Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
HUANG Leping ,LI Shidong ,ZHANG Jiabei ,PAN Chenchen ,ZHAO Jinchao
(1.School of Material Science and Engineering,Wuhan Textile University,Wuhan 430200,China;2.Hubei Provincial Engineering Laboratory for Clean Production and High Value Utilization of Bio-Based Textile Materials,Wuhan Textile University,Wuhan 430200,China)
Abstract: The millimeter-scale capsules with controllable morphology,ultra-low permeability and excellent mechanical stability were fabricated by millifluidics.Viscosity of inner phase was adjusted to control the morphology and properties of the capsules.In detail,as the concentration of polyvinyl alcohol (PVA)increased from 0 to 8% in the inner phase of the capsules,the diameter of capsules decreased from 3.33 ± 0.01 mm to 2.97 ± 0.01 mm,the shell thickness of capsules decreased from 0.183 ± 0.004 mm to 0.155 ± 0.003 mm.While the capsules had round shape and high sphericity.Notably,the capsules with 2% PVA in the inner phase had remarkably decreased water permeability and good morphological stability.Specifically,the end-time of water losing of the capsules was up to 49 days,while the dehydrated capsules maintained spherical appearance,and crushing force of the capsules was up to 13.73 ± 0.79 N,which ensured stability during processing and transportation.This research provides a new strategy for stable encapsulation of small molecules.
Key words: capsule;millimeter-scale;millifluidics;polyvinyl alcohol;viscosity
1 Introduction
Compared with micro and nano scale capsules,millimeter scale capsules own high encapsulation efficiency,uniform monodispersity,and excellent dimensional stability,have important application in high energy physics[1,2],food engineering[3],pollutant detection and collection[4],biological medicine[5,6],cell immobilization[7,8]and tissue engineering[9].Conventional preparation method of micro and nano scale capsules,such as emulsion polymerization[10],complex coacervation[11],membrane emulsification[12]and spray drying[13]method,can not be used to fabricate the millimeter scale capsules.The millifluidics[14,15],orifice-bath coagulation[16,17],liquid marbles[18,19]and template[20]method have been developed.Upscaled from microfluidics,millifluidics consists of an assembly of commercially available tubing of millimeter size and chromatography connectors which is a versatile and easy to use approach[21,22].
Particularly as for complex three-phase fluids,such as O/W/O and W/O/W,the millifluidics contributes to formation of the compound droplets.Englet al[23]controlled the shape of the droplets by varying the tube diameter,and enabled droplets in the shape of rods,disks and more complicated shapes.The effect of flow rate ratio on droplets size and thickness had been systematically described by Vladisavljevićet al[24]and Liuet al[25],the results showed that the shell thickness decreases with the increase of the flow rate ratio of middle to inner fluids.Shaoet alinvestigated the effect of the flow rate ratio of inner to middle fluids (x) on the droplet diameter[15],and found that the diameter increased withxwhenx<0.5,decreased withxwhenxwas between 1 and 2,and did not change withxwhenx>3.Panet al[2]also studied the effects of flow rate,interfacial tension and viscosity on droplet diameter and shell thickness,and established empirical correlation with prediction error of less than 10%.Liuet al[26]improved the uniformity of the shell thickness by density matching,which improved the yield of capsules with shell thickness variations of less than 5 μm,increased from 15% to 84%.And the effects of polymer in the outer phase on droplet formation were investigated,such as polymer of PVA and PAA[27].It was found that lower interfacial tension and higher bulk viscosity reduced the occurrence of fracture and coalescence of droplets.For the certain setup,the shape and size of the capsules were mainly controlled by the viscosity,interfacial tension,and the flow rate of the three-phase liquids[2].
Although there had been many efforts devoted to millifluidics,most of studies still focused on controlling of the capsule morphology,the stability and lifetime of the capsules had not been taken into account.Thus,a one-step three phases with vertical setup of millifluidics combined with UV-curing technology was established to realize the fabrication of the millimeterscale aqueous core capsules.The effect of polyvinyl alcohol in inner phase on the capsules was investigated.The relationship between morphology,properties and stability of the capsules was mainly discussed.
2 Experimental
2.1 Materials
Polyurethane acrylate (PUA,UA-2166),diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (TPO) were obtained from Zhongshan Jesida Fine Chemicals Co.,Ltd,China.Polyvinyl alcohol (PVA) (degree of polymerization: 1750 ± 50) was purchased from Sinopharm Chemical Reagent Co.,Ltd,China.Reactive brilliant red was purchased from Nanjing dulai Biotechnology Co.,Ltd,China.Above reagents were used directly without further purification.Deionized water was used throughout the experiments.
