Pyronaridine combined with diminazene aceturate inhibits Babesia in vitro and in vivo
Shimaa Abd El-Salam El-Sayed ,Mohamed Z.Sayed-Ahmed ,Shaimaa Ahmed Awad Ali ,Nourah Alsadaan ,Nawazish Alam ,Mahmoud S.Alkhoudary ,Ikuo Igarashi,Mohamed Abdo Rizk
1National Research Center for Protozoan Diseases,Obihiro University of Agriculture and Veterinary Medicine,Inada-Cho,Obihiro,Hokkaido 080-8555,Japan
2Department of Biochemistry and Chemistry of Nutrition,Faculty of Veterinary Medicine,Mansoura University,Mansoura 35516,Egypt
3Department of Clinical Pharmacy,College of Pharmacy,Jazan University,Jazan 45142,Saudi Arabia
4Medical-Surgical Department,College of Nursing,Jouf University,Sakaka 72388,Saudi Arabia
5Critical Care and Emergency Nursing,Faculty of Nursing,Mansoura University,Egypt
6College of Nursing,Jouf University,Sakaka 72388,Saudi Arabia
7Faculty of Pharmacy,Mansoura University,Mansoura 35516,Dakahlia,Egypt
8Department of Internal Medicine and Infectious Diseases,Faculty of Veterinary Medicine,Mansoura University,Mansoura 35516,Egypt
ABSTRACT Objective: To evaluate the combination therapy of pyronaridine tetraphosphate and diminazene aceturate against Babesia in vitro and in vivo.Methods: Bioinformatic analysis was performed using atom pair fingerprints.An in vitro combination test was performed against Babesia bovis and Theileria equi.Moreover,the in vivo chemotherapeutic efficacy of pyronaridine tetraphosphate in combination with diminazene aceturate was investigated against the growth of Babesia microti in mice using a fluorescence inhibitory assay.Results: Pyronaridine tetraphosphate and diminazene aceturate exhibited nearly similar molecular weights.The in vitro combination of pyronaridine tetraphosphate and diminazene aceturate was synergistic on Babesia bovis and additive on Theileria equi.In addition,5 mg/kg pyronaridine tetraphosphate combined with 10 mg/kg diminazene aceturate inhibited Babesia microti growth significantly compared with those observed after treatment with 25 mg/kg diminazene aceturate alone from day 6 post treatment to day 12 post treatment.The combination therapy also normalized the hematological parameters of infected mice.Conclusions: An oral dose of pyronaridine tetraphosphate combined with a subcutaneous dose of diminazene aceturate inhibits Babesia in vitro and in mice,suggesting it might be a new paradigm for the treatment of babesiosis.
KEYWORDS: Babesia;Pyronaridine tetraphosphate;Diminazene aceturate;Babesiosis;Theileria✉To whom correspondence may be addressed.E-mail: mzakaria@jazanu.edu.sa (MZ.Sayed-Ahmed);dr_moh_abdo2008@mans.edu.eg (M.Abdo Rizk)
Significance
The conventional medications used to treat babesiosis have reached their limitations in terms of parasite resistance and host toxicity.The findings of the present study demonstrated that Babesia in vitro and in mice were significantly inhibited by a combination of an oral dosage of pyronaridine tetraphosphate and a subcutaneous dose of diminazene aceturate.It might provide a new paradigm for the treatment of babesiosis.
1.Introduction
Babesiosis is an infectious disease that infects different animal species leading to significant financial losses in the global livestock market and pet trade[1].Theileria equi (T.equi) and Babesia caballi in equines,and Babesia bovis (B.bovis) and Babesia bigemina in cattle are the primary causes of the disease[2-4].Additionally,Babesia microti (B.microti) and Babesia divergens are blood parasites that infect rodents and cattle,respectively,and have zoonotic importance[4,5].In the United States,the primary etiologic agent known to cause babesiosis in humans is B.microti[5].
