Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
WU Wanghua ,CHEN Shuichang ,YE Haodong ,LI Shiqian ,LIN Yuanzhi ,CHEN Qinghua ,XIAO Liren
(1.College of Chemistry and Materials Science,Fujian Normal University,Fuzhou 350117,China;2.College of Materials and Package Engineering,Fujian Provincial Key Lab of Coastal Basin Environment,Fujian Polytechnic Normal University,Fuqing 350300,China;3.College of Agriculture,Guangxi University,Guangxi 530004,China)
Abstract: Polyurethane/desulfurization ash (PU/DA) composites were synthesized using "one-pot method",with the incorporation of a silane coupling agent (KH550) as a "molecular bridge" to facilitate the integration of DA as hard segments into the PU molecular chain.The effects of DA content (φ) on the mechanical properties,thermal stability,and hydrophobicity of PU,both before and after the addition of KH550,were thoroughly examined.The results of microscopic mechanism analysis confirmed that KH550 chemically modified the surface of DA,facilitating its incorporation into the polyurethane molecular chain,thereby significantly enhancing the compatibility and dispersion of DA within the PU matrix.When the mass fraction of modified DA (MDA) reached 12%,the mechanical properties,thermal stability,and hydrophobicity of the composites were substantially improved,with the tensile strength reaching 14.9 MPa,and the contact angle measuring 100.6°.
Key words: polyurethane;silane coupling agent;desulfurization ash;modification;mechanical property;hydrophobicity;thermal stability
1 Introduction
Since the 1950s,the production of plastics has experienced exponential growth,triggering a persistent global challenge known as the white pollution crisis.To tackle this pressing issue,numerous countries have adopted plastic recycling programs.For instance,as reported by the National Chemical Laboratory and the PET Clean Environment Packaging Association in India,Europe boasts a recycling rate of approximately 59% for end-use PET,while the United States stands at 31%.Notably,Japan leads with an impressive 93% recycling rate,closely followed by China at an astounding 83%[1].So far,researchers have made significant progress in developing highly efficient and environmentally friendly recycling and remediation methods,which involve depolymerizing plastics under milder conditions[2].These advancements present a practical and sustainable solution to recycle plastic waste,aligning with the goal of responsible waste management.
Polyester polyols from waste PET can be used to synthesize a key material,PU,a promising class of polymers noted for their excellent thermal insulation,lightweight properties and exceptional durability[3].PU exhibits a complex chemical structure featuring a multiphase block copolymer configuration achieved through a well-balanced combination of polyol,chain extender,and polyisocyanate components.The main chain of PU comprises both soft and hard segments,with the former derived from polyol contributing to the material’s flexibility,while the latter,originating from polyisocyanate and chain extender,imparting crucial mechanical and physical properties[4,5].Consequently,by precisely adjusting the ratio of these raw materials,the parameters such as hardness,viscosity,and swelling of PU can be finely tuned,enhancing its versatility and compatibility with a diverse range of fillers[6].However,Challenges such as its susceptibility to water absorption,high production costs,and the potential generation of hazardous substances during manufacturing have constrained its broader usage in construction applications.
Considering the challenges in fabricating polyurethane panels and the potential environmental repercussions stemming from its production process,researchers have recently directed their attention towards a captivating avenue of exploration-the integration of inorganic filler or recycled materials,including mica and zeolite,to partially substitute polyurethane (Fig.1 shows the classification of inorganic fillers and their applications in PU and Table 1 shows some physical properties of inorganic fillers and applications of inorganic filler/PU composites).This emerging domain has garnered considerable interest.For instance,Bouzmane[7]and colleagues investigated the contribution of feldspar and quartz minerals to the mechanical properties of TPU-based composites.The researchers observed a significant enhancement in the mechanical properties of the composites,attributed to the homogeneous dispersion and improved interfacial adhesion of mineral particles to the TPU phase.Meanwhile,A study conducted by Wanget al[8]explored the development of palygorskite/RPUFs nanocomposites through the strategic integration of nanoclay minerals into rigid polyurethane foams.Impressively,When the content of the modified Pal was 8wt% of polyether polyol,the elastic modulus and compressive strength of the Pal/RPUFNs were increased by ca.131% and 97%,respectively.Although ongoing research focuses on inorganic fillers for polyurethane plastic composites primarily centered around minerals,with limited exploration involving direct composites utilizing industrial by-products like fly ash[9].To the best of our acknowledge,there is no report of PU/DA composite.