2.2 Preparation of the millimeter-scale aqueous core capsules
As shown in Fig.1,a millifluidics was constructed to fabricate compound fluids.The inner,middle and outer sizes of the needle were 19 G/14 G/10 G.The needle was connected to a quartz glass tube with inner diameter and length of 7.5 and 670 mm,respectively.The PVA solution was used as the inner phase fluid,where the solution contained 0.03wt% brilliant red dye for observation.The mixture of PUA and TPO were used as the middle phase fluid.The 6% PVA solution was used as the outer phase fluid.Three syringe flow pumps were used to control the flow rates of inner,middle and outer phase fluids separately.The compound droplets were irradiated by UV light installed on side of the quartz glass tube.The wave length of UV light was 365 nm.The UV intensity was 2800 mW·cm-2.Finally,the millimeter-scale aqueous core capsules were obtained.
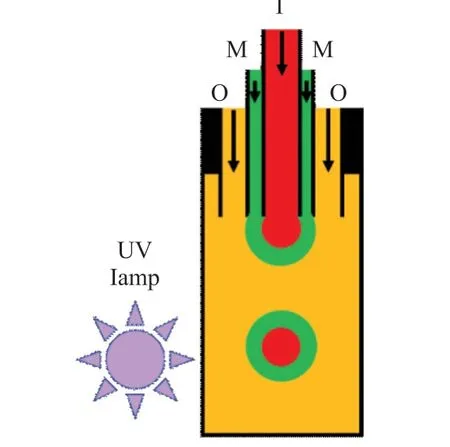
Fig.1 Experimental setup for preparation of the millimeter-scale aqueous core capsules
The viscosity of inner phase(μI) was adjusted.The PVA was added in the inner phase.The concentrations of PVA were adjusted as 0%,2%,4%,6%,and 8%.In middle phase,the concentration of TPO in PUA was 5%.The flow rates of inner,middle and out phases were 200,40 μL·min-1,and 10 mL·min-1.
2.3 Characterization
The viscosities of fluids were measured by a rheometer (MCR 302,Anton Paar®,Austria) with a shear rate of 10 s-1at 25 ℃.Images of the capsules were obtained by a digital microscope (SZ61TR,Olympus,Japan).The dimensions of capsules were measured and the coordinate points were marked by software of OLYMPUS Stream.The data were the averages of 10 measurements for dimension analysis of the capsules.As shown in Fig.2(a),nlines in capsule shell are taken and denoted asL1,L2,⋅⋅⋅,Ln.Thickness(t) of the capsule shell is defined as Eq.(1),
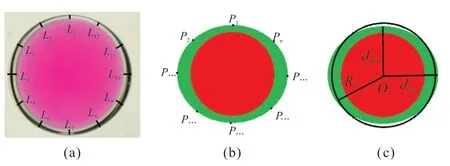
Fig.2 Fitting of approximate circle to characterize morphology of the capsule: (a) Image of capsule;(b) and (c) Fitting schematics
In Fig.2(b),coordinates of multiple points on the outer wall of the capsule shell are taken and denoted asP1,P2,⋅⋅⋅,Pn.Use least squares to fit these points to an approximate circle.The coordinate center and radius of the circle are obtained.As shown in Fig.2(c),center of the circle isO1and the corresponding radius isR1.Diameter of the capsule (DC) is defined as twice ofR1.The sphericity (S) of the capsules is defined as Eq.(2),
where,dmaxis the distance from the farthest point ofPntoO1,anddminis the distance from the closest point ofPntoO1.
The Universal Testing Systems (5943,Instron,America) was used to test the crushing force of capsules with a compression speed of 1 mm∙min-1.The constant temperature and humidity chamber(150LGS,Beijing Labonce Thermostatic Technology Company,China) was used to keep the capsules in 25℃,35 RH% environments.Weight of 50 capsules was measured by an analytical balance (ME104E,Mettler Toledo,Switzerland),then the results were averaged.The weight of capsules was measured in every 7 days until the difference of weight was less than 1 mg for two consecutive times.The water retention ratio (ωm)of the capsules is calculated by the Eq.(3),
where,m1is the initial weight,mnthe weight measured for the last time andmithe weight measured oniday which ranges from 1 ton.