Typically,a Babesia spp.infection is accompanied by a high fever and erythrocyte rupture,which can result in hemolytic anemia,hemoglobinuria,and pronounced splenomegaly[4,5].The two drugs that are currently on the market for treating Babesia infections in animals,imidocarb dipropionate (ID) and diminazene aceturate(DA),have demonstrated their limits regarding host toxicity and parasite resistance[5].Atovaquone (AV),clindamycin,azithromycin,quinine,and tetracycline have all been used to treat severe cases of human babesiosis;however,some of these treatments have been reported to be ineffective[5].Finding and creating safer and more effective antibabesial drugs has therefore become imperative.In this regard,combination therapies consisting of low doses of the recently developed and commonly used antibabesial drugs are recommended.In this regard,the 1970-created drug pyronaridine tetraphosphate (PYR) is currently being used in combination therapy with artemisinin to treat malaria[6].Additionally,a fixed-dose combination of artesunate and PYR is being studied for the treatment of blood-stage Plasmodium vivax and simple Plasmodium falciparum malaria[6].Considering the close biological similarities between Babesia and Plasmodium,in the present study,bioinformatic analysis was performed for PYR and other antibabesial drugs including DA,ID,and AV to search for the best use of PYR in combination therapy to treat babesiosis.Bioinformatics is an interdisciplinary scientific subject that creates software tools and methods for interpreting biological data,particularly complicated and large-scale data sets[1-6].Accordingly,we evaluated the chemotherapeutic efficacy of two drug interactions on the in vitro growth of B.bovis (as a bovine Babesia model),and T.equi (as an equine piroplasm model).Then,the combination therapy that exhibited the best interaction is used for the treatment of B.microti in mice.
2.Materials and methods
2.1.Ethical statement
The Animal Care and Use Committee at Obihiro University of Agriculture and Veterinary Medicine approved all experimental methods used in this study (Approval No.27-65).The IDs for the pathogen experiment were: bovine Babesia: 201708-4;equine piroplasm parasites: 201910-2;and B.microti: 20170905.
2.2.Chemical reagents
Using a fluorescence SGI assay,SYBR Green I (SGI) nucleic acid stain (Lonza,USA;10 000 ×) was frozen and then thawed for use to assess the inhibitory effects of selected hits.This study used a lysis buffer that included Tris (130 mM;pH 7.5),ethylene diamine tetraacetic acid (EDTA) (10 mM),saponin (0.016%;w/v),and TritonX-100 (1.6%;v/v) to lyse red blood cells (RBCs) from cattle,horses,and mice.As a 100 mM stock solution,DA (Novartis,Japan),ID,AV,and PYR (both from Sigma-Aldrich,Japan) were made and kept at -30 ℃ until needed.
2.3.Parasites and in vitro cultures
A Texas strain of B.bovis[7],and a United States Department of Agriculture (USDA) strain of T.equi[8] were grown in a microaerophilic stationary-phase culture utilizing purified bovine or horse RBCs[9,10].The two parasites were cultured using medium 199 (Sigma-Aldrich).Supplements to the media included 40%normal horse serum (for T.equi) or 40% normal bovine serum(for B.bovis),in addition to 60 units per mL of penicillin G,60 mg/mL of streptomycin,and 0.15 mg/mL of amphotericin B (all from Sigma-Aldrich).Importantly,13.6 g of hypoxanthine (ICN Biomedicals,Inc.,USA) per mL was added to the T.equi culture.In an environment with 5% CO2,5% O2,and 90% N2,cultures of parasitized RBCs (pRBCs) were incubated at 37 ℃.
2.4.Bioinformatic analysis
To determine the structural similarities between the PYR and drugs often used to treat babesiosis (DA,ID,and AV),we used atom pair fingerprinting (APfp)[11].To calculate APfp,each compound’s chemical ID was obtained from PubChem.The ChemMine tools software was then used to determine the APfp of each compound based on the chemical IDs[11].Hierarchical clustering analysis was performed on the APfp using the ChemmineR program[11].
