Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
DONG Defu,WANG Wei,ZHAN Chun,LI Chenglong,ZHOU Qisheng,XIAO Shengqiang
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,Wuhan University of Technology,Wuhan 430070,China)
Abstract: By replacing hexyl chains in poly(3-hexylthiophene) (P3HT) with 2-propoxyethyls,four poly(3-(2-propoxyethyl)thiophene) (P3POET) homopolymers with comparable polydispersity indexes(PDI) and regioregularities were prepared herein in addition with step increment of about 7 kDa on numberaverage molecular weight (Mn) from around 11 to 32 kDa (accordingly denoted as P11k,P18k,P25k,and P32k).When doped in film by FeCl3 at the optimized conditions,the maximum power factor (PFmax)increases greatly from 4.3 μW·m-1·K-2 for P11k to 8.8 μW·m-1·K-2 for P18k,and further to 9.7 μW·m-1·K-2 for P25k,followed by a slight decrease to 9.2 μW·m-1·K-2 for P32k.The close Seebeck coefficients(S) at PFmax are observed in these doped polymer films due to their consistent frontier orbital energy levels and Fermi levels.The main contribution to this PFmax evolution thus comes from the corresponding conductivities (σ).The σ variation of the doped films can be rationally correlated with their microstructure evolution.
Key words: conjugated polymer;molecular weight;microstructure;thermoelectric performance
1 Introduction
Conjugated polymers have garnered increasing attention in the field of electronic devices due to their advantages such as light weight,processability from solution as well as tunable electronic and optical properties via molecular engineering[1,2].Recently,doped conjugated polymers with improved conductivity have rapidly emerged in the organic thermoelectric community as promising candidates for near-room-temperature thermoelectric materials[3,4].Compared to their inorganic counterparts,doped conjugated polymers exhibit their merits like nontoxicity,intrinsically low thermal conductivity and mechanical flexibility[5,6].Consequently,thermoelectric devices based on doped conjugated polymers are highly anticipated to serve as self-generating power supplies for wearable and portable electronics[7,8].However,their passable thermoelectric performance renders it imperative to be further improved for expanding their application scenarios and enhancing their commercial value.Due to the microstructure complexity with locally varying degrees of order and disorder in conjugated polymers,the transport behaviors of charge carriers within them have been demonstrated to be unique[9,10].Moreover,the effects of dopants and doping processing on their film morphology would further increase the complexity of the structure-performance relationship when doping conjugated polymers[3,11].In recent years,some reports have demonstrated that the microstructure characteristics (eg,crystallinity,intermolecular stacking distance and orientation)in a neutral conjugated polymer film have a great influence on its doping efficiency as well as charge transport and energy distribution (eg,Fermi energy and transport energy) after doping[12-16].These factors would synergistically determine the conductivity (σ)and Seebeck coefficient (S) in a doped polymer film and thus its final thermoelectric performance due to their complex dependence on carrier concentration and microstructure[5,12,17].From this perspective,realizing an appropriate state of microstructure matched with real thermoelectric conversion steps is one of the keys for a specific conjugated polymer to maximize its performance[18].
As a crucial structure parameter for a conjugated polymer,molecular weight has been proven to play an important role on its film structure[19,20].Molecular weight control on conjugated polymers thus has been demonstrated efficient to tune film structures for more efficient device performance especially in fields such as organic solar cells and organic fieldeffect transistors[21-24].Quet alonce investigated thermoelectric performance of the homopolymer of poly(3-hexylthiophene) (P3HT) with different numberaverage molecular weight (Mn) of 9.2,21.1,and 42.6 kDa and corresponding polydispersity indexes (PDI)of 1.5,2.3,and 2.4[25].The 21.1 kDa sample provided betterσandSafter doping by Fe(TFSI)3.It was claimed that the suitable chain length of this sample allowed charges to transport through both ordered and amorphous regions with enough connectivity whereas much folding of chains in the 42.6 kDa P3HT film had a negative impact on transport and consequently led to a deteriorated thermoelectric performance.In a recent work of Yoonet althree difluorobenzothiadiazoledithienosilole based D-A copolymers (PDFD-T) withMnof 19,32,and 41 kDa respectively and comparable PDI of around 1.8 were obtained to present the corresponding highest occupied molecular orbital(HOMO) energy levels at -5.30,-5.10,and -5.10 eV[26].The 41 kDa sample after doping by FeCl3achieved the highestσand power factor (PF),which was found to inherit crystallinity and good charge transport path from its neutral film.Obviously,the evolution trend of thermoelectric performance of the above two kinds of polymers is not fully paralleled to each other with increasedMn.The effect of molecular weight on thermoelectric performance of conjugated polymers therefore is urgent to be further disclosed from a viewpoint on the fundamental correlations among molecular weight,microstructure and device performance,especially based on the premise on controlling PDI and frontier orbital energy levels of involved conjugated polymers.
