Capsosiphon fulvescens suppresses LPS-stimulated inflammatory responses by suppressing TLR4/NF-κB activation in RAW264.7 murine macrophages
Seon Yeong Ji ,EunJin Bang, Hyun Hwangbo ,Min Yeong Kim ,Da Hye Kim ,Su Hyun Hong ,Shin-Hyung Park,Chang-Young Kwon,Gi-Young Kim,You-Jin Jeon,Suengmok Cho,Yung Hyun Choi✉
1Department of Biochemistry,Dong-eui University College of Korean Medicine,Busan 47227,Republic of Korea
2Basic Research Laboratory for the Regulation of Microplastic-Mediated Diseases and Anti-Aging Research Center,Dong-eui University,Busan 47227,Republic of Korea
3Department of Pathology,Dong-eui University College of Korean Medicine,Busan 47227,Republic of Korea
4Department of Oriental Neuropsychiatry,Dong-eui University College of Korean Medicine,Busan 47227,Republic of Korea
5Department of Marine Life Science,Jeju National University,Jeju 63243,Republic of Korea
6Department of Food Science and Technology/Institute of Food Science,Pukyong National University,Busan 48513,Republic of Korea
ABSTRACT Objective: To evaluate the effects of Capsosiphon fulvescens (C.fulvescens) ethanolic extract on inflammation in lipopolysaccharide(LPS)-induced RAW296.7 macrophages.Methods: The protective effects of C.fulvescens ethanolic extract on LPS-induced inflammation in RAW264.7 macrophages were assessed using biochemical analysis,including enzyme-linked immunosorbent assay,quantitative reverse transcription-polymerase chain reaction,and Western blot analysis.To examine reactive oxygen species (ROS) production,flow cytometry analysis,and immunofluorescence staining were used.Furthermore,the modulatory effect of C.fulvescens ethanolic extract on NF-κB activation was investigated.Results: C.fulvescens ethanolic extract significantly attenuated LPS-induced levels of pro-inflammatory cytokines and notably reduced the secretion and mRNA levels of LPS-mediated matrix metalloproteinases.In addition,C.fulvescens ethanolic extract decreased ROS production and suppressed the TLR4/NF-κB signaling pathway.Conclusions: C.fulvescens ethanolic extract alleviates inflammation as well as oxidative stress by modulating the TLR4/NF-κB signaling in LPS-induced RAW264.7 macrophages.C.fulvescens can be used as a potential therapeutic agent to suppress inflammation and oxidative stress-associated diseases.
KEYWORDS: Capsosiphon fulvescens;Inflammation;Oxidative stress;NF-κB;Nrf2;TLR4
Significance
Capsosiphon fulvescens is a filamentous green alga that has been consumed in the form of food due to various beneficial biological activities.The results of this study demonstrate that Capsosiphon fulvescens ethanolic extract ameliorated inflammation and oxidative stress in lipopolysaccharide-stimulated RAW264.7 macrophages by decreasing the mRNA and protein expressions and production of pro-inflammatory cytokines,matrix metalloproteinase,and reactive oxygen species by modulating the TLR4/NF-κB signaling and nuclear factor erythroid-2-related factor 2.This suggests that Capsosiphon fulvescens ethanolic extract could be a valuable therapeutic supplementary food to improve inflammation and inflammation-associated diseases.
1.Introduction
Inflammation is an immune response to harmful stimulation such as toxic compounds and pathogens.Although controlled inflammatory responses can help maintain homeostasis in the body,uncontrolled excessive inflammatory responses can disturb homeostasis and cause hyperactivation in the body,leading to detrimental effects[1,2].Excessive inflammatory response is known as one of the main factors in various diseases such as autoimmune diseases and cancer[3].In the immune system,macrophages are a critical part of the initial immune response for host defense against many pathogens.Lipopolysaccharide (LPS),an important part of the outer membrane of Gram-negative bacteria,stimulates acute inflammation by activating macrophages and releasing various proinflammatory factors[4,5].Therefore,LPS is commonly used to trigger inflammatory responses and subsequently tissue destruction by stimulating the production of inflammation-related molecules,including nitric oxide (NO),prostaglandin E2(PGE2),tumor necrosis factor (TNF)-α,interleukin (IL)-6,IL-1β,matrix metalloproteinases(MMPs),and proteinoids generally through Toll-like receptor 4(TLR4)[6,7].These pro-inflammatory mediators and cytokines are abnormally upregulated in various inflammatory pathological conditions and are commonly found in acute and chronic inflammatory conditions[8].
Under normal conditions,reactive oxygen species (ROS) are required for maintaining normal physiological function and host defense responses[9].However,excessive production of ROS can induce a pro-inflammatory cellular environment and consequently lead to disease development[10].In general,LPS stimulates ROS production in macrophages,contributing to the manifestation of inflammation.Elevated oxidative stress and inflammation are common pathological conditions.Nuclear factor-κB (NF-κB) is the most critical transcription factor involved in the expression of inflammatory regulators[11,12].It has been well established that LPSinduced TLR4 activation can induce NF-κB activation which is associated with diverse inflammatory diseases[13,14].In addition,excessive ROS and related species such as superoxide (O2-) and hydrogen peroxide (H2O2) can produce signaling responses that induce activation of the NF-κB transcription factor[15,16].Various therapeutic agents are widely prescribed in efforts to alleviate inflammatory symptoms.However,long-term administration of these agents has side effects.Therefore,safe and effective agents or functional foods for treating various inflammation-related diseases need to be discovered.