2.5.In vitro drug combination
Using two-drug combination therapies (PYR+DA,PYR+ID,and PYR+AV) against the in vitro growth of B.bovis (as a model of bovine Babesia and one of the primary causative agents of bovine babesiosis) and T.equi (as a model of equine Theileria),a pharmaceutical combination experiment was conducted[10].There were four different combination ratios used,ranging from 0.75:0.75 to 0.50:0.50.IC50values in the in vitro inhibitory assay were used to determine the preparation of all combination ratios[3,7,8] as previously described[8,12,13].In a 96-well plate (Nunc,Roskilde,Denmark),the combination ratios were added in triplicate to the well that contained the infected RBC.Following the plate’s incubation in a humidified incubator,the released fluorescence signals were measured using a fluorescence assay[4,7].For every parasite that was screened,initiation by 1% of parasitemia was used in the experiment.For bovine Babesia parasites,2.5% hematocrit (HCT) was utilized,and 5% HCT was used for other Babesia and Theileria parasites.Each well was treated with a lysis buffer containing a nucleic acid stain SGI to measure the fluorescence signals that were released.The experiment was conducted in triplicates.The obtained fluorescence values were used for quantifying the inhibition caused by the screened combination therapy in terms of the fractional inhibitory concentration (FIC) index,which is defined as the sum of the FIC values of two drugs in combination[3,7].An example of the method used to calculate the SFIC is as follows: For two anti-babesial substances (A and B) both alone and in combination: FIC(A)=IC A in presence of B devided on IC A,FIC(B)=IC B in presence of A devided on IC B,and∑FIC=FIC(A)+FIC(B).
2.6.In vivo chemotherapeutic effect of PYR
The PYR in vivo inhibition assay for B.microti (Munich strain)[10]was carried out using a fluorescence assay in 25 female BALB/c mice that were 8 weeks old (CLEA Japan,Tokyo,Japan)[14].Five groups of mice (each with five animals) were used.The first group remained uninfected and untreated,serving as a negative control.All other animals were given 1 × 107B.microti-RBCs intraperitoneally[8,10,14].The selected medications were administered to each specified group when parasitemia reached roughly 1%.As a positive control,mice in the second group were given the solvent,double distilled water.Mice in the third group were given five subcutaneous doses of DA(Novartis,Japan).Non-toxic five successive oral doses of PYR(Sigma-Aldrich,Japan) were administrated to the mice in the fourth group.Mice in the fifth group were treated with five successive oral and subcutaneous doses of PYR and DA,respectively.PYR and DA were administrated at the same time.Using a pipette,venous tail blood was extracted from each mouse’s tail every two days.Subsequently,the drawn blood was carefully combined with 50 µL of lysis buffer in triplicate in RPMI 1640 Medium within a 96-well plate.Subsequently,each dilution was directly treated with 50 µL of lysis buffer that had been gently mixed with a 2× SGI (10 000×)nucleic acid stain.This allowed us to determine the fluorescence signals that were released,as previously described in our published paper[14].The experiment was repeated three times.
2.7.Determination of hematological parameters
Every 96 hours[14],a Celltac MEK-6450 computerized hematology analyzer (Nihon Kohden Corporation,Tokyo,Japan) was used to measure the hematological parameters [HCT,hemoglobin (HGB),and RBC counts] in the blood of every animal.
2.8.Statistical analysis
To identify significant differences between the groups,a one-way ANOVA test was conducted using GraphPad Prism (version 5.0 for Windows;GraphPad Software,Inc.,San Diego,CA,USA).P-values below 0.05 were regarded as statistically significant.
3.Results
3.1.Atom pair fingerprints analysis
In the current study,the maximum structural similarity,distance matrix,and molecular weight between PYR,and the commonly used antibabesial drugs were calculated.The hierarchical clustering analysis revealed that PYR (CID: 156867) showed the maximum structural similarity with AV (CID: 74989).Interestingly,PYR and AV exhibited nearly similar molecular weights with an AP Tanimoto value of 0.20 (Figures 1 and 2),followed by nearly similar molecular weights between PYR and DA (Figure 1).PubChem fingerprint for similarity workbench revealed that benzylamine was the difference between PYR and DA (Figure 2).Of note,AP Tanimoto value between PYR and ID was the highest (Figure 2).