The paradigmatic homopolymer of P3HT has been widely adopted as a platform in the organic thermoelectric field[27,28].Through molecular engineering and film processing optimization on P3HT and its derivatives,some progress has been achieved on investigating the doping mechanism of thermoelectric polymers and developing strategies for performance improvement in recent years[16,29].We once reported a homopolymer of poly(3-(2-propoxyethyl)thiophene) (P3POET) prepared from Rieke polymerization method by replacing the side hexyl chains of the benchmark thermoelectric P3HT with 2-propoxyethyls[30].Compared to P3HT,P3POET offered a more prominent edge-on orientated intermolecular stacking with superior crystallinity in film and better charge transport.In addition,Kumada catalyst-transfer polycondensation (KCTP) is of great power on controlling molecular weight of P3HT and its variants with high regioregularity (RR) and narrow PDI[31].P3POET therefore can rationally employed KCTP method to realize molecular weight tunability with controlled PDI,RR.In this work,four polymers of P3POET with an increment of about 7 kDa onMnfrom 11 to 32 kDa were prepared from KCTP method and marked accordingly as P11k,P18k,P25k,and P32k.Their PDI,RR were all successfully controlled to be comparable to each other.Furthermore,their frontier orbital energy levels are also consistent.Thus,Mnwas controlled as a sole variable to reliably investigate its effect on thermoelectric performance of the resulted homopolymers.Differences on their aggregated structures can be apparently observed among the corresponding neutral films.Thep-type doping of the four polymers in film was performed by immersing them into a solution of FeCl3in acetonitrile.The evolution ofσatPFmaxat the optimized doping conditions is found to be highly related to their film microstructures asMnincreases.
2 Experimental
2.1 Materials
3-Thiophene ethanol was purchased from Beijing Ouhe Technology Co.,Ltd.All other chemicals were purchased from Sinopharm and Energy Chemical,and utilized as received.Anhydrous tetrahydrofuran was obtained by distilling with sodium and benzophenone.The monomer of 2,5-dibromo-3-(2-propoxyethyl)thiophene was prepared from 3-thiophene ethanol according to the literature and the polymer samples were then prepared via KCTP method from this monomer[32].

Scheme 1 Synthetic route of P3POET
Preparation of P3POET samples with different molecular weights: The monomer 2,5-dibromo-3-(2-propoxyethyl)thiophene (656.1 mg,2.0 mmol) was dissolved in 4.0 mL of anhydrous THF in a 50 mL dry double-necked flask with stirring under N2and cooled in an ice bath.1.5 mL of isopropylmagnesium chloride lithium chloride complex solution in anhydrous THF (1.3 M,2.0 mmol) was added dropwise into the reaction solution.After warming the reaction mixture to room temperature and stirring for 4 hours,10.0 mL of anhydrous THF was added to dilute the reaction solution.A certain amount of Ni(dppp)Cl2catalyst at a controlled monomer/catalyst ratio was rapidly introduced into the reaction system,and the reaction system was placed in an oil bath at a set temperature for 12 hours.Specifically,the monomer/catalyst ratio for P11k,P18k,P25k,and P32k are 50,150,300,and 300,respectively.Their corresponding temperature of polymerization are 25,60,60 ℃,and reflux,respectively.When quenching the polymerization with 10.0 mL of HCl solution in water (1 M),a purpleblack polymer solid precipitated out from the reaction mixture and an appropriate amount of methanol was then added into the reaction mixture to further promote coagulation of the polymer.The polymer was collected by filtration and washed with methanol.The polymer was then sequentially extracted with methanol,hexane,and chloroform in a Soxhlet extractor.The targeted product of P3POET was obtained from the extract in chloroform by removing the solution under reduced pressure and further dried under vacuum.