Capsosiphon fulvescens (C.fulvescens) is a filamentous green algae that is abundant in North Atlantic and Northern Pacific,including Southwest coast of Republic of Korea[17].C.fulvescens has been consumed in the form of food with a characteristic taste and soft texture.It was originally used to treat stomachache[18].This seaweed has shown potential as a functional food material with a variety of biological activities.For example,C.fulvescens extract shows an inhibitory effect on melanin production by blocking the activity of tyrosinase in B16F10 cells[19].In addition,polysaccharides extracted from C.fulvescens can induce apoptosis in AGS gastric carcinoma cells[20].It has been reported that consumption of C.fulvescens powder can reduce cholesterol levels in hypercholesterolemic rats[21].Fang et al.have isolated several compounds with antioxidant activity from C.fulvescens and reported their properties[22].Recently,Oh et al.have reported that glycoproteins extracted from C.fulvescens can increase probiotic-induced cognitive improvement in an aging mouse model and that such improvement is correlated with nuclear factor erythroid-2-related factor 2 (Nrf2) activation,which is a major redox transcriptional factor[23].Regarding its anti-inflammatory effects,pheophorbide,an anti-glycation compound isolated from C.fulvescens,possesses scavenging activity against intracellular ROS and significantly suppresses inflammation-related cytokines and chemokines[24].However,the preventive effects of C.fulvescens on inflammation and oxidative stress and its action mechanisms in macrophages have not been fully elucidated yet.Therefore,the current study was conducted to evaluate the suppressive effects of an ethanol extract of C.fulvescens on inflammation and oxidative stress in LPS-treated RAW264.7 murine macrophages and assess the involvement of TLR4/NF-κB and Nrf2 in mediating these beneficial effects of C.fulvescens extract.
2.Materials and methods
2.1.C.fulvescens preparation
C.fulvescens used in this study was provided by Jeju DongMoon Merchandise (Jeju,Republic of Korea).For preparation of C.fulvescens,C.fulvescens sample (200 g) was submerged in 70%ethanol for 24 h with shaking at 24-27 ℃.After shaking for 24 h,the C.fulvescens extract was put into centrifugation at 15 000 rpm for 15 min at 4 ℃.The collected supernatant was filtered to remove solid materials.The process of incubator shaking to filtration was repeated three times.Filtered extracts were concentrated and freeze-dried at -80 ℃.C.fulvescens extract was diluted in dimethyl sulfoxide(DMSO,Thermo Fisher Scientific,Waltham,MA,USA) before use in experiments.
2.2.Cell cytotoxicity assay
The cytotoxicity of C.fulvescens was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay following a previous study[25] with appropriate modifications.Briefly,seeded RAW 264.7 cells (American Type Culture Collection,Manassas,VA,USA) were stimulated with various concentrations (50,100,and 200 μg/mL) of C.fulvescens extract.After treatment with C.fulvescens,cells were treated with MTT reagent (Thermo Fisher Scientific) for 2 h.After removing media,formazan was dissolved using DMSO,and absorbance was measured at 540 nm with a microplate reader (BioTek Instruments Inc.Winnoski,VT,USA).
2.3.NO measurement
NO concentrations were measured using Griess reagent assay following a previous study[26] with appropriate modifications.Briefly,cells were seeded into a 6-well plate at a concentration of 6 ×105cells/well and pretreated with C.fulvescens extract (50,100,and 200 μg/mL) or dexamethasone (10 μM,Sigma-Aldrich Chemical Co.,St.Louis,MO,USA) for 1 h before additional treatment with LPS (100 ng/mL,Sigma-Aldrich Chemical Co.) for 24 h.Culture supernatant and Griess reagent were mixed at 1:1 and reacted for 10 min at room temperature.The optical absorbance was detected at 540 nm with a microplate reader (BioTek Instruments Inc).
2.4.PGE2,cytokines,and MMPs measurements
RAW 264.7 cells were seeded into 6-well plates at a concentration of 6 × 105cells/well and pre-treated with C.fulvescens extract (50,100 and 200 μg/mL) or dexamethasone (10 μM) or BAY7085 (0.5 μM,Sigma-Aldrich Chemical Co.) for 1 h before treatment with 100 ng/mL of LPS (Abcam,Waltham,MA,USA) for 24 h.Cell culture supernatants were gathered to detect concentrations of PGE2,IL-1β,IL-6,TNF-α,MMP-2,and MMP-9 using Enzyme-Linked Immunosorbent Assay (ELISA) kits (PGE2,Cayman Chemical,Ann Arbor,Michigan,USA;IL-1β,R&D Systems,Minneapolis,MN,USA;IL-6,R&D Systems;TNF-α,R&D Systems;MMP-2,Abcam;MMP-9,R&D Systems).
2.5.Heme oxygenase-1 (HO-1) activity assay
RAW 264.7 cells were pre-treated with C.fulvescens extract (200 μg/mL) for 1 h prior to treatment with LPS (100 ng/mL) for 24 h.After treatment,cells were collected and resuspended in a cell extraction buffer.Cell extracts were used to evaluate HO-1 activity using a Mouse Heme Oxygenase 1 ELISA kit (Abcam) following the product protocol.
2.6.Western blot analysis
Western blot analysis was conducted following a previous study[27]with appropriate modifications.Briefly,cells were collected and lysed by using a cell lysis buffer for 1 h.After incubating cells with a cell lysis buffer,lysates were subject to centrifugation at 12 000 rpm for 15 min at 4 ℃.The protein fraction was collected and same amounts of protein samples were used for Western blot analysis.Separated proteins by sodium dodecyl sulfate-polyacrylamide gels were moved to polyvinylidene difluoride membranes (Amersham,Staffordshire,UK).After blocking the membrane in 5% nonfat milk for 1 h at room temperature,membranes were incubated with appropriate primary antibodies overnight at 4 ℃ on a rotator.Primary antibodies used for Western blot analysis were iNOS(610333,BD Biosciences,Franklin Lakes,NJ,USA),COX-2 (sc-19999,Santa Cruz,Dallas,TX,USA),MyD88 (ab219413,Abcam),IL-1β (12242s,Cell Signaling Technology,Danvers,MA,USA),IL-6 (12912s,Cell Signaling Technology),TNF-α (sc-52746,Santa Cruz),p65 (6956P,Cell Ssignaling Technology),p-Nrf2 (Ser40)(PA5-67520,Invitrogen,Carlsbad,CA,USA),HO-1 (Ab13248,Abcam),TLR4 (NB100-56566,NOVUS,St.Louis,MO,USA),β-actin (BS6007M,Bioworld,Bloomington,MN,USA),and lamin B (sc-6216,Santa Cruz).All primary antibodies were diluted with a 1:1 000 ratio in 1 × phosphate buffered saline (PBS) with Tween®20 (Sigma-Aldrich Chemical Co.).After membrane washing,membranes were visualized with an enhanced chemiluminescence solution (Thermo Fisher Scientific) and exposed to a Vilber Fusion FX Image System (Vilber,Collégien,France) for protein detection.