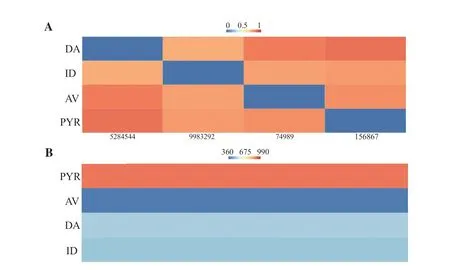
Figure 1.A heatmap displaying the relationships between (A) the distance matrix and (B) molecular weight for pyronaridine tetraphosphate (PYR) and the three antibabesial medications that are often used [atovaquone (AV),imidocarb dipropionate (ID),and diminazene aceturate (DA)].
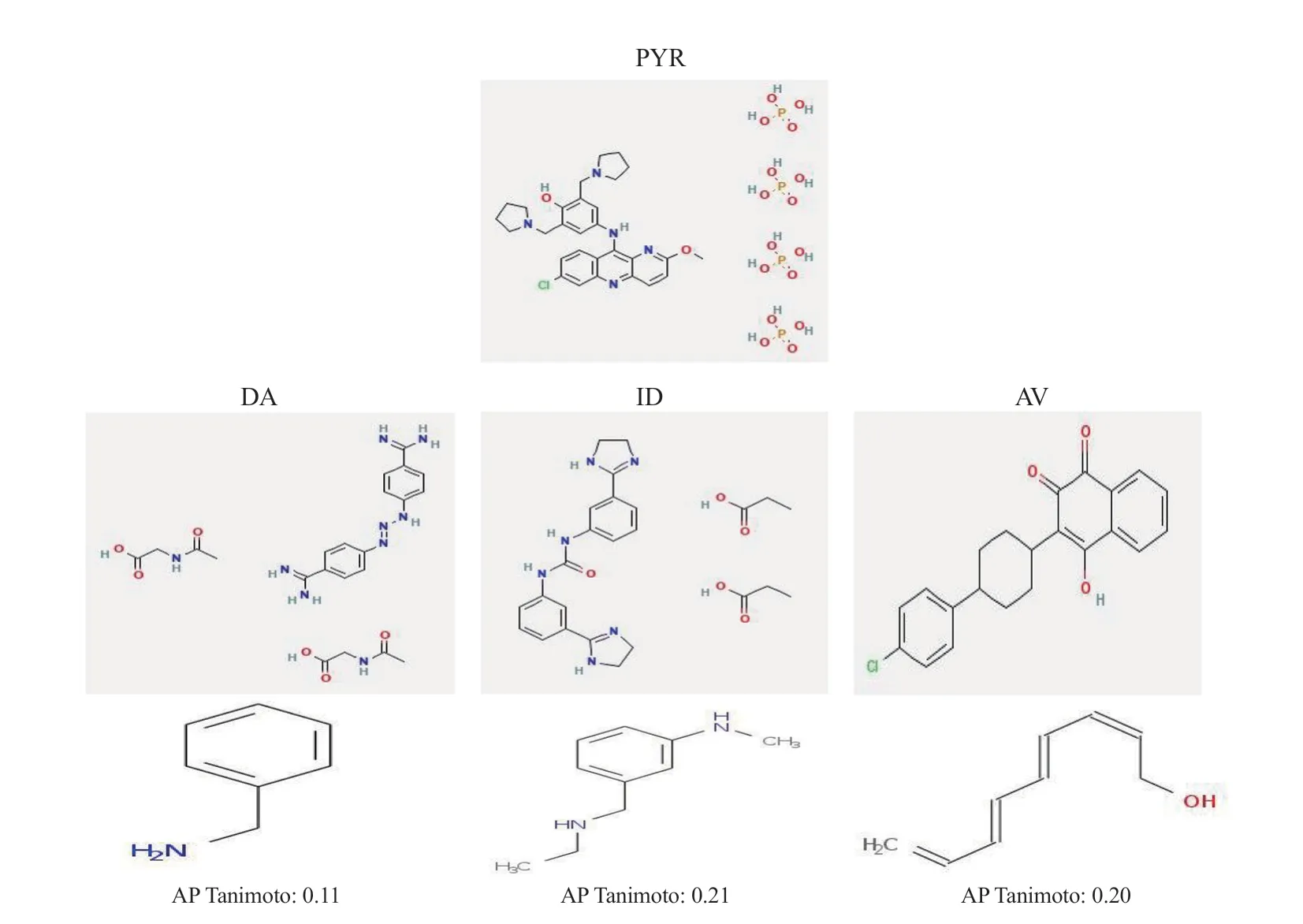
Figure 2.The PubChem fingerprint for similarity workbench compares PYR to the widely used antibabesial medications.