2.2 Processing doped polymer films
The glass substrate was cleaned sequentially with distilled water,acetone and isopropanol for 20 minutes each and treated by UV-Ozone for 10 minutes.The polymer film fabrication and immersion-doping were then both carried out in the N2filled glove box (O2: <0.1 ppm and H2O: <0.1 ppm).For processing a polymer film,a polymer was dissolved in chlorobenzene with a concentration of 30 mg·mL-1.A polymer film with a thickness of 180 ± 3 nm was then obtained by spincoated onto the glass substrate with the controlled spinning rate of 500-800 rpm for 1 minute.For immersion-doping,anhydrous FeCl3was dissolved with concentrations of 1,2.5,and 5 mM in anhydrous acetonitrile respectively.The prepared films of the polymer samples were then immersed in one FeCl3solution for different time.The doped polymer films were then used for TE performance evaluation.
2.3 Measurements and instruments
The molecular weights of the P3POET polymers were determined by gel permeation chromatography(GPC) on a Waters-2414 system.The1H NMR spectra were recorded on a Bruker AV500 at 500 MHz.The thermogravimetric analysis (TGA) was performed on a NETZSCH syncthermogravimetric analyzer(STA449F3) at a heating rate of 10 ℃·min-1under N2.The UV-visible (UV-Vis) absorption spectra of P3POET polymers in dilute chlorobenzene solutions (1×10-5g·mL-1) were conducted on a SHIMADZU UV-1800 spectrophotometer.The UV-visible-Near Infrared (UVVis-NIR) absorption spectra of neutral and doped films were taken on a HITACHI UH4150 spectrophotometer.The cyclic voltammetry (CV) measurements were performed on a CHI 660D electrochemical analyzer with the supporting electrolyte of a 0.1 M solution of tetrabutylammonium hexafluorophosphate(Bu4NPF6) in anhydrous acetonitrile under N2,using a conventional three-electrode configuration with a platinum wire as a counter electrode,platinum disk electrode as a working electrode,and ferrocene as the internal reference.The scan rate for CV measurement was 80 mV·s-1.The ultraviolet photoelectron spectroscopy (UPS) measurements of doped films were measured with AXIS SUPRA under vacuum (4.0 × 10-8Torr) with an unfiltered He I gas discharge lamp source(21.22 eV).The X-ray photoelectron spectroscopy(XPS) measurements of doped films were conducted on AXIS SUPRA.Atomic force microscopy (AFM) was performed with ScanAsyst mode on Bruker Dimension FastScan.The film thickness was measured on a Bruker DEKTAK XT profilometer.
The metal-insulator-semiconductor (MIS)devices have an architecture of ITO/doped polymer films/insulator/Al.The insulator layers were prepared according to the Refs.[33,34].The top Al electrode with 100 nm thickness was thermally evaporated.The capacitance-voltage (Cp-V) measurement was conducted at a frequency of 100 Hz for ion gel-based devices for AC bias.The carrier density (n) was extracted by Mott-Schottky analysis[27,34].The Grazing incident wide-angle X-ray scattering (GIWAXS)measurements were performed by Xeuss 3.0 SAXS/WAXS laboratory beamline with a Cu X-ray source(8.05 keV,1.54 Å) and an Eiger2R 1M detector in a vacuum environment.The incidence angle was 0.22°.To quantify the orientation ratio in a polymer film,the pole figure was drawn at its (100) peaks (atq=0.3 -0.5 Å-1)[15,35,36].TheσandSof the doped polymer films were measured with Cryoall CTA-3 apparatus under a helium atmosphere.
3 Results and discussion
3.1 Fundamental performance of materials
The molecular weights of the four polymer samples were measured by GPC.The related molecular structure parameters are listed in Table 1.TheMnof these four polymers were found to be 10.6 (P11k),17.9 (P18k),24.5 (P25k),and 32.3 kDa (P32k),with an average PDI value of 1.4 and a standard deviation of 0.1.By comparing the integrated proton peaks ofα-CH2linked to the thiophene unit that corresponding to regioregular (δ=3.09 ppm) and non-regioregular(δ=2.85 ppm) couplings from1H NMR spectra in Fig.1,the RR of the polymer samples were calculated to be about 91%[37].Namely,both PDI and RR values in these polymer samples have been well controlled with little fluctuation.Notably,the conjugation length of P11k with the lowestMnis up to around 62 monomers which greatly surpasses the effective conjugation length of polythiophenes of 20-22 monomers[38,39].TGA measurements in Fig.2(a) indicate that all of the polymers exhibit a weight loss of less than 5%on heating to 360 ℃,suggesting their good thermal stability.