2.7.Quantitative reverse transcription-polymerase chain reactions (qRT-PCR)
qRT-PCR analysis was conducted following a previous study[28]with appropriate modifications.In brief,total RNA was isolated from cells by using TRIzol reagent (Bioneer,Daejeon,Republic of Korea).Complementary DNA was prepared using total RNA (2 μg)and AccuPower®CycleScript™RT PreMix (Bioneer).Quantitative PCR was conducted on a CFX Duet Real-Time PCR System (Bio-Rad Lab.,Hercules,CA,USA) using SsoAdvanced Universal SYBR Green Reagent (Bio-Rad Lab.) and specific primers (Table 1).
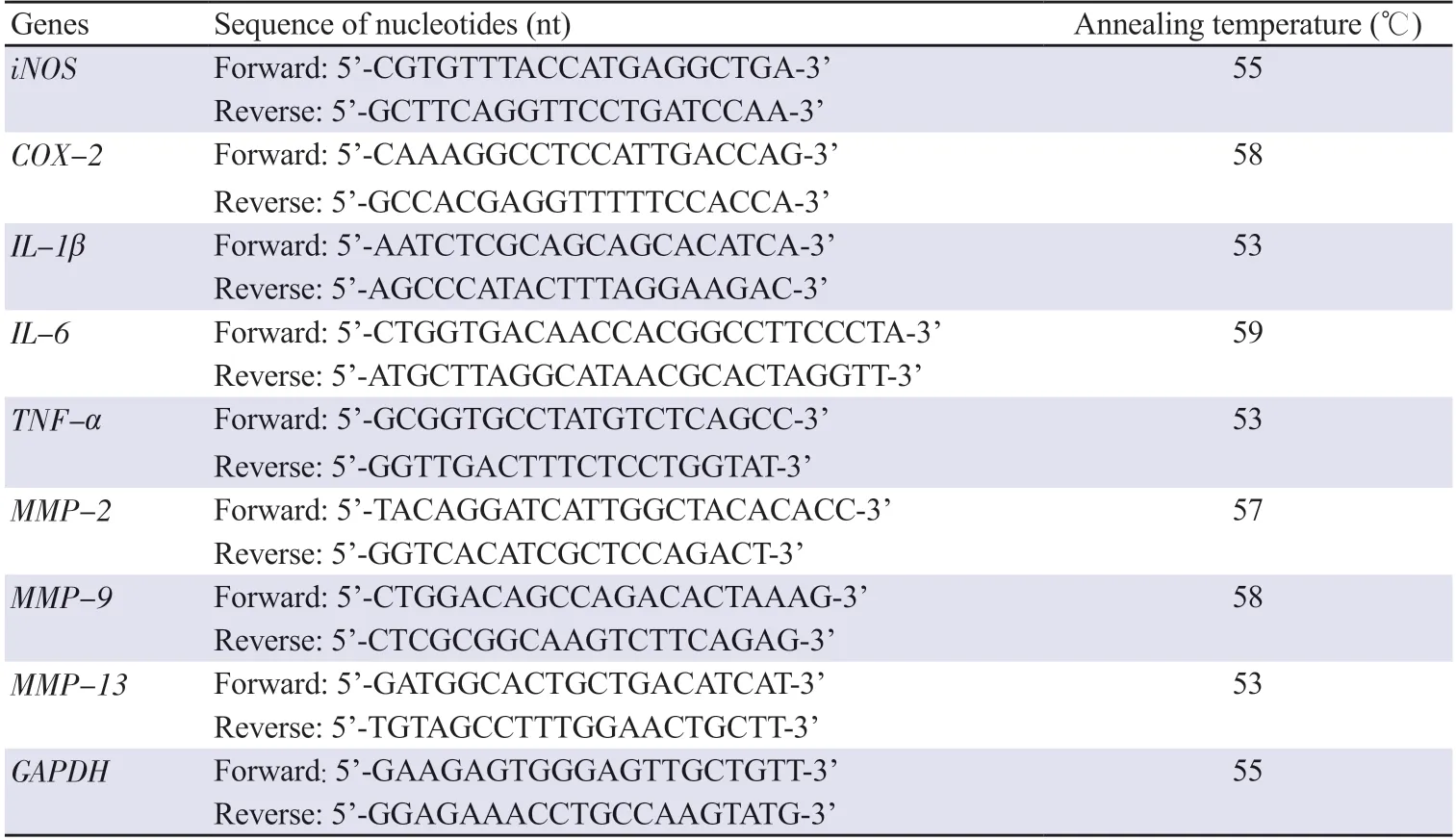
Table 1.Primer sequences used for qRT-PCR analysis.
2.8.FITC-LPS binding affinity measurement
Fluorescein isothiocyanate (FITC)-LPS binding affinity measurement was conducted following a previous study[29] with appropriate modifications.Briefly,RAW264.7 cells were pre-treated with C.fulvescens extract for 1 h and further treated with FITC-LPS(100 ng/mL,Sigma-Aldrich Chemical Co.) for 24 h.After treatment,cells were collected and washed with 1 × PBS.The number of bound FITC-molecules per cell was then measured using a flow cytometer(BD Bioscience) at TRCORE,Dong-eui University (Busan,Republic of Korea).
2.9.ROS production
ROS production was measured following a previous study[30] with appropriate modifications.For measurement of ROS production,cells were washed and stained with H2DCF-DA (Thermo Fisher Scientific) at 37 ℃ with 5% CO2for 20 min.After staining,the intensity of DCF fluorescence was analyzed with a flow cytometer.Furthermore,H2DCF-DA fluorescence images were observed using an EVOS FL Auto 2 Imaging System (Thermo Fisher Scientific).