3.2.In vitro combination assay
The drug combination analysis aimed to ascertain whether the medications work antagonistically (for a lesser effect),additively (for a similar effect),indifferently (equal satisfaction to an individual),or synergistically (for a greater benefit).According to our findings,the combination of PYR and DA was synergistic against B.bovis and additive against T.equi (Table 1).The combination of PYR and AV was additive on B.bovis when treated with high concentration ratios of PYR,and indifference on T.equi (Table 1).The combination of PYR and ID was indifferent and antagonistic against the screened bovine Babesia and horse Theileria parasites,respectively (Table 1).
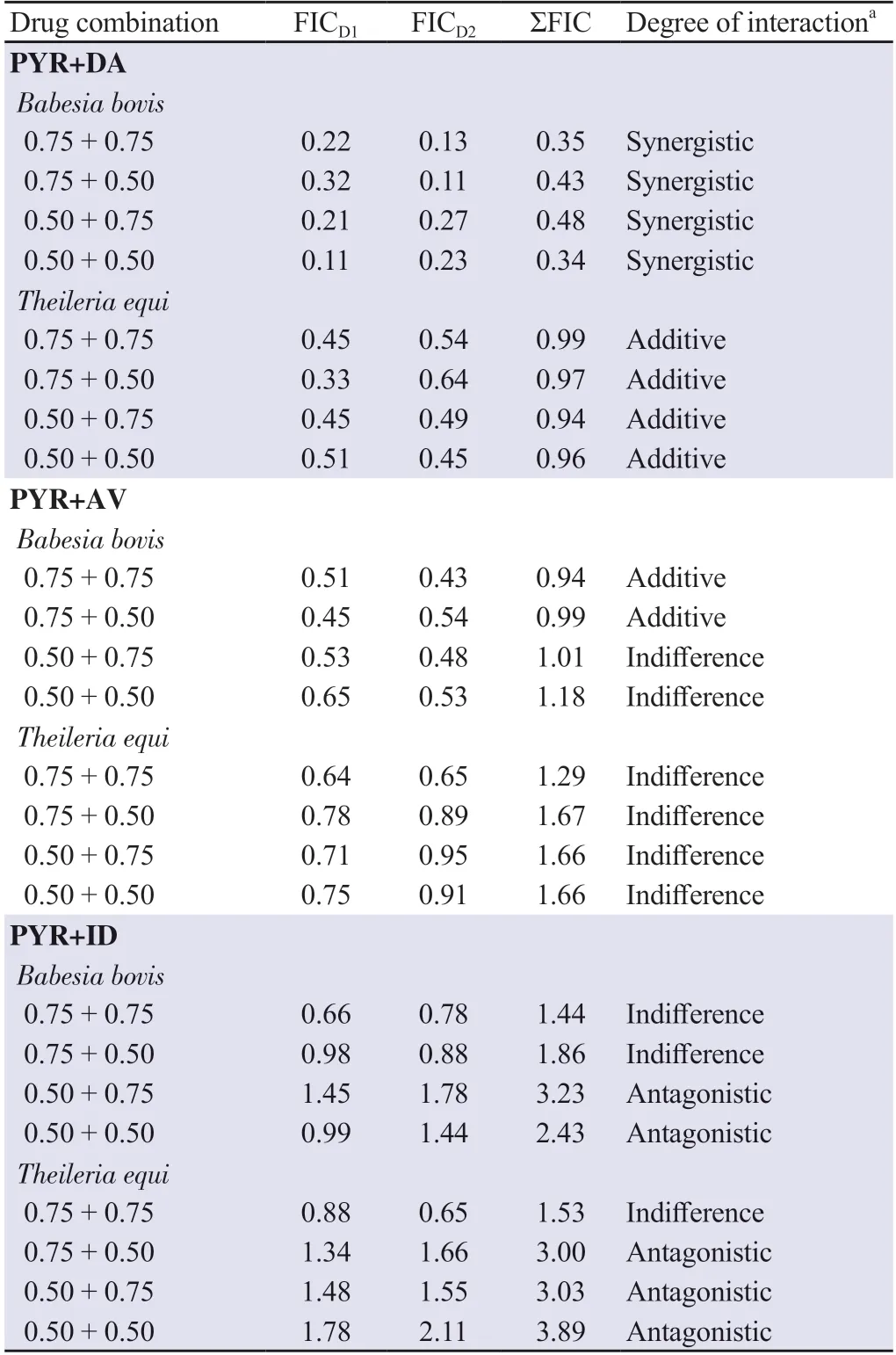
Table 1.Two medication interactions between diminazene aceturate (DA),imidocarb dipropionate (ID),and atovaquone (AV) and pyronaridine tetraphosphate (PYR) against the in vitro growth of Babesia bovis and Theileria equi.