Table 1 Some physical properties of inorganic fillers and applications of inorganic filler/PU composites
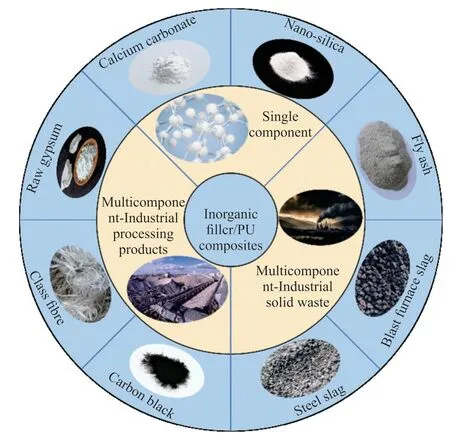
Fig.1 The classification of inorganic fillers and their applications in PU
DA is generated by introducing lime water or lime powder into the desulfurization tower,where a high atomization nozzle aids in its dispersion.The lime-laden DA interacts with the high-temperature flue gas within the sealed confines of the building,effectively neutralizing sulfur dioxide and giving rise to industrially valuable by-products,primarily comprising CaSO3and CaSO4.Meanwhile,minor quantities of fly ash,Ca(OH)2,and CaCO3are present in the resultant mixture.The conventional disposal of DA via landfill poses inherent groundwater and soil contamination risks.Nonetheless,owing to its substantial SO3content and volcanic ash activity,DA finds an alternative purpose in fabricating gypsum board,desulfurized gypsum,and other building materials.This sustainable approach repurposes DA and mitigates its negative environmental impact.In recent research endeavors,scholars have harnessed the unique attributes of DA,such as its fine particle size,recyclability,rapid production capabilities,minimal waste residue,and absence of wastewater,to craft highly efficient and environmentally friendly wall materials[10].Furthermore,a desulfurization ash-based grouting fire prevention material was prepared by Fenget al[11]according to the ingredient design,its compressive strength could reach 6.8 MPa and had suitable fire prevention properties.It notably curtails SO3emissions while conserving precious polyurethane raw materials and reducing energy consumption.
However,the physical attributes of composites comprising inorganic fillers and polymers are strongly contingent on the physical properties of particles but also the degree of particle dispersion achieved within the matrix.To improve the dispersion of organic filler within the PU matrix,researchers have tried to enhance the interaction between the polymer and organic filler of layered silicate components with various techniques such as ultrasonication[12],solution blending[13],in-situ polymerization[14,15],the use of ionic liquid[16].Notably,the realization of organic-inorganic hybrid materials through coupling reactions has gained considerable traction[17].For instance,Wuet al[18]undertook the development of fly ash-filled composites by employing polyurethane-modified epoxy resin as the matrix.In fractography observation of composites,it was evident that the integration of a silane coupling agent yielded enhancements in the interfacial bonding interactions between the fly ash particles and the polymer matrix.All of the above show that DA has good environmental protection characteristics when applied to polyurethanebased building panels.
In this study,the structural features and behavior of desulfurization ash-filled polyurethane-based building panels were systematically investigated.The DA was modified by adding KH550 to improve the densification and thermal stability of PU/DA composites.The micro-mechanisms of the PU/DA composite system before and after modification were characterized using Fourier transform infrared spectroscopy (FTIR),X-ray diffraction (XRD),scanning electron microscopyenergy dispersive spectrometry (SEM-EDS),and thermo-gravimetric analysis (TGA).This work utilized DA fillers further to improve the overall performances of PU-based building panels.And it provided a theoretical basis for applying inorganic solid waste and recycled polyester polyol as raw materials for effective lightweight and green PU-based building panels.