2.10.Immunofluorescence staining
Immunofluorescence staining was conducted following a previous study[31] with appropriate modifications.For immunofluorescent staining of TLR4,RAW264.7 cells were pre-treated with C.fulvescens extract for 1 h and then further treated with LPS (100 ng/mL) for 4 h.These cells were reacted with TLR4 antibody(NOVUS) and Alexa Fluor 594-conjugated antibody (Invitrogen).After counterstaining the nuclei with 4′,6-diamidino-2-phenylindole(DAPI,Sigma-Aldrich Chemical Co.),images were obtained using an EVOS FL Auto 2 Imaging System.To evaluate p65 translocation in the nucleus,cells reacted with p65 antibody (Cell Signaling Technology) and Alexa Fluor 594-conjugated antibody (Invitrogen).After counterstaining the nuclei with DAPI,cells were washed and images were obtained by using the EVOS FL Auto 2 Imaging System.
2.11.Statistical analysis
All results were subjected to one-way analysis of variance(ANOVA) and Tukey’s post hoc test using GraphPad Prism software ver.5.03 (GraphPad Software,Inc.,La Jolla,CA,USA).The quantitative data are expressed as mean ± standard deviation.
3.Results
3.1.C.fulvescens extract suppresses LPS-induced production of inflammation-associated mediators
RAW264.7 cells were treated with different concentrations of C.fulvescens extract (50,100,and 200 μg/mL) for 24 h,and cellular cytotoxicity was evaluated by performing an MTT assay.The various concentrations of C.fulvescens extract tested in this study did not show cell toxicity to RAW264.7 cells (Figure 1A).Thus,two or three of the tested concentrations of C.fulvescens extract were selected to examine the dose-dependent preventive effect of C.fulvescens extract on inflammation-associated responses,including production of proinflammatory factors and mediators,and their protein expression levels.As shown in Figure 1B,NO concentration stimulated by LPS was remarkably decreased by C.fulvescens extract.The decrease of NO concentration by C.fulvescens extract was greater than that by dexamethasone.Similarly,PGE2concentration increased by LPS stimulation was significantly reduced by C.fulvescens extract in a concentration-dependent manner,the effect of which was greater than that of dexamethasone (Figure 1C).Subsequently,we investigated whether C.fulvescens extract could suppress the levels of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) expression under LPS stimulation.Western blot and qRT-PCR results revealed that the protein and mRNA expressions of iNOS and COX-2 were decreased notably by C.fulvescens extract at a high concentration (200 μg/mL) (Figure 1D-1F).
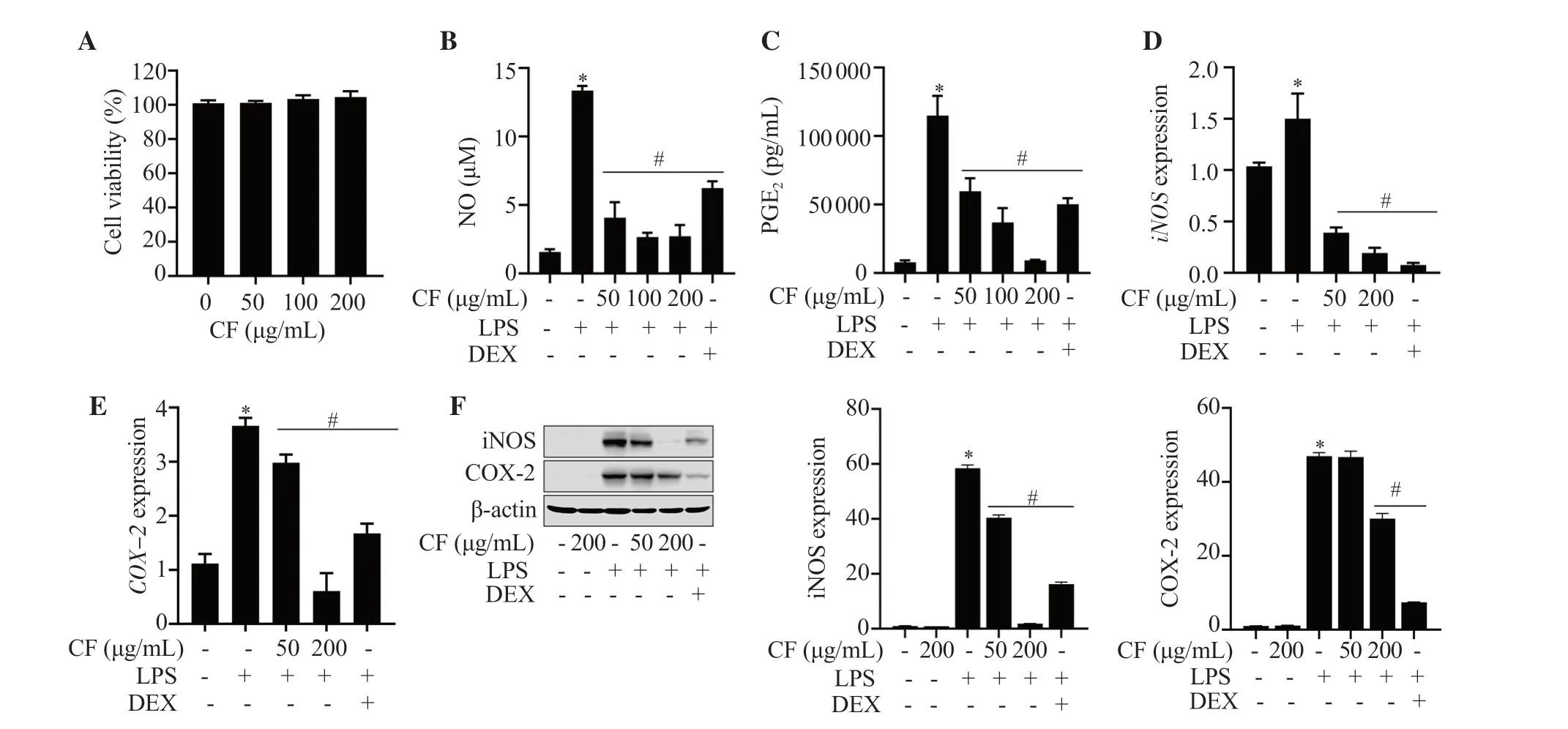
Figure 1.Effects of Capsosiphon fulvescens (C.fulvescens) ethanolic extract on LPS-induced cellular cytotoxicity and inflammation-associated mediators in RAW264.7 cells.Cells were exposed to C.fulvescens at different concentrations for 24 h and assessed using an MTT assay (A).Cells were pretreated with C.fulvescens or dexamethasone (10 μM) for 1 h and further treated with LPS (100 ng/mL) for 24 h (B-F).*P <0.05 vs.control cells;#P <0.05 vs.LPS-treated cells.LPS: lipopolysaccharide;CF: C.fulvescens extract;DEX: dexamethasone;NO: nitric oxide;PGE2: prostaglandin E2.