3.3.Combined effect of PYR and DA on B.microti in mice
When compared to control mice,B.microti-infected mice treated with an oral dose of PYR showed a significant (P<0.05) suppression of the fluorescent signals they emitted from days 6 to 14 post treatment(Figure 3A).PYR monotherapy caused 31.11% inhibition in parasite growth at day with peak parasitemia (Day 8) (Figure 3).Interestingly,from day 6 post treatment until day 12 post treatment,the combination of PYR with DA showed a greater reduction of B.microti growth than those shown following treatment with 25 mg/kg DA (Figure 3).
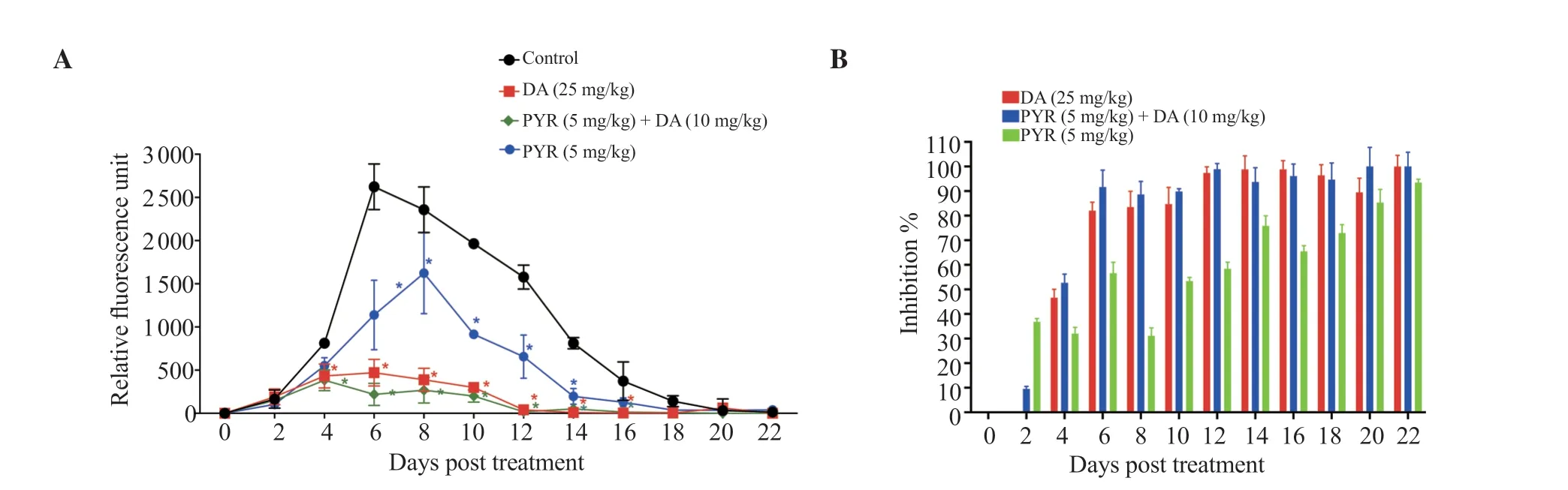
Figure 3.The in vivo chemotherapeutic efficacy of PYR alone or in combination with DA on Babesia microti proliferation.(A) The inhibitory impact of PYR.Values are represented as mean ± standard deviation of three expermental trials.*P<0.05 compared with the untreated infected mice.(B) Percentages of inhibition in the growth of Babesia microti in mice.Each treated group’s percentage of parasite inhibition was computed as a ratio to the positive control group.