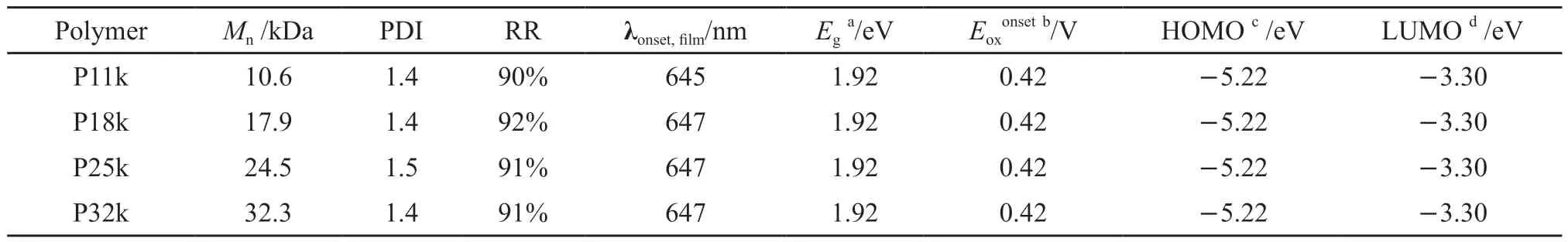
Table 1 Molecular structure parameters,photophysical and electrochemical properties of P11k,P18k,P25k,and P32k
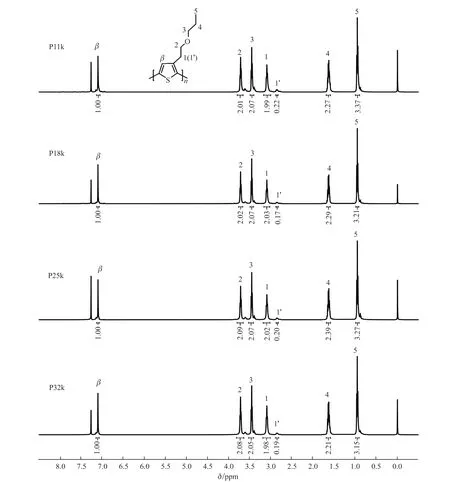
Fig.1 The 1H-NMR spectra of P11k,P18k,P25k,and P32k
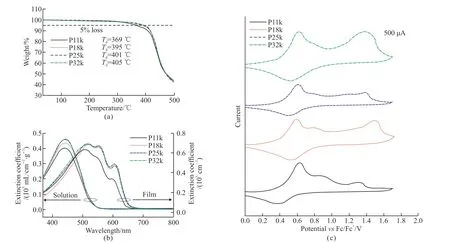
Fig.2 For P11k,P18k,P25k,and P32k: (a) The TGA curves,(b) UV-Vis absorption spectra in chlorobenzene solutions at 1×10-5 g·mL-1 and in films with a thickness of 180 nm and (c) cyclic voltammograms with a scanning rate of 80 mV·s-1
The UV-Vis absorption spectra of the four polymer samples were measured in dilute solutions with a concentration of 1×10-5g·mL-1in chlorobenzene and solid films with a thickness of 180 nm at room temperature.As shown in Fig.2(b),the four polymers all exhibit a single absorption peak at 442 nm originating from the π-π* transition on the conjugated backbone in diluted solution,consistent with the fact that the effective conjugation lengths have all reached saturation in these polymers.When going from solution to solid film,P11k shows three characteristic absorption peaks at 509,543,and 599 nm while a stronger and redshifted (8 nm) absorption feature can be apparently observed for P18k,P25k and P32k asMnfurther increases,indicative of improved ordering of interchain stacking within the latter three films[40].CV measurements (Fig.2(c)) determine the highest occupied molecular orbital (HOMO) energy levels consistently located at -5.22 eV for all four polymers,calculated from their oxidation onset potential (Eonset,ox)versus the internal standard Fc+/Fc located at -4.8 eV with the equationEHOMO=-(Eonset,ox+4.8) eV.Given the same optical band gap (Eg) of 1.92 eV estimated from the onset absorption wavelengths (λonset,film) for all films,the corresponding lowest unoccupied molecular orbital (LUMO) energy levels were then calculated according to the equationELUMO=(EHOMO+Eg) to be-3.30 eV for all polymers.The consistent HOMO and LUMO levels of the four polymer samples suggest the negligible influence ofMnon their frontier orbital energy levels as their chain length has surpassed the effective conjugation length.