3.2.C.fulvescens extract suppresses LPS-induced production of inflammation-associated cytokines
As indicated in Figure 2A-2C,pretreatment with C.fulvescensextract significantly suppressed LPS-induced IL-1β,IL-6,and TNF-α levels.To assess whether the inhibition of production of these cytokines in C.fulvescens extract-pretreated RAW264.7 cells was associated with changes in gene expression level,mRNA and protein expression levels were investigated under these conditions.Similarly,C.fulvescens extract suppressed both protein and mRNA levels of these cytokines stimulated by LPS at the indicated concentrations(Figure 2D-2G).
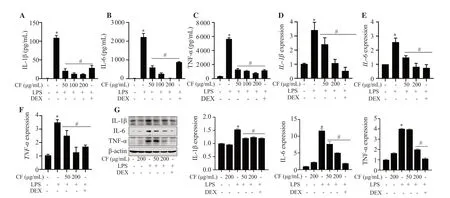
Figure 2.Protective effects of C.fulvescens ethanolic extract on LPS-induced production of inflammation-associated cytokines in RAW264.7 cells.Cells were pretreated with the indicated concentrations of C.fulvescens ethanolic extract or dexamethasone for 1 h and subsequently stimulated with LPS for 24 h.Secretion of IL-1β (A),IL-6 (B),and TNF-α (C) was assessed using ELISA kits.(D-G) Protein and mRNA levels of these cytokines were assessed using Western blot and qRT-PCR,respectively.*P <0.05 vs.control cells;#P <0.05 vs.LPS-treated cells.
3.3.C.fulvescens extract suppresses LPS-induced secretion of MMPs
The concentrations of MMP-2 and MMP-9 were remarkably upregulated in LPS-treated RAW264.7 cells.However,pretreatment with C.fulvescens extracts significantly inhibited LPS-stimulated secretion of MMP-2 and MMP-9,with the most significant effect observed at a concentration of 200 μg/mL (Figures 3A and 3B).Similarly,C.fulvescens extract significantly decreased the mRNA expression of MMP-2,MMP-9,and MMP-13 in LPS-stimulated RAW264.7 cells (Figure 3C-3E).
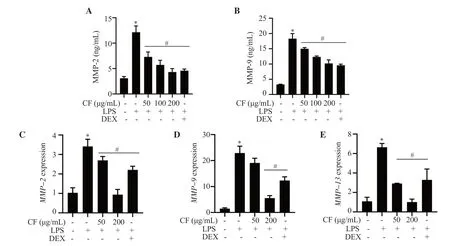
Figure 3.Protective effect of C.fulvescens ethanolic extract on LPS-induced production of MMPs in RAW264.7 cells.Cells pretreated with C.fulvescens ethanolic extract or dexamethasone for 1 h were treated with LPS for 24 h.(A-B) The levels of MMP-2 and MMP-9 were evaluated using ELISA kits.(C-E)The mRNA expressions of MMP-2,MMP-9,and MMP-13 were evaluated using qRT-PCR.*P <0.05 vs.control cells;#P <0.05 vs.LPS-treated cells.MMP:matrix metalloproteinase.
3.4.C.fulvescens extract suppresses LPS-induced ROS generation
As oxidative stress is elevated in inflammatory responses,we assessed whether C.fulvescens extract potentially modulates LPS-stimulated ROS generation.Flow cytometry analysis was conducted using H2DCF-DA probe.Resultsdemonstrated that the ROS production stimulated by LPS was significantly alleviated by pretreatment with C.fulvescens extract at 200 μg/mL (Figure 4A).This suppressive effect of C.fulvescens extract on ROS production was superior to that of N-acetyl-L-cysteine (NAC),a positive control,under LPS-stimulated conditions.In accordance with this result obtained from flow cytometry analysis,the increase of ROS production by LPS was notably inhibited by pretreatment with C.fulvescens extract at 200 μg/mL (Figure 4B).To evaluate potential involvement of redox regulator protein HO-1 in C.fulvescens extractmediated ROS suppression in LPS-stimulated RAW264.7 cells,HO-1 activity was assessed using an HO-1 ELISA kit.HO-1 activity was significantly increased by C.fulvescens extract at 200 μg/mL in LPS-stimulated RAW264.7 cells (Figure 4C).To further evaluate the effects of C.fulvescens extract on intracellular ROS levels,the levels of HO-1 expression and its upstream regulator,redox-sensitive transcription factor Nrf2,were assessed using Western blot analysis.The phosphorylated Nrf2 (p-Nrf2) and HO-1 expression were upregulated by C.fulvescens extract in a concentration-dependent manner (Figure 4D).