2 Experimental
2.1 Materials
Polyurethane two-component A and B materials(PU-A,PU-B) were supplied by Beijing HUATENG Hightech Corp.Table 2 shows their behaviour and main chemical compositions.One of the main components of PU-A,poly (1,2-propylene glycol adipate),was derived from waste PET.DA was produced by Sanming Steel group,and it mainly consisted of CaSO3·0.5H2O (59.34%),SO3(4.78%),CaSO4(2.2%),Ca(OH)2(2.18%),and a small amount of SiO2and Al2O3.Fig.2 shows DA’s particle size distribution and scanning electron microscope diagram.The modifier of DA was silane coupling agent KH550(γ-aminopropyltriethoxysilane,purity 98%),purchased from Shanghai Taitan Scientific Corp.

Table 2 Properties of PU-A and PU-B and their main chemical composition

Fig.2 DA’s particle size distribution (a) and SEM image (b)
2.2 Sample preparation
In the context of this experiment,direct surface modification of DA proves unsuitable due to its amorphous agglomerate nature.Consequently,the“one-pot method” is adopted,wherein intermixing DA with KH550 is employed to effectuate the desired modification of DA.The process for preparing PU/DA composites is depicted in Fig.3,and comprehensive details are presented below.
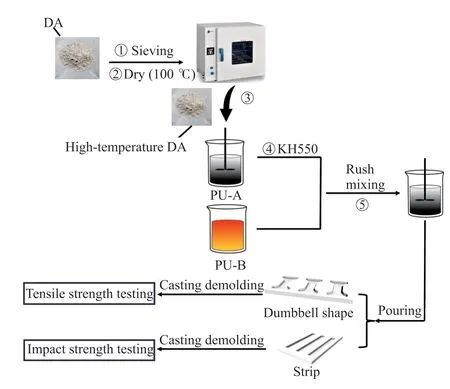
Fig.3 Schematic diagram of the preparation process of PU/DA composites
According to the formulation shown in Table 3,the corresponding raw materials were weighed.Initial treatment involved dehydrating DA in a drying oven at 100 ℃ for 12 h.Subsequently,the DA was added to PU-A in a specific proportion and mixed evenly.Afterward,1% of the DA mass of KH550 was introduced to the mixture.Finally,PU-B was added to PU-A at a fixed ratio and stirred until the reaction became exothermic.Stirring was then terminated,and the slurry was gradually poured into polytetrafluoroethylene (PTFE) molds whose surfaces were treated with a release agent.The paste was cast into dumbbells and strips as required.The samples were then cured in a 20±2 ℃ oven to ensure dimensional stability.After 9 hours of curing,the molds were demolded,and the samples were left to cure naturally in an indoor environment for 2 days.
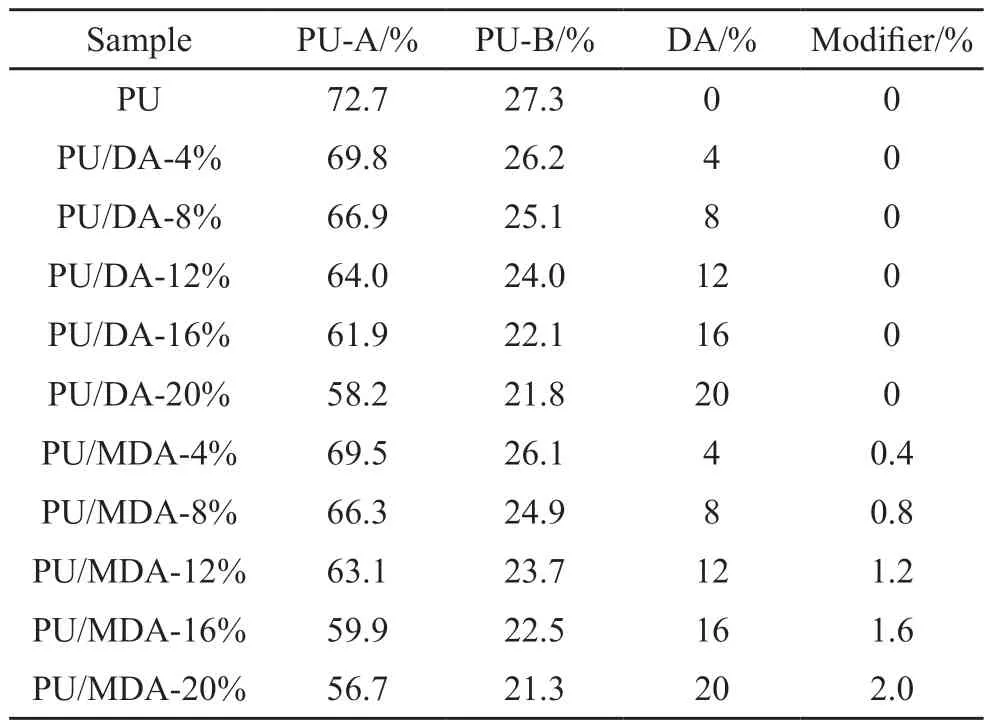
Table 3 Formulations of PU and PU/DA composites
To ensure the utmost fineness and uniformity of DA,we underwent meticulous pre-screening utilizing an 80-mesh standard test sieve.
2.3 Basic properties of samples