Additionally,the effect of PYR combined with DA in treating hemolytic anemia was evaluated.Mice given low doses of PYR and DA showed hematological characteristics similar to those of mice given the DA monotherapy (25 mg/kg) (Figure 4).
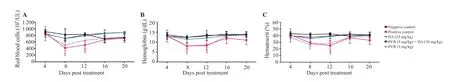
Figure 4.Anemia monitoring in Babesia microti-infected mice receiving combination therapy (PYR+DA).(A) red blood cells,(B) hemoglobin,(C) hematocrit.Values are represented as mean ± standard deviation of three expermental trials.*P<0.05 compared with the untreated infected mice.
4.Discussion
In cheminformatics,quantifying the similarity of two molecules is a fundamental idea and a common task[11].Virtual screening is one of the many disciplines it is applied in,especially in medicinal chemistry[12].The hierarchical clustering analysis revealed that PYR and AV showed the maximum structural similarity followed by a nearly similar molecular weights correlation between PYR and DA.As a result,a drug combination test was performed to determine the two-drug interaction against Babesia and Theileria parasites.The combination test showed that PYR and DA exhibited synergistic and additive interactions on B.bovis and T.equi,respectively.While other screened combination therapies showed either indifference or antagonistic interaction against the tested Babesia and Theileria parasites.The obtained findings were contrary to an additive and antagonistic interaction caused by DA+ID on B.bovis,and T.equi,respectively[12].Comparably,the combined application of PYR/MMV396693 demonstrated both additive and indifferent interactions against B.bovis’s in vitro growth.PYR/clofazimine exhibited antagonistic and indifferent interactions with B.bovis in vitro growth[6].
Based on the in vitro combination test results obtained in the present study,PYR+DA was evaluated further for their in vivo inhibitory efficacy using a B.microti-infected mouse model.When PYR was administered orally,its inhibitory effect[7] was shown in mice.Inhibition of B.microti growth was greater in mice treated with low doses of PYR combined with DA than in those treated with 100 mg/kg enoxacin,150 mg/kg norfloxacin,and 700 mg/kg ofloxacin[5],125 mg/kg PYR[14],and 50 mg/kg thymoquinone[8].Taken together,the ability of PYR combined with DA to get parasitemia close to 0%at the day with peak parasitemia (Day 6) post treatment,gives this combination merit over the recently developed antibabesial drug,resveratrol[15],fluoroquinolones[5],thymoquinone[8],and myrrh oil[2].The inhibitions in the growth of B.microti in mice observed after treatment with PYR and DA either in days with peak parasitemia or all days post treatment are higher than the 67% inhibition rates for 50 mg/kg enoxacin and 10 mg/kg DA[5],68.56% inhibition rates for a 15 mg/kg oral dose PYR and 15 mg/kg clofazimine[6],and 53.25% inhibition rates for an intramuscular dose of 85 mg/kg PYR combined with 10 mg/kg DA[14].Thus,PYR when administrated in the oral route combined with DA caused around 30% more inhibition against B.microti growth than those caused after treatment with an intramuscular dose of PYR[14] combined with DA.Interestingly,a very low dose of PYR (5 mg/kg) when administrated by oral route combined with a low dose of DA (10 mg/kg) caused 23% more inhibition in B.microti growth than those caused after treatment with a 15 mg/kg oral dose of PYR combined with a 15 mg/kg oral dose of clofazimine[6].