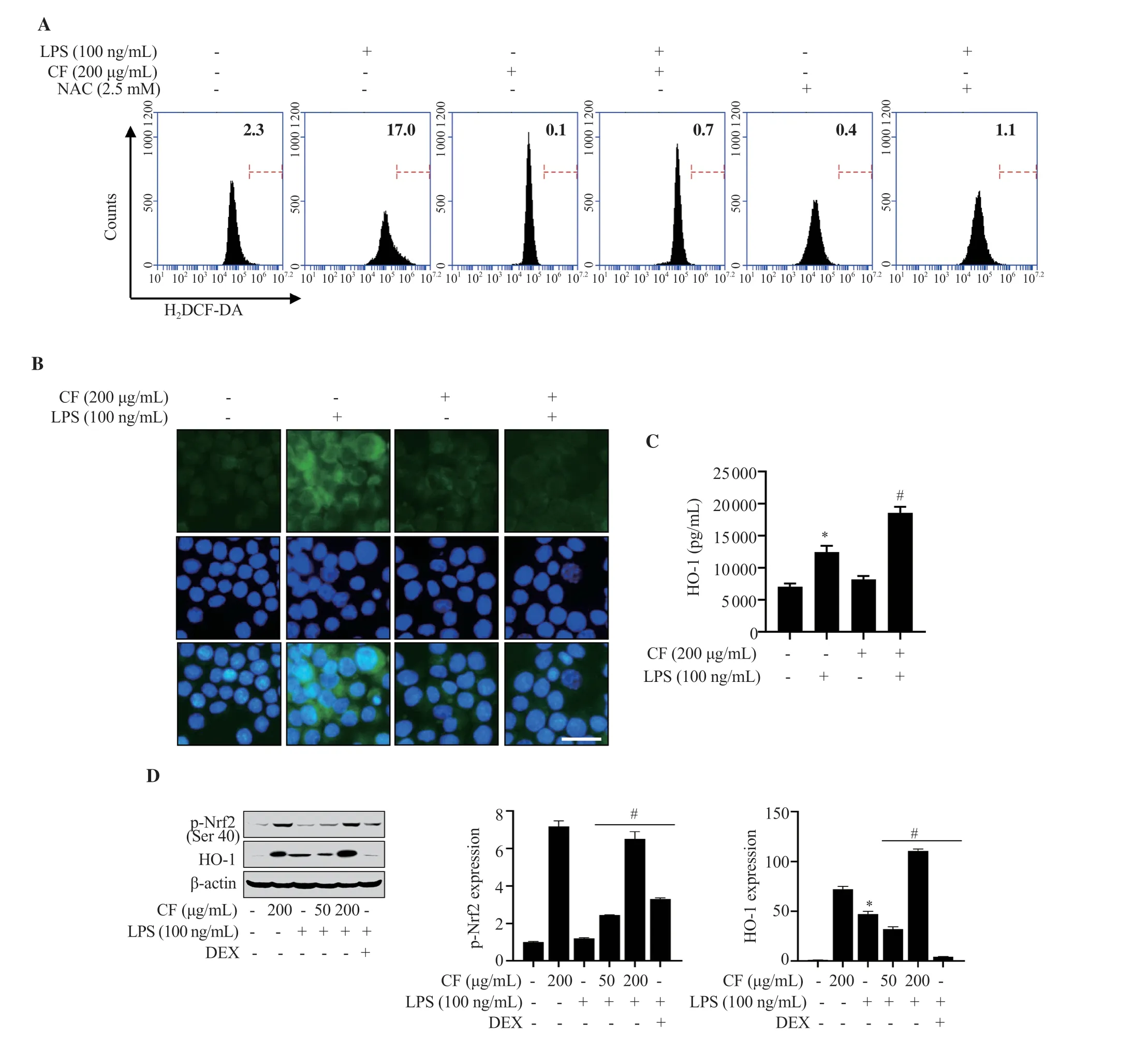
Figure 4.Effect of C.fulvescens ethanolic extract on oxidative stress in LPS-treated RAW264.7 cells.(A) ROS concentration was assessed using H2DCF-DA and flow cytometry analysis.(B) ROS level (green) was measured using H2DCF-DA and fluorescence microscope imaging.(C) HO-1 activity was evaluated using a Mouse HO-1 ELISA kit.(D) Protein expression levels of p-Nrf2 and HO-1 were investigated using Western blot analysis.*P <0.05 vs.control cells;#P<0.05 vs.LPS-treated cells.
3.5.C.fulvescens extract suppresses LPS-induced TLR4 and NF-κB activation
To understand mechanisms involved in the protective effects of C.fulvescens extract on inflammation and oxidative stress,we evaluated whether C.fulvescens extract could modulate LPS-induced TLR4 signaling pathway.As expected,when RAW 264.7 cells were treated with LPS,LPS could bind to cells with a high affinity.This LPS-stimulated heightened binding affinity was significantly prevented by pretreatment with C.fulvescens extract at 200 μg/mL(Figure 5A).Since LPS can activate the NF-κB signaling pathway after binding to TLR4,we examined the modulating effect of C.fulvescens extract on TLR4 expression in LPS-treated RAW264.7 cells.As shown in Figure 5B and 5C,the expression of TLR4 and myeloid differentiation factor 88 (MyD88),a downstream factor of TLR4,were significantly upregulated after treatment with LPS.However,they were effectively decreased by C.fulvescens extract with increasing concentration.Since NF-κB is a major regulating transcription factor of pro-inflammatory molecules and cytokines,whether C.fulvescens extract could block LPS-stimulated activation of NF-κB was further examined.As demonstrated in Figure 5D,nuclear NF-κB p65 expression was largely accumulated in the LPSinduced group in comparison to that in the control group.However,this nuclear translocation of NF-κB was significantly prevented by pretreatment with C.fulvescens extract.LPS-induced NF-κB nuclear translocation and the preventive effect of C.fulvescens extract were further confirmed at protein levels.As shown in Figure 5E,the protein level of NF-κB p65 in the nucleus of LPS-treated cells was notably increased,while its level in the cytoplasm was slightly decreased.However,C.fulvescens extract suppressed LPS-induced nuclear accumulation of NF-κB p65 protein in RAW264.7 cells.
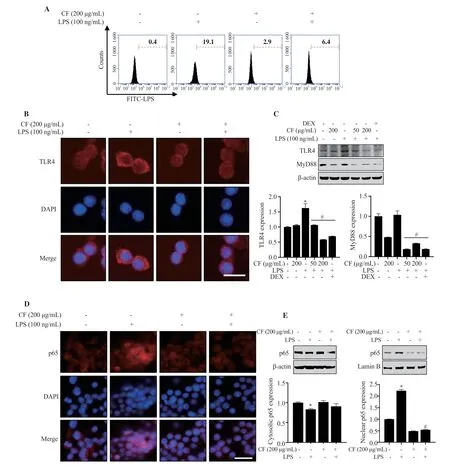
Figure 5.Inhibitory effects of C.fulvescens ethanolic extract on TLR4 and NF-κB activation in LPS-treated RAW264.7 cells.(A) Macrophage-LPS binding affinity was measured using FITC-LPS and flow cytometry analysis.(B-C) Protein levels of TLR4 and MyD88 were evaluated using immunofluorescence and Western blot analysis,respectively.(D-E) Protein expressions of p65 in the cytosol and nucleus were evaluated using immunofluorescence and Western blot analysis,respectively.