3.2 Thermoelectric performance at room temperature
Thep-doping of the four polymer films was achieved by immersing film samples into a FeCl3solution in anhydrous acetonitrile at room temperature.The optimization on the FeCl3concentration (1,2.5,and 5 mM) and the immersion time have been carried out (Fig.3).TheSvalues of the doped P11k,P18k,P25k,and P32k polymers (abbreviated as d-P11k,d-P18k,d-P25k,and d-P32k,respectively) decrease gradually as immersion time increases under each FeCl3concentration.Meanwhile,the correspondingσvalues of these four doped polymer films show a trend of first rising and then slightly falling over immersion time.ThePFmaxs for the d-P11k,d-P18k,d-P25k,and d-P32k films were obtained as immersing accordingly in the FeCl3solution with a concentration of 2.5 mM for 120,120,240,and 240 s.These doping conditions for achievingPFmaxare determined as the optimized doping conditions in this work.As presented in Fig.4(a)and Table 2,the d-P18k film offers a twice largerPFmax(8.8 μW·m-1·K-2) than that of d-P11k film (4.3 μW·m-1·K-2).WhenMnfurther increases,a slight increase onPFmaxis observed in the d-P25k film (9.7 μW·m-1·K-2),followed by a slight decrease to 9.2 μW·m-1·K-2for the d-P32k film.Namely,the three polymer samples reaching 18 kDa provide closePFmaxafter doping,in which P25k is slightly higher than the other two.At the optimized doping conditions,the correspondingSvalues show a slight fluctuation with an average value of 34.05 μV·K-1and a standard deviation of 1.78.Scan be defined as the average entropy per charge carrier weighted by the contribution of the carrier to conduction[17].If the carriers (holes) are only transported in the valence band,Sis given by
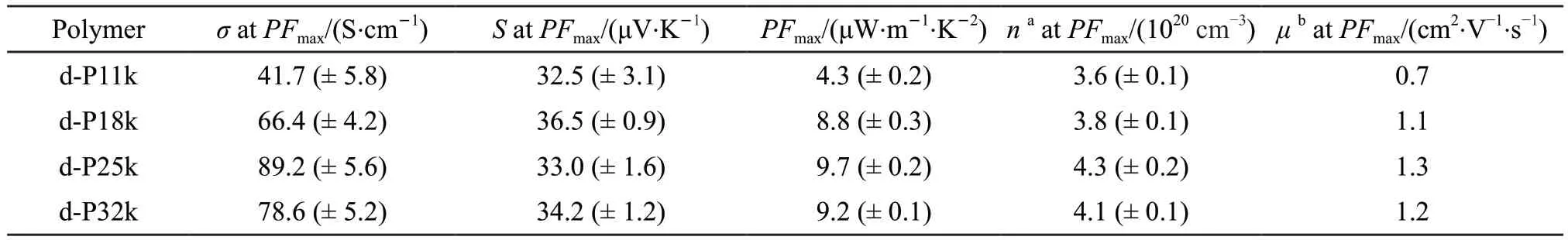
Table 2 The PFmax,the corresponding σ,S,n,and μ at the optimized doping conditions for the doped polymer films

Fig.3 The σ (a-c),S (d-f) and PF (g-i) of the P11k,P18k,P25k,and P32k films immersed in FeCl3 solutions with concentrations of 1.0,2.5,and 5.0 mM respectively for different time (The results for each doped polymer film are the average of at least three film samples)

Fig.4 (a) The PFmax,the corresponding σ and S at the optimized doping conditions vs the according molecular weight of the polymers;(b)UPS scurves of the doped P11k,P18k,P25k,and P32k films at the optimized doping conditions
where,kis the Boltzmann constant,qis the elementary charge,EFis the Fermi level,ETis the transport level which is located near the HOMO level,andAis a dimensionless numerical factor and related to the weighted conductivity density of states[41-45].Notably,for the P3HT films doped by F4-TCNQ at different levels,a simplified version of the mobility edge model equation ofS=(EF-ET)/qTwas deduced by Zouet alfrom neglectingAbecause their Fermi level was at a large distance to the corresponding energy band edges (EF-ET>>kT)[46,47].TheETs of the four polymers are considered to be similar because their consistent HOMO levels.TheSof the four doped polymer films thus depend on theirEFs.The ultraviolet photoelectron spectroscopy (UPS) measurements were performed to investigate theEFvalues of the doped polymer films at the optimized doping conditions.As shown in Fig.4(b),the d-P11k,d-P18k,d-P25k,and d-P32k films provide the similarEFvalues for 5.12 eV.These closeEFandETvalues among the doped polymer samples result in their consistent values ofEF-ETand their closeSvalues can thus be reasonably explained.Compared to theSvalues with small fluctuations,theσvalues at the optimized doping conditions obviously respond to the increasedMnof the four polymers.Theσvalues of the d-P11k,d-P18k,and d-P25k films show an upward trend for 41.7,66.4,and 89.2 S·cm-1respectively while a slightly decreasedσis observed as 78.6 S·cm-1from the d-P32k films (Fig.4(a)).It thus can be concluded that thePFmaxevolution is dominated by the correspondingσin the doped films.