The PU/DA composite is shown in Fig.4.The samples exhibited exceptional structural integrity without any signs of chipping,and quantifying linear dimensional changes and mass variations rigorously evaluated their dimensional stability.In-depth data analysis revealed that the amount of DA added exerted no discernible influence on the dimensional stability of the PU/DA composites.Remarkably,the values of linear dimensional changes remained consistently close to 1%,while the mass changes hovered around 0.5% for all samples.These observed values fall well within the acceptable limits prescribed by relevant construction materials standards,where the dimensional stability of samples should ideally be less than 3% (measured over 24 hours at 70 ℃)[26].The analyzed samples met the above requirements and were ready for the following mechanical tests.

Fig.4 A sample of PU (a) and PU/DA composites(b)
2.4 Performance testing
The tensile and impact strength of the samples were tested according to the Chinese Standard GB/T 2567-2021.A microcomputer-controlled precision electronic servo tensile testing machine (HS-3001A-S),and its tensile maximum capacity was 500 kN.The samples at 25 ℃ and 50% relative humidity were tested until they were damaged at the tensile loading speed of 100 mm/min.A simple beam impact tester (H-S807B)performed impact tests on the samples at an impact rate of 2.9 m/s.Meanwhile,surface wettability was analyzed by measuring the contact angle of deionized water with the material surface using the Kreuz DSA25 goniometer.
2.5 Characterization method
XRD (Uitima Ⅳ,China) was used to qualitatively identify the mineralogical phases of the samples with 2θ=5°-80°.To characterize the internal mineral crystal structure and organic-inorganic phase interface structure of the samples,the morphologies of the samples were observed by using scanning electron microscopy (SEM,Regulus 8100,Japan).EDS elemental mapping was also collected using an energy spectrometer (Octane Elect Plus,USA).FTIR analyses of the materials were carried out using a Nicolet 380 FTIR (USA) in the range of 500-4 000 cm-1with a resolution higher than 0.5 cm-1.TG analysis was performed using a TGA2 thermal analyzer (Switzerland) with the samples in a nitrogen atmosphere with a flow rate of 50 mL/min at 30-800 ℃ and a ramp rate of 10 ℃/min.This analytical method was used to estimate the decomposition temperature and content of the samples quantitatively.