3.6.Anti-inflammatory effect of C.fulvescens extract in LPS-treated RAW264.7 cells is dependent on NF-κB
To further confirm the regulatory role of C.fulvescens extract in NF-κB activation in LPS-treated RAW 264.7 cells,we used BAY7085,an inhibitor of NF-κB known to suppress LPS-mediated NF-κB activation.As shown in Figure 6A,LPS-stimulated NO concentration was effectively alleviated by C.fulvescens extract pretreatment,which was superior to that after BAY7085 pretreatment.Furthermore,C.fulvescens extract effectively alleviated LPS-induced production of pro-inflammatory cytokines (Figure 6BD),which was superior to those after BAY7085 pretreatment.
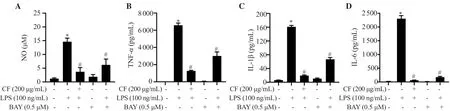
Figure 6.Inhibitory effects of C.fulvescens ethanolic extract on inflammation-associated mediators in LPS-stimulated RAW264.7 cells.(A) NO concentration was measured using Griess assay.The levels of TNF-α (B),IL-1β (C),and IL-6 (D) were assessed using ELISA kits.*P <0.05 vs.control cells;#P <0.05 vs.LPS-treated cells.
4.Discussion
NO is a soluble endogenous gas that can function as a signaling molecule,playing a major role in inflammation-associated diseases[32].Under normal physiological conditions,NO provides an anti-inflammatory effect.However,NO is considered a proinflammatory mediator that can promote inflammatory responses when it is overproduced under abnormal physiological conditions,which could further contribute to apoptosis[33].In addition,NO is known to contribute to the development of inflammatory pathological conditions of the joints,gut,and lungs,in which NO inhibitory compound might exert an important therapeutic effect and modulate inflammatory diseases[34].COX enzymes can catalyze the production of PGE2through conversion from arachidonic acids,a group of hormone-like molecules that play multiple physiological roles[35].However,PGE2overproduction due to COX-2 hyperactivity could result in various inflammatory stimulations[36].Therefore,modulating overly produced inflammation-associated mediators is considered a therapeutic strategy that could prevent or delay inflammation-associated diseases.The current results indicated that the release of NO and PGE2in LPS-treated RAW264.7 macrophages was significantly suppressed by increasing concentrations of C.fulvescens extract.C.fulvescens extract also decreased iNOS and COX-2 levels and suppressed inflammatory responses.
The development of cytokines is important to the immune function of macrophages that develop from innate to adaptive immunity[37].Hypersecretion of cytokines has been implicated in various disease states with chronic inflammatory conditions.In macrophages,LPS can stimulate immune responses by inducing cytokines production via NF-κB activation[38].Therefore,the levels of inflammationassociated cytokines are considered key markers to evaluate antiinflammatory effectiveness.In our study,C.fulvescens extract alleviated the secretion of inflammation-associated cytokines such as IL-1β,IL-6,and TNF-α as well as their mRNA levels in LPStreated RAW264.7 macrophages.These results demonstrate the potential anti-inflammatory effect of C.fulvescens extract on proinflammatory cytokines in LPS-stimulated macrophages.MMPs are a family of neutral endopeptidases.These enzymes can degrade all parts of the extracellular matrix (ECM),which plays a role in transmitting signals important for recruiting inflammatory cells[39].MMPs are upregulated in the process of inflammatory responses.They are critical for regulation of immune system[40].MMPs level needs to be under regulation in order to avoid excessive tissue destruction and pathological inflammatory conditions.MMPs are not only produced by structural cells but also by macrophages[39].In this study,C.fulvescens extract alleviated the expression of MMPs at the transcriptional level,preventing their increased production under LPS stimulation.This indicates that C.fulvescens extract might inhibit the ability of macrophages to degrade ECM by blocking reactions that produce MMPs.Because the activity of MMPs on ECM metabolism is counteracted by the expression of their inhibitors such as tissue inhibitors of metalloproteinases[41],additional studies are still necessary to determine how C.fulvescens extract regulates the production of MMPs.
ROS production is an important biological change in the progression of various inflammation and inflammation-related diseases[9].ROS can function as signaling molecules and inflammation mediators.Under normal conditions,ROS could control cell growth,differentiation,apoptosis,and senescence[42].However,overproduction or chronic ROS generation is considered a key contributor to the progression of inflammatory diseases.C.fulvescens extract effectively suppressed ROS production in LPStreated RAW264.7 cells by oxidized flow cytometry analysis and H2DCF-DA analysis.Nrf2 is a transcription factor that functions in regulating intracellular redox balance as a protective antioxidant[43].The presence of an antioxidant response element (ARE) suggests that a group of antioxidant genes,including thioredoxin reductase 1,NAD(P)H-quinone oxidoreductase 1,and HO-1,can be regulated through Nrf2 binding to the ARE sequence[44].Based on our results,p-Nrf2 expression level was significantly upregulated by C.fulvescens extract at 200 μg/mL.These results indicate that C.fulvescens extract could exert antioxidative effects through Nrf2 activation.However,antioxidative target genes upregulated by Nrf2 activation need to be determined in further studies.