3.3 Generation of charge carriers and doping efficiency
The UV-Vis-NIR absorption measurement was performed on the four polymer films upon doping with different immersion time as shown in Fig.5 to monitor the generation of charge carriers within the films under the FeCl3solutions with a concentration of 2.5 mM.After immersing for 20 s,a great absorbance decrease can be apparently observed for the characteristic absorption band in the range from 400 to 650 nm from the neutral films.Simultaneously,two new absorption bands appear with the maximum wavelengths around 770 and 2 200 nm,which can be ascribed to the typical P2 and P1 bands of polarons respectively.As the immersion time gradually prolonged from 20 to 480 s,a modest decline was observed continuously in the absorbance of the peak at 520 nm while the absorbance of the P1 and P2 polaron bands kept increasing in each doped polymer film.Notably,only one isosbestic point can be found at around 615 nm for the d-P11k,d-P18k,d-P25k,and d-P32k films,indicating the generation of polarons as the carriers throughout the whole doping process in all films[48].
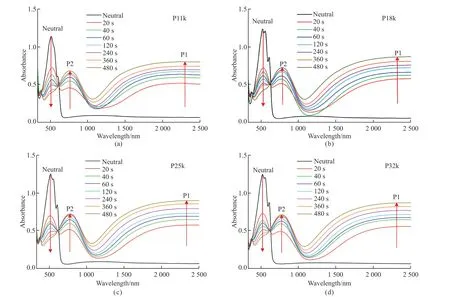
Fig.5 The UV-Vis-NIR absorption spectra of (a) P11k,(b) P18k,(c) P25k,and (d) P32k films doped by immersing in a FeCl3 solution with a concentration of 2.5 mM for 20,40,60,120,240,360,and 480 s
The carrier concentrations (n) of each doped polymer were then extracted from the MIS admittance spectroscopy with the Mott-Schottky equation,
where,e,ε0,andεrare the elementary charge,dielectric constant of vacuum and relative dielectric constant of active layer,respectively[33,34].Theεrof 3.5 was approximately adopted for P3POET films[27].The Mott-Schottky curves of the doped polymer films under the optimized doping conditions are shown in Fig.6.Thenvalues of the d-P11k,d-P18k,d-P25k,and d-P32k films are 3.6 × 1020,3.8 × 1020,4.3 × 1020,and 4.1× 1020cm-3respectively,which are similar to each other.The carrier mobility (μ) was then calculated from the equationσ=neμ.The d-P18k film (1.1 cm2·V-1·s-1) show a 1.5 timesμvalue of the d-P11k film (0.7 cm2·V-1·s-1).WhenMnfurther increases,theμvalue increase slightly to 1.3 cm2·V-1·s-1for the d-P25k film and then decrease slightly to 1.2 cm2·V-1·s-1for the d-P32k film.This variedμis caused by the different molecular stacking orientation tuned byMnin their doped polymer films,which is supported by GIWAXS data (will be discussed further below).Briefly,the largerσvalues of the doped polymers with increasedMncan be mainly attributed to their improvedμthan d-P11k.

Fig.6 The Mott-Schottky plots of doped P11k,P18k,P25k,and P32k films at the optimized doping conditions
Upon doping the polymer films,Fe3+from FeCl3would be reduced to Fe2+by oxidizing the thiophene units within the polymer chains.The oxidation doping efficiency by FeCl3therefore can be alternatively achieved by quantifying the relative content of Fe3+and Fe2+species within the doped films,which can be easily performed from XPS characterizations.The broad Fe 2p XPS peaks from the doped polymer films corresponding to theirPFmaxwere deconvoluted into a Fe2+peak and Fe3+peak as shown in Figs.7(a-d).The doping efficiency was then roughly estimated by comparing the peak integration ratio of Fe2+to the total Fe3+(including reduced Fe2+and unreacted Fe3+)with an accuracy of 10%[49].As shown in Fig.7(e),the doping efficiency of the d-P11k film is 50% while those of the d-P18k,d-25k,and d-32k films all improve to 60%.The lowest carrier concentration of the d-P11k film thus can be partly explain by its inferior doping efficiency.