3 Results and discussion
3.1 Mechanistic analysis
DA undergoes a high temperature drying process,resulting in the main component,CaSO4.The CaSO4readily hydrates in the slurry,crystallizing into CaSO4·2H2O,characterized by two crystalline water molecules.These water molecules’ hydroxyl groups(-OH) combine with calcium ions (Ca2+) and sulfate ions (SO42+),leading to the presence of -OH on the surface of DA.Fig.5 presents the synthesis of PU/MDA and the crosslinking mechanism involving KH550 and the PU matrix on DA.When KH550 is vigorously stirred in the slurry,it undergoes hydrolysis,generating silanol groups that subsequently condense with the -OH groups on the surface of DA,forming a strong -SiO-M(M represents DA) covalent bond.Simultaneously,the silanol groups further condense,forming an interfacial layer that covers the surface of DA,resulting in a highly organized surface.This entire experimental process facilitates the transformation of DA from hydrophilic to hydrophobic,effectively increasing the compatibility between the inorganic and organic phases.In addition,the amino group at the end of KH550 reacts with the cyano group at the other end of MDI,generating the ureido group,which promotes the increase of crosslinking density of the organic phase[27].Therefore,KH550 plays a crucial role in influencing the properties of PU/DA composites.
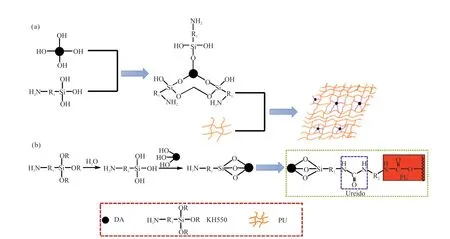
Fig.5 Schematic of the synthesis of PU/MDA(a) and the crosslinking mechanism involving KH550 and the PU matrix on DA (b)
3.2 Mechanical properties of PU/DA composites
Tensile and impact strength are crucial indicators of fracture resistance and toughness,respectively,in materials.Therefore,a comprehensive study of these two mechanical properties in composites is paramount,especially when considering their application as a mechanical support in construction materials.The influence of differentφof DA on the mechanical properties of PU/DA composites,both before and after the addition of KH550,is elucidated in Fig.6.
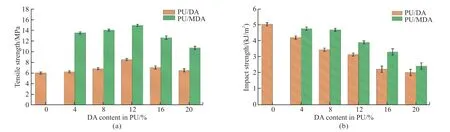
Fig.6 Effect of different weight percent of DA on the mechanical properties of PU/DA composites before and after the addition of KH550(effect on tensile strength (a) and effect on impact strength (b))
Starting with Fig.6(a),the tensile strength of the composite material initially shows an increase and then begins to decline asφincreases from 0 to 20%.This phenomenon can be owing to the inherent strength of DA,which contributes to reducing deformation and strengthening hardness in the composite.However,excessive amounts of DA tended to agglomerate,increasing porosity within the polyurethane matrix.Consequently,the cross-sectional area of the specimen diminished,and stress concentration occurred,resulting in a decline in the mechanical properties of the composite.Fig.6(b) exhibits that the impact strength gradually decreases with increasing filler content.However,a noteworthy observation was that the introduction of KH550 significantly improved the samples’ tensile strength and impact strength compared to their unmodified counterparts.At a modified filler content of 12%,the samples’ tensile strength reached a peak value of 14.9 MPa,exhibiting a remarkable 75.3%increase over the PU/DA-12% configuration.Similarly,the impact strength experienced a 13.3% increase upon modification with KH550.The escalated performance can be attributed to forming a thin,dense interfacial layer between the filler and the matrix facilitated by KH550.This interfacial layer efficiently transfers stress and enhances the interface’s adhesive strength and interaction capacity[28].
3.3 Hydrophobic analysis of PU/DA composites
Contact angle measurements were performed to analyze the behavior of different DA concentrations as filler on PU (Fig.7).Fig.7(a) shows the apparent contact angle measurements of PU/DA composites.With the increase inφ,the apparent contact angle gradually increases,signifying the strengthened hydrophobicity of PU upon adding DA.It was essential to note that the introduction of KH550 further improved the composites’ hydrophobicity.However,whenφ>12%,the porosity of the PU/DA composites became substantially large,resulting in the absorption of water droplets by the pores on the solid surface.As a result,the hydrophobicity of PU/MDA-16% experienced a decline.Fig.7(b) showcases the apparent contact angles of PU and PU/MDA-12% at 59.0° and 100.6°,respectively.It is the presence of polar groups such as urethane groups,ester groups,and ether bonds in the chain segments of the PU molecule chain that makes large surface energy,indicating that the hydrophobicity of the unfilled polyurethane is poor.But the contact angle increased when the PU was modified by silane and DA.The result shows that the reaction of polar groups in the PU molecular chain with KH550 and the introduction of non-polar molecule such as SO3.Thus,the roughness and surface energy of PU/DA composites had been ameliorated,and the hydrophobicity of PU/DA composites was clearly improved.These characteristics prove to be beneficial for combining the DA and the PU matrix,further improving the properties of PU/DA composites.