3 Results and discussion
Water and water vapor transport through polymeric barrier materials are of considerable importance in packaging materials[28].Because the water and aqueous solution which can facilitate the encapsulation of water-soluble vitamins and anticancer drugs,carrier of cell culture and microreactor in aqueous phase[29-32]have abroad application in biological,medicine and chemical engineering.However,water permeates to a large extent than permanent gases due to the larger solubility and diffusivity[33].Therefore,resins with high compactness and easy processing are preferred as capsule shells.In this work,formation of compound droplets in millifluidics devices utilized independently controlled flows of immiscible liquid.Water was used as a candidate of inner core to investigate the stability and permeability of millimeter-scale capsules,and UVcurable PUA resin,which had high reactivity,excellent flexibility and solvent resistance,was used as shell to enhance encapsulation of water.PVA was used to adjustingμI.Effect of concentration of PVA on theμIis shown in Fig.3.The viscosity of the inner phase fluid without PVA is 1.06 mPa∙s,as the concentration of the PVA increases from 2% to 8 %,theμIincreases significantly from 4.5 to 310 mPa∙s.In addition,the viscosity of the intermediate phase is 9466.01 mPa∙s.
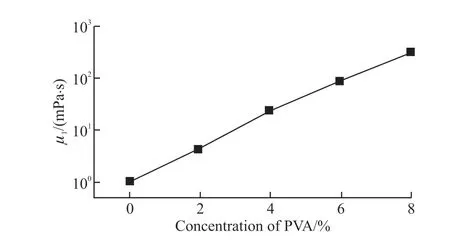
Fig.3 Effect of concentration of PVA on μI
The images of the millimeter-scale aqueous core capsules are shown in Fig.4.It appears that the millimeter-scale aqueous core capsules are beautiful concentric spheres of which the inner phase with plenty of water is encapsulated by the thin layer of middle resin phase.As shown in Figs.4(a)-4(c),the randomly selected capsules have uniform spherical appearance can be fabricated successfully before and after addition of PVA.As enlarged images of the single capsules shown in Figs.4(a1)-(c1),core and shell of the capsules are clearly visible.The capsules change from large to small as the concentration of PVA gradually increases from 0% to 8%.In detail,with increasing ofμIfrom 1.06 to 310 mPa∙s,DCdecreases from 3.33 mm ±0.01 mm to 2.97 ± 0.01 mm by decreasing of 10.81%(Fig.5(a)).Because the drag force is increased with the increase of the viscosity and flow rate of the liquid[25,34].AsμIincreases,increasing of drag force of inner phase leads to easy detachment of the compound droplets,thusDCof the capsules decreases.

Fig.4 Images of the millimeter-scale aqueous core capsules,where the concentration of PVA are (a/a1) 0%,(b/b1) 4%,and (c/c1)8%,respectively.The length of the scale bar for a-c is 3 mm,and for a1-c1 is 1 mm
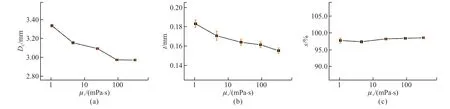
Fig.5 Effects of μI on (a) DC,(b) t and (c) s of the capsules
Theoretically,the shell thickness,t,of compound droplets can be calculated as Eq.(4).
And assuming theDI(diameter of inner phase droplets Eq.(5)) andDC(Eq.(6)) are unchanged before and after UV-curing of middle phase.TheQIandQMare the flow rates of the inner phase and the middle phase,respectively.
where,twis the time interval of droplets formation.
Based on Eq.(4),tof the capsules relies onDC,QMandQI[35].WhenQMandQIkeep constants,tis dependent onDCof the droplets.In Fig.5(b),tof the capsules decreases gradually from 0.183 ± 0.004 mm to 0.155 ± 0.003 mm as theμIincreases from 1.06 to 310 mPa∙s.
Effects ofμIon thesof the capsules are demonstrated in Fig.5(c).As theμIincreases from 1.06 to 310 mPa∙s,thesis always high,between 97.37%± 0.43% and 98.54% ± 0.25%.It indicates thatμIhas little effect ons.