Fig.7 The XPS spectra of the Fe 2p peaks for (a) P11k,(b) P18k,(c) P25k,and (d) P32k films after doping at the optimized conditions as well as (e) the estimated doping efficiencies of the polymer films accordingly
3.4 Molecular stacking and morphology of neutral and doped films
The multi-scale microstructure within a polymer film is the foothold for carrying out the heat-toelectricity conversion process,which is highly coupled with the thermoelectric performance of the corresponding polymer after doping[12,50].GIWAXS was thus carried out on both the neutral and doped films of the four polymers to explore the effect ofMnon their molecular stacking.The corresponding 2D GIWAXS patterns and 1D profiles along the out-of-plane (OOP)and in-plane (IP) directions are shown in Fig.8.The details on the scattering peaks in 1D profiles are listed in Table 3.In the neutral films,the lamellar stacking distance of the four polymers are both close in the IP and OOP direction.However,an obvious decrease was observed both on the OOP and IP lamellar stacking coherence length (Lc) from the P11k film to the P18k films.The IP and OOP lamellar stackingLcthen exhibits a slight decrease in the P25k and P32k films asMnfurther increases.In addition,the neutral P11k films exhibit a larger IP π-π stacking distance of 3.83 Å than those of the neutral P18k,P25k,and P32k films (3.74 Å).This looser intermolecular π-π stacking in the P11k film is consistent with its weaker absorption feature in Fig 2(b).

Table 3 Summary of detail information on the characteristic scattering peaks and the coherence lengths (Lc) for the neutral polymer films and their according doped films at the optimized doping conditions
When the four polymer films were doped with FeCl3at the optimized conditions,an increase on lamellar stacking distance along with a decline on π-π stacking distance are observed in each polymer film (Table 3).It indicates that FeCl3dopants prefer to interpose into side chain lamellae within the films,which has also been widely observed by the other groups[26,52].Moreover,the observed tighter π-π stacking for all of the four doped films,which may result from the planarization of the polymer backbone via the doping process or coulombic interactions between the positively charged conjugated polymer and negatively charged dopant[26,45,53].The increase of lamellar distance (Δd) for d-P11k is obviously smaller than those of d-P18k,d-P25k,and d-P32k in both IP and OOP directions (Fig.9),suggesting that fewer FeCl3dopants have inserted into the side chain lamellae within the d-P11k film.It could be related to the highest crystallinity of side chain lamellar stacking in the P11k film,which could prevent FeCl3from interposing into the side chain lamellae for effective doping[54].In other words,the weaker side chain lamellae crystallinity of the polymer films,more FeCl3dopants would insert into the side chain lamellae.As such,the Δdvalue reaches the maximum in d-P25k and d-P32k films.Combing the results from the Mott-Schottky analysis and the XPS measurement at the optimized doping conditions,the lower carrier concentration in the d-P11k film thus can be reasonably explained by its least inserted FeCl3dopants and the inferior doping efficiency upon doping.
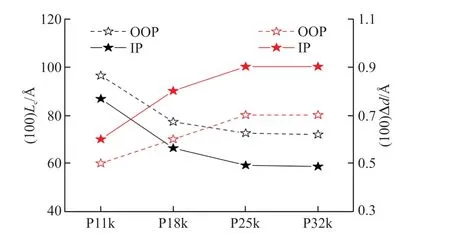
Fig.9 The lamellar stacking Lc along the OOP and IP directions in the neutral films and the Δd in the films after doping
To further investigate the molecular orientated stacking in the neutral and doped polymer films,the pole figures of their (100) peaks were constructed to quantify the fractions of edge-on,face-on and isotropic molecular stacking (fedge-on,fface-on,andfisotropic) (Fig.10)[35,36].In neutral films,thefface-onin each polymer film is around 8%,which is much smaller thanfedge-onandfisotropic.The P11k film shows a lowerfedge-onof 26.2% while a higherfisotropicof 67.5%.AsMnincreases,thefedge-onin the P18k,P25k and P32k films increases to 49.3%,51.4%,and 49.2% while theirfisotropicdecreases to 41.0%,39.9%,and 42.2%,respectively.It indicates the improved structural ordering in the films of these three polymers with higherMn.After doping with FeCl3,thefedge-onvalues of the four polymer films decrease greatly while thefisotropicvalues increase obviously,additionally with a slight decrease onfface-onvalues.Among them,the d-P11k film shows the largestfisotropicvalue of 78.8% as well as the smallest values both onfedge-onandfface-onfor 16.7% and 4.5% respectively,which suggests the most disordered domains in this film.It can be the reasons for its lowestμvalue than the other three.For the d-P18k,d-P25k,and d-P32k films,their longer conjugated chains could facilitate the effective connections between the order domains through the increased disorder zones,which is beneficial for charge transport[55].Moreover,the remainedfedge-onin the d-P18k,d-P25k,and d-P32k films (~30%) is about twice that of the d-P11k film,which is supposed to be favorable for in-plane charge transport in a thermoelectric film[56,57].Notably,the slightly higherμof the d-P25k film can be reasonably explained by its higher remainedfedge-onof 32.4% as well as the lowerfisotropicof 60.4% than the d-P18k and d-P32k film.