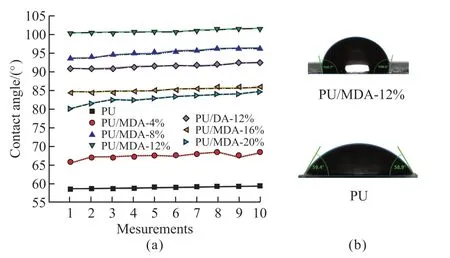
Fig.7 (a) PU/DA composites apparent contact angle and apparent contact photos;(b) PU/MDA-12% and PU
3.4 Microstructure assessment of PU/DA composites
To further verify the reinforcement and hydrophobicity mechanism of the desulfurization ashfilled polyurethane-based composite system,PU/DA composites were selectively selected to characterize and analyze the constitution and structure of the samples by XRD,SEM-EDS,FTIR,and TG.
The XRD correlation patterns of PU/DA composites are shown in Fig.8.The prominent hydrated products are CaSO4·2H2O.Minor characteristic peaks of Ca(OH)2and CaSO3·0.5H2O also are discernible in the material’s curve after incorporating DA.It is worth noting that PU itself possesses a certain level of crystallinity.The PU chain segments in crystalline PU can adopt an ordered crystal structure,resulting in multiple diffraction peaks due to PU’s crystallinity and grain size variations.Diffraction peaks are observed near 2θ=29°,which indicates stereotyped crystallization of the hard segments in PU.This phenomenon primarily arose from the rotation of urethane bonds,leading to a non-coplanar molecular backbone.The crystalline diffraction peak at 2θ=48°primarily stemmed from the crystalline diffraction peak formed by substituting the benzene ring on the MDI.Furthermore,a comparative analysis of the XRD curves of PU/DA-12% and PU/MDA-12% highlights the influence of KH550 addition.It is evident that the introduction of KH550 results in an increased content of hard segment crystals in the composites,thereby favorably heightening the materials’ mechanical strength.This insightful correlation between the XRD patterns and the mechanical properties elucidates the role of KH550 in reinforcing the PU/DA composites.

Fig.8 XRD correlation patterns of PU/DA composites
The above phenomenon is supported by the SEMEDS,as shown in Fig.9,which presents the SEM micrographs and elemental distributions of PU/DA composites.The fractures belong to brittle fractures,as illustrated in Figs.9(a)-9(d).The fracture surface of pure PU is flat and smooth,with many cells (Fig.9(a)).The DA particles are uniformly distributed in the organic matrix with few cells,and the fracture surfaces of the materials are relatively rough in Figs.9(b)-9(d).As a nucleation site,DA particles hinder cell growth and lead to an increase in the viscosity of the system[29].With the filling of DA,significant agglomeration and interfacial fault occur,indicating that no chemical crosslink is formed between the DA and the PU matrix (Fig.9(b)).When the composites are subjected to an external force,the stress cannot be effectively transmitted from the PU matrix to the rigid DA particles,resulting in stress concentration at the interface of the partial phase and a decline in the overall strength of the composites.The cross-sectional morphologies of PU/DA composites modified by KH550 are shown in Figs.9(c)-9(e).On the one hand,it can be found that MDA is fully coated by organic PU matrix at this time (Fig.9(c)).The interface between the two phases is closely bonded.The addition of KH550 eliminates the discontinuity at the boundary,thus reducing the stress concentration.On the other hand,the adhesive structure is observed from the further enlarged graph in Fig.9(e).When KH550 is successfully connected to DA and PU,the DA particles are not entirely stripped from the organic PU by external force,implying that composites toughness to be improved.However,whenφ>12%,some large agglomeration may damage the structure of the PU matrix,resulting in the opening of the vesicle walls[30].