Packaging performance and mechanical stability of the millimeter-scale aqueous core capsules are importance for practical application.Although water is commonly used carrier for active material and energy,water permeability of the capsule shell always has been ignored in previous report.In our previous researches[17,36],water permeability of the millimeter scale aqueous core capsules with calcium alginate shell was investigated.The capsules with hydrogel shell loosed completely inner water within 3 000 min even the hydrophobic modified shell.In this work,the UV-light curing resin is used as capsule shell causes tremendous decline of water permeability of the capsules.An interesting phenomenon is founded that the dehydrated capsules without PVA in inner phase are collapsed as bowls (Fig.6(a)).However,the dehydrated capsules can maintain spherical appearance when the PVA is adding in the inner phase (Fig.6(b)).It is proved that the PVA helps stabilizing and strengthening of inner/middle interface.Effect of the PVA in inner phase onωmof the capsules is shown in Fig.6(c).The capsules without PVA in inner pase lose water completely in 28 days.As 2% PVA is adding in the inner phase,the end-time of water losing prolongs to 49 days althoughtof the capsules decreases.There are two reasons for the prolongation of the end-time of water losing.On the one hand,the PVA solution which has high viscosity and hydrophilicity decreases movement and volatilization rate of water molecular.On the other hand,the capsules do not collapse during the process of water losing after the addition of 2% PVA,at which time,the gas-pressure inside the capsules is lower than atmospheric pressure,which makes it more difficult for water molecules to permeate outside the capsules.As the concentration of PVA increases from 2% to 8%,the end-time of water losing decreases from 49 days to 42 days which ought to ascribe to decreasing oft.However,the end-time of water losing for all capsules with PVA added is higher than 28 days for the capsules without PVA added,which proves that the addition of PVA facilitates retaining water.
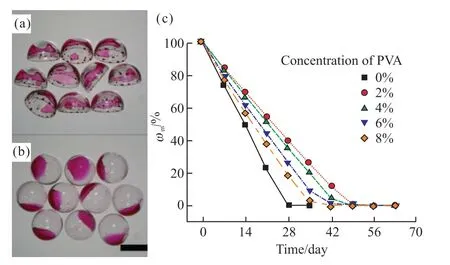
Fig.6 Effects of PVA in inner phase on (a,b) morphology of the dehydrated capsules and (c) ωm of the capsules,where (a) and(b) are images of sample with 0% and 2% PVA,respectively.The length of the scale bar is 3 mm
Same as packaging performance,mechanical property of the capsules is enhanced by using of UV-light curing resin as shell material.As shown in Fig.7(a),three capsules are fixed on the back of Petri dish by double-sided tapes to avoid rolling.1 kg weight stands steadily on the capsules which are not broken.Effect of concentration of PVA on crashing force of the capsules is investigated.From Fig.7(b),it is found that crushing force of the capsules increases obviously from 7.64 ± 0.65 N to 13.73 ± 0.79 N by 79.71%increasing,as adding of 2% PVA in the inner phase althoughtdecreases,indicating that the addition of PVA can increase the crushing force of the capsules.As concentration of PVA continue increases,crushing force of the capsules decreases gradually from 13.73 ± 0.79 N to 10.57 ± 0.51 N ascribed to decreasing oft.However,all of them are higher than the 7.64 N of the capsules without PVA addition,indicating that mechanical stability of the capsule is enhanced by PVA.
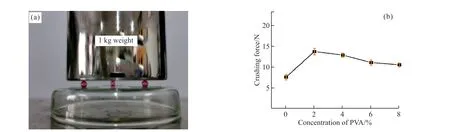
Fig.7 (a) Image of 1 kg weight standing steadily on the capsules of sample 2;(b) Effects of concentration of PVA on crushing force of the capsules
4 Conclusions
In summary,the millimeter scale capsules with aqueous core were prepared with a millifluidics combined with UV-light polymerization method.The results showed that the obtained capsules which owned spherical shape and millimeter-scale diameter had aqueous core surrounded by thin layer of resin shell.In details,μIhad great influences onDC.AsμIincreased from 1.06 to 310 mPa∙s with increasing of concentration of PVA from 0% to 8%,DCreduced from 3.33 ± 0.01 mm to 2.97 ± 0.01 mm by 10.81%decreasing.tof the capsules fluctuated withDC.μIhad little influence ons.Importantly,the permeability of the capsules could be remarkably reduced by adding of PVA in the inner phase.The end-time of water losing of the capsules increased from 28 to 49 days with addition of 2% PVA in the inner phase.While the dehydrated capsules with 2% PVA in the inner phase could maintain spherical appearance.The crushing force of the capsules could be controlled between 7.64 ± 0.65 N to 13.73 ± 0.79 N.
In general,the millimeter-scale aqueous core capsules with controllable dimension,remarkably enhanced packaging performance and excellent mechanical strength were obtained.It will demonstrate significant research and application value in wide range of industrial fields.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