Fig.10 The pole figures of (100) peaks as well as the fedge-on,fface-on,and fisotropic for the neutral (a-d) and their according doped films (e-h) at the optimized doping conditions were obtained by performing geometrical correction,sin(χ)I(χ)
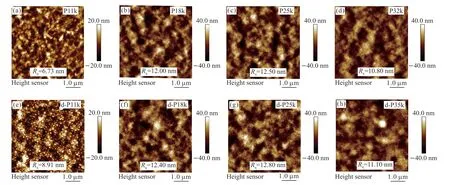
Fig.11 AFM height images of the neutral (a-d) and doped polymer films (e-h) under the optimized doping conditions
The surface morphology of both the neutral and doped polymer films was further studied by AFM.As shown in Figs.11(a-d),the isotropic nodular aggregation structures can be observed in all neutral films.The more uniform film morphology with the smaller nodular aggregation structures resulted in the lowest root mean square roughness (Rq) of the neutral P11k films (6.73 nm) than those of P18k (12.00 nm),P25k (12.50 nm),and P32k (10.80 nm) films.After doping with FeCl3at the optimized doping conditions,apparent particles appeared and dispersed within the original nodular structures of the doped polymer films(Figs.11(e-h)),suggesting the aggregation of FeCl3on the film surfaces[45,52,58].More aggregated particles with larger sizes are observed on the d-P11k films than the other three polymer films.TheRqvalue of the d-P11k films thus exhibits a larger increase of 2.18 to 8.91 nm while those of the d-P18k,d-P25k,and d-P32k films slightly increased to 12.40,12.80,and 11.10 nm,respectively with a rather limited increase of~0.3 nm.This over-aggregation of FeCl3on the d-P11k film surface suggests that less dopants interposed in,which is strongly in line with the results from the GIWAXS measurements.
4 Conclusions
In summary,the doped films of the P3POET samples with variedMnoffer the comparableSowing to their consistent HOMO levels andEFs.The effect ofMnon the thermoelectric performance of P3POET thus primarily manifests through theσvariation as a consequence of the film microstructure evolution.The crystallinity of the side chain lamellar stacking both in IP and OOP directions decrease greatly from P11k to P18k but slightly from P18k to P25k and P32k.Under the optimized doping conditions,a great decrease onfedge-onand increase onfisotropicwere observed in all doped polymer films due to the interposing dopants.The higherfedge-onof 30% in addition with the lowerfisotropicof~60% is maintained in each doped polymer film with increasedMnover 11 kDa,which favors inplane charge transport to obtain its improvedμand thus higherσ.The d-P18k,d-P25k,and d-P32k films therefore provide the closePFmaxfor 8.8,9.7,and 9.2 μW·m-1· K-2respectively,which are about 2.1 times higher than that of the d-P11k film (4.3 μW·m-1·K-2).The d-P25k film shows higherμwith a higher remainedfedge-onand a lower remainedfisotropic,which result in its slightly higherσandPFmaxthan the d-P18k and d-P32k films.This work demonstrates the effectiveness ofMncontrol on tuning film microstructure of a conjugated polymer to improve thermoelectric performance.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
- Mussel-inspired Methacrylic Gelatin-dopamine/Ag Nanoparticles/Graphene Oxide Hydrogels with Improved Adhesive and Antibacterial Properties for Applications as Wound Dressings
- Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
- Synthesis and Characterization of Nonionic Waterborne Polyurethane and Application to Wool Fabric Finishing
- Fluorescent Double Network Hydrogels with Ionic Responsiveness and High Mechanical Properties for Visual Detection
- Damage Mechanism of Ultra-thin Asphalt Overlay (UTAO)based on Discrete Element Method
