhead/winged-helix and is a key factor in the development and maintenance of Treg functions.Foxp3 gene can not only inhibit the production of inflammatory mediators such as IL-17,IL-2,and TNF-α through a series of regulatory pathways,but also increase the expression of other genes such as CD5,CTLA-4,and IL-10,and regulate the expression of precursor T cell differentiation,thereby maintaining Treg autoimmune tolerance and inhibiting chronic inflammation.Consistent with our previous results,we found that IL-6 and IL-17 RNA and protein levels were significantly increased in the lymphocytes of the ASO rat model,while the anti-inflammatory genes Foxp3 and IL-10 were significantly decreased.In clinical samples,it was found that Foxp3 and IL-10 in postoperative lymphocytes were significantly increased,while IL-6 and IL-17 were significantly decreased.The results further illustrate that lymphocytes regulate the immune response in ASO.
However,this study has several limitations.Firstly,the sample size is small,which may have statistical bias.Secondly,the research method used does not employ a double-blind or triple-blind multicenter approach,which could potentially be influenced by subjective factors.Thirdly,the study is limited in terms of inflammatory indicators measured,and future research should consider including additional indicators.Finally,there is a lack of research on surgical methods,post-treatment follow-up and long-term efficacy evaluation.
In summary,the Th17/Treg balance,M1/M2 ratio,and the corresponding inflammatory factors play an important role in the occurrence and development of ASO,which can reflect the improvement effect of surgery on the inflammatory response of ASO to a certain extent.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This work is supported by Natural Science Foundation of Hainan Province (820MS135),Hainan Provincial Health Commission 2023 Provincial Key Clinical Discipline (Clinical Medical Center)Construction Unit Fund Project (Qiongwei Yihan [2022] No.341),and Hainan Provincial Health Technology Innovation Joint Project(WSJK2024MS209).
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’ contributions
CW and YQ participated in study design and data collection.ZD.Liu,ZX,and HQ carried out data analysis.XH drafted the final manuscript.ZZ.Li and ML revised the manuscript.All authors read and approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2024年3期
Asian Pacific Journal of Tropical Biomedicine2024年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Pyronaridine combined with diminazene aceturate inhibits Babesia in vitro and in vivo
- Rosmarinic acid improves tracheal smooth muscle responsiveness and lung pathological changes in ovalbumin-sensitized rats
- Icariin ameliorates viral myocarditis by inhibiting TLR4-mediated ferroptosis
- Capsosiphon fulvescens suppresses LPS-stimulated inflammatory responses by suppressing TLR4/NF-κB activation in RAW264.7 murine macrophages