Interestingly,the effect of a 5 mg/kg oral dose of PYR combined with 10 mg/kg DA on RBC count was better than those observed either after treatment with an 85 mg/kg intramuscular dose of PYR combined with a 10 mg/kg subcutaneous dose of DA[14] or with a 15 mg/kg oral dose PYR and 15 mg/kg clofazimine[6].
When administered via various routes,even the same medication demonstrated varying chemotherapeutic effects[15].Previous research conducted in our laboratory[8,16] revealed that when administered in two different ways,thymoquinone or clofazimine had varying inhibitory effects on the growth of B.microti in mice.When clofazimine was administered orally as opposed to intraperitoneally,the effect was significantly greater.On the other hand,the results for thymoquinone were the opposite.Such different chemotherapeutic effects with different administrative routes may explain the high inhibitory effect of PYR when administrated in an oral dose in the present study over those observed in our previous study[14] where we used an intramuscular dose of PYR.
PYR,when taken as a whole,is a blood schizonticide that works against malaria by preventing the ability of parasite’s digestive vacuole to produce hemozoin pigment[17].In addition to its antimalarial action stemming from its inhibition of hemozoin production,PYR is also known to intercalate into DNA and block DNA topoisomerase 2 enzymes[17].It is currently unknown how DA,an aromatic diamidine,works against Babesia.However,since it has been seen in Trypanosoma and Leishmania species,it is believed to have an impact on the parasite’s aerobic glycolysis and DNA synthesis[12].Subsequently,the observed synergetic interaction in the present study might be attributed to that both drugs have an inhibitory effect on the parasite’s DNA synthesis.
It should be noted that there are some limitations to the present study.Although the obtained combination test showed synergistic interaction between PYR and DA on B.bovis,there is no clear reason behind such interaction.Therefore,further future studies are required to clarify the mechanism by which both drugs inhibit B.bovis and to confirm the aforementioned suggested theory on the effect of both drugs on the parasite’s DNA synthesis.Also,future studies are required to explore why the combination of PYR with DA treated the hemolytic anemia associated with babesiosis.Future studies are needed to clarify the efficacy of this combination therapy in removing the remnants of parasite nucleic acid from the treated animal body using PCR assay.Further studies are required to evaluate the inhibitory effect of PYR combined with DA in clinically infected animals,as well as investigate the in vivo inhibitory effects of PYR when used in combination with other antibabesial drugs such as AV and ID.
In conclusion,the hierarchical clustering analysis revealed that PYR and DA showed a nearly similar molecular weight correlation.A subcutaneous dose of DA potentiates the anti-B.microti efficacy of an oral dose of PYR in vivo.Low doses of PYR and DA caused either a significant inhibition in the growth of B.microti in mice or recovery from hemolytic anemia,which is better than those observed after treatment with DA alone.PYR and DA might be more effective if used as a combination therapy rather than a single therapy for the treatment of babesiosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to great thank Prof.Naoaki Yokoyama,National Research Center for Protozoan Diseases,Obihiro University of Agriculture and Veterinary Medicine,Inada-cho,Obihiro,Hokkaido,Japan for his scientific support and discussion.
Funding
This research work is supported by Deputyship for Research&Innovation,Ministry of Education in Saudi Arabia through the project number: ISP23-73.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’ contributions
MAR and II supervised and designed the study.MAR,and SAESES performed experimental analysis.MZSA,MSA,SAA,N.Alsadaan,N.Alam,and SAESES performed the analytic calculation.MAR,MZSA,and II provided the resources.MAR,SAESES,MSA contributed to the final version of the manuscript.MAR and II supervised the project.
 Asian Pacific Journal of Tropical Biomedicine2024年3期
Asian Pacific Journal of Tropical Biomedicine2024年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Rosmarinic acid improves tracheal smooth muscle responsiveness and lung pathological changes in ovalbumin-sensitized rats
- Icariin ameliorates viral myocarditis by inhibiting TLR4-mediated ferroptosis
- Capsosiphon fulvescens suppresses LPS-stimulated inflammatory responses by suppressing TLR4/NF-κB activation in RAW264.7 murine macrophages
- Th17/Treg balance and macrophage polarization ratio in lower extremity arteriosclerosis obliterans