LPS can stimulate TLR4 and further stimulate the secretion of critical inflammation-associated mediators and cytokines involved in potentiation of immune responses[7].In canonical signaling pathway,LPS binds to TLR4,further activating NF-κB signaling activation.In the nucleus,transcriptionally active NF-κB can enhance the expression of NF-κB dependent pro-inflammatory target genes[45].Therefore,LPS-mediated TLR4/NF-κB is a major signaling pathway that can promote inflammatory conditions and various inflammatory diseases[11].Our results demonstrated that LPS binding to macrophages was significantly suppressed by C.fulvescens extract at 200 μg/mL.Furthermore,LPS-mediated NF-κB p65 translocation from cytosol into nucleus was significantly inhibited by C.fulvescens extract as demonstrated in immunofluorescence and Western blotting analysis.In addition,C.fulvescens extract suppressed the production of NO,IL-1β,IL-6,and TNF-α greater than NF-κB inhibitor BAY7085.These results suggest that C.fulvescens extract can alleviate inflammatory response by blocking the TLR4/NF-κB signaling pathway and activating Nrf2 in LPS-stimulated RAW264.7 macrophages (Figure 7).Therefore,C.fulvescens extract is a potential candidate for treating inflammatory conditions and diseases.However,the anti-inflammatory and antioxidative effects of this plant extract need to be further evaluated in vivo.
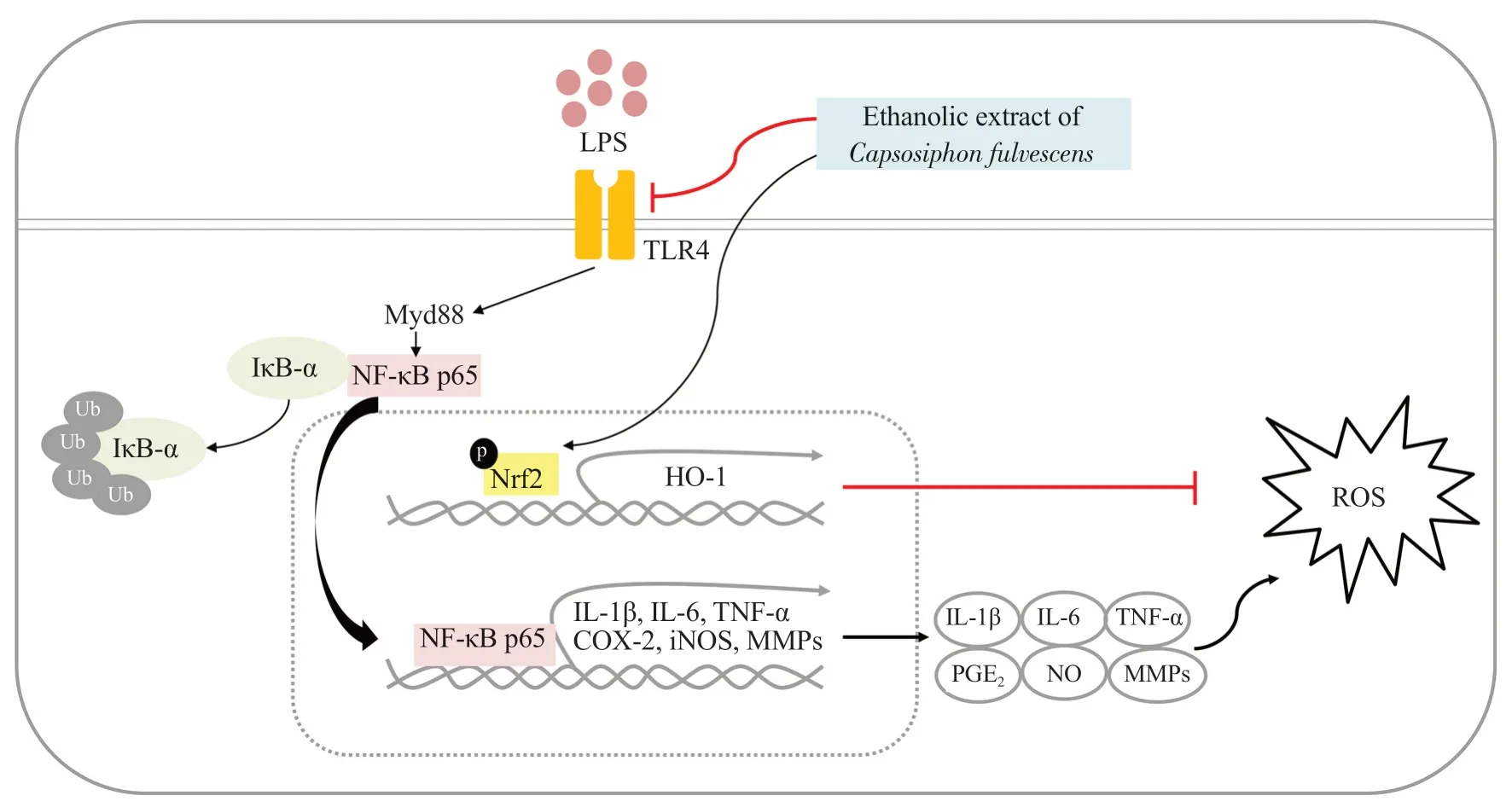
Figure 7.Schematic diagram reflecting the anti-inflammatory effect of C.fulvescens ethanolic extract in LPS-treated RAW296.7 macrophages.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Funding
This research was funded by Korea Institute of Marine Science &Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries,Korea (20220488).
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’ contributions
SYJ,SHH,and YHC designed and conceptualized the study.SYJ planned and performed experiments and analyzed the data.EJB,HH,MYK,and SHP validated and reviewed the analysis.EJB reviewed and investigated the data.HH,MYK,DHK,SHP,CYK,GYK,YJJ,and SC provided resources for the experiment.EJB wrote the original draft.EJB and YHC reviewed and edited the manuscript.SHH visualized data in manuscript format.YHC supervised the study.CYK,GYK,YJJ,and SC administered the study project.YHC acquired the funding.All authors have read and agreed to the published version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2024年3期
Asian Pacific Journal of Tropical Biomedicine2024年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Pyronaridine combined with diminazene aceturate inhibits Babesia in vitro and in vivo
- Rosmarinic acid improves tracheal smooth muscle responsiveness and lung pathological changes in ovalbumin-sensitized rats
- Icariin ameliorates viral myocarditis by inhibiting TLR4-mediated ferroptosis
- Th17/Treg balance and macrophage polarization ratio in lower extremity arteriosclerosis obliterans