Fig.9 SEM and SEM-EDS images of PU/DA composites: PU (a);PU/DA-12% (b);PU/MDA-12% ((c),(e),(f),(g));PU/MDA-20% (d)
Moving to Fig.9((f),(g)),the CaSO4·2H2O crystals of PU/DA composites present acicular structures,which may be due to the alkalinity of the liquid phase at the early stage of hydration of composite pastes such as Ca(OH)2in DA[10].It mainly contains Ca,O,and S.The EDS surface scan shows that Ca,O,and S are not uniformly distributed.This crystal contains vital ionic bonding substances with high filling efficiency and reinforcement[31].Thus,the formation of CaSO4·2H2O crystals is favorable to building up the compactness and strength of the composites.
The major chemical groups and organic compositions of PU/DA composites were analyzed by FTIR before and after the addition of KH550.As shown in Fig.10(a),the absorption peak at 1 731 cm-1is attributed to C=O in polyurethane prepolymer,the telescopic vibration peak of N-H at 3 337 cm-1,and the telescopic vibration peak of N-H of amide at 1 597 cm-1,which are the characteristic peaks of urethanes.Fig.10(b) shows absorption peaks at 2 931 cm-1and 1 450 cm-1attributed to the asymmetric stretching vibration peak of C-H and the stretching vibration peak of C=O in urethane esters,respectively,which indicates that there are allophanates formed in the polyurethane molecular chain.In Fig.10(c),the Si-O-C stretching vibration peak at 1 180 cm-1and the absorption peak of the urea group at 1 641 cm-1were added,and these peaks were only weakly broadened due to the relatively small amount of KH550 added;the -NH2stretching vibration peak of KH550 was not found at 3 400 cm-1,suggesting that the occurrence of -NH2and -NCO addition reaction,and KH550 has been successfully grafted onto the PU molecular chain[32].The crosslinking results of KH550 molecules on DA particles and PU molecular chains can be further confirmed by thermogravimetric analysis.

Fig.10 FTIR analysis of PU/DA composites: (a) PU;(b) PU/DA-12%;(c) PU/MDA-12%
3.5 Thermal stability of PU/DA composites
The thermal degradation behavior of DA,PU,and PU/DA composites under the N2atmosphere was analyzed by TGA.Fig.11 shows the thermogravimetric(TG) and differential thermogravimetric (DTG) curves.The following thermal parameters calculated by the TG/DTG curve are shown in Table 4-T5%represents the initial degradation temperature corresponding to a 5% weight loss;T1andT2represent the temperature corresponding to the maximum degradation rate of the first and second degradation phases,respectively;andR1andR2represent the maximum degradation rate of the first and second degradation phases,respectively.
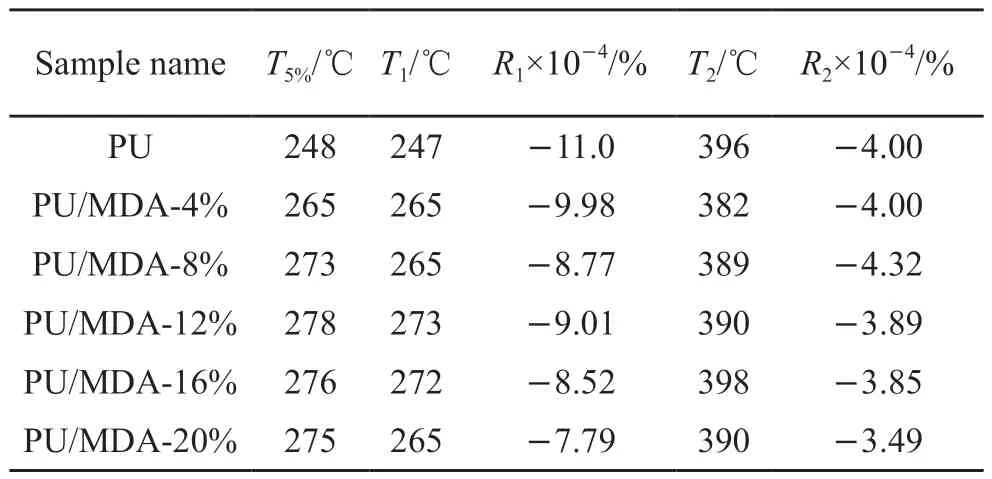
Table 4 Characteristics of thermal degradation of PU/DA composites
Upon analyzing the TG curves,the heat treatment process of DA exhibits two distinct stages of weight loss.In the initial stage (T<570 ℃),an 8% weight loss was primarily attributed to the decomposition of Ca(OH)2,accompanied by the volatilization of water of crystallization and SO3.In the subsequent stage (570-695 ℃),the weight loss resulted from the decomposition of CaCO3and CaSO3[33].
The thermal degradation of PU involves four thermal decomposition platforms.The first weight loss (10%) occurs in the temperature range of 200-280℃,which could be resulted from the evaporation of water,catalysts,and volatiles remaining in the samples rather than the decomposition of PU itself[34].The twostage character of PU pyrolysis has been documented extensively in the literature.During the decomposition of PU,the initial stage involves degradation,mainly the breaking of carbamate and urea bonds in the hard chain bond[35].The maximum decomposition rate at this stage typically occurs between 280-300 ℃.Subsequently,the degradation was linked to the distribution of bonds in the soft segments of PU[36].Moreover,weight loss still occurs at the 600-750 ℃ stage,which was caused by some additives and impurities contained in PU-A.At the end of the thermogravimetric test,PU left residues such as unburned charcoal and most inorganic salts.
As shown in the Table 4,theT5%of the PU/DA composites was higher than that of PU,indicating that the addition of DA increased the initial thermal degradation temperature of PU and delayed the thermal degradation of PU.The positive effect of introducing the inorganic filler into the PU was also observed by Usta.Net al[37].In addition,the composites’T1increased with the introduction of MDA,while the thermal degradation rates of the PU/DA composites were lower than those of PU within this temperature range.This phenomenon occurred because the barrier effect produced by the chemical cross-linking of MDA with PU[38].On the other hand,the introduction of high-energy covalent bonds such as silicon-oxygen bonding (Si-O bonding energy of 550 kJ/mol),whose synergistic effect ultimately led to the improvement of the thermal stability of PU/DA composites.It is noted that T2increased significantly withφ,indicating an interaction between the soft chain segment of PU and DA,thereby improving the thermal stability.In the literature,the heat-resistant performance of the obtained PU composites was found to be comparable or higher than those of the conventional PU.These outcomes allowed to claim that PU/DA composites can be potentially applied as heat-resistant materials in the building field.
4 Conclusions
In this study,a PU/DA reinforcement material was prepared by the one-pot method by adding DA and 1% KH550 and compositing them with PU.After the modification,its mechanical properties and hydrophobicity have been greatly improved.Specifically,its tensile strength and apparent contact angle increase by 75.3% and 69.5%,respectively.The results of mechanical tests and SEM further verify that KH550 effectively mitigates the interfacial differences between organic and inorganic phases,improves the crosslink density and reduces stress concentration.Meanwhile,the TGA results showed that the thermal degradation rate of the PU/DA composites was slowed down,and the thermal stability was improved.The results of microscopic mechanism analysis show that one end of the KH550 molecule crosslinks with the DA surface through hydrolytic condensation,while the amino group and the cyano group at the other end crosslink with the organic matter through the generation of urea groups.In summary,it provides a theoretical basis for improving the overall performance of the samples.Therefore,a lightweight,green PU/DA composite can be developed as a building panel.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
- Photocatalytic Activity Enhancement in Organic Dyes Degradation by Loading Ag Nanoparticles onto α-Fe2O3/ZnOs
