Fluorescent Double Network Hydrogels with Ionic Responsiveness and High Mechanical Properties for Visual Detection
ZHENG Wan,LIU Lerong,LÜ Hanlin,WANG Yuhang,LI Feihu,ZHANG Yixuan,CHEN Yanjun,WANG Yifeng
(School of Materials Science and Engineering,Wuhan University of Technology,Wuhan 430070,China)
Abstract: We developed a fluorescent double network hydrogel with ionic responsiveness and high mechanical properties for visual detection.The nanocomposite hydrogel of laponite and polyacrylamide serves as the first network,while the ionic cross-linked hydrogel of terbium ions and sodium alginate serves as the second network.The double-network structure,the introduction of nanoparticles and the reversible ionic crosslinked interactions confer high mechanical properties to the hydrogel.Terbium ions are not only used as the ionic cross-linked points,but also used as green emitters to endow hydrogels with fluorescent properties.On the basis of the “antenna effect” of terbium ions and the ion exchange interaction,the fluorescence of the hydrogels can make selective responses to various ions (such as organic acid radical ions,transition metal ions) in aqueous solutions,which enables a convenient strategy for visual detection toward ions.Consequently,the fluorescent double network hydrogel fabricated in this study is promising for use in the field of visual sensor detection.
Key words: visual detection;ionic responsiveness;fluorescent hydrogels;double network hydrogels;mechanical property
1 Introduction
As functional polymers,stimulus-responsive hydrogels can change their structures and properties in respond to external stimuli,so they are useful in sensors[1],controlled drug release[2],regenerative medicine[3],and other technologies[4-6].Combining stimulus-responsive hydrogels with fluorescent materials(mainly including organic fluorescent dyes[7],semiconductor quantum dots[8],and noble metal nanocrystals[9])can endow hydrogels with fluorescence,and their fluorescent properties usually exhibit selective responses to external stimuli.Therefore,fluorescent hydrogels have broad practical applications in drug delivery[10],sensors[11],and tissue regeneration[12].For example,Zhanget al[8]introduced spherical carbon nanodots (CDs) into hydrophobically associating hydrogels to prepare a new cross-linked hydrogel (HCD).HCD hydrogel had excellent fluorescent performance and photostability,and the fluorescent intensity decreased linearly with the increase of H2O2concentration in the range of 0.01-25 M.Wenget al[13]designed a new europium (Eu)-containing polymer hydrogel using Eu-iminodiacetate (Eu-IDA) coordination in a hydrophilic poly (N,N-dimethylacrylamide) matrix.The hydrogel showed interesting switchable ON/OFF luminescence along with the solgel transition through the reversible formation and dissociation of Eu-IDA complexes upon various stimuli.
Lanthanide ions,as fluorescent materials,have unique characteristics such as high photochemical stability,narrow band emission and low toxicity.The fluorescent hydrogels formed by introducing lanthanide ions into polymer networks have been widely used as intelligent detection sensors,due to the advantages of long service life[14,15]and high color purity[16.17].For example,Zhanget al[18]complexed poly-ligand with Eu3+and Tb3+ions to obtain the red color-emitting poly-ligand-Eu and green color-emitting poly-ligand-Tb hydrogels,respectively.The resulted hydrogels not only possessed excellent fluorescent property (fluorescence quantum yields are high above 60% in the solid state),but also were applied as fluorescence sensors to detect acidic and alkaline vapors reversibly.Zhaoet al[19]prepared a fluorescent hydrogel via the coordination of Ln3+ions to nitrogen atoms from terpyridine moieties tethered to the polymer chains composed of polydimethylsiloxane and hexamethylene diisocyanate segments.The emitting color of the hydrogels was simply modulated by changing the excitation wavelengths and altering the Eu3+/Tb3+molar ratio.It is worth noting that lanthanide ions can coordinate with ligand molecules to form polyvalent lanthanide complexes due to their abundant valence electrons.Therefore,lanthanide ions can be used as ionically cross-linked agents for the preparation of ionically cross-linked hydrogels which have great application value in the field of ionic detection[20].For example,Yuet al[21]constructed luminescent lanthanide metal-organic frameworks to prepare a hydrogel based on the combination of 2’-amino-[1,1’:4’,1’’-terphenyl]-3,3’,5,5’’-europium(EuTPTC-NH2) and agarose.The hydrogels were used for the quantitative visual detection of F-ions in water.
Physically cross-linked hydrogels with excellent flexibility and rapid response characteristics[22,23]have great application prospects in the field of flexible sensor detection[24-26].Physically cross-linked hydrogels formed by nanoscale materials (such as laponite clay and CDs) as cross-linked points are generally referred to as nanocomposite (NC) hydrogels.For the NC hydrogels containing laponite clay (laponite NC hydrogel),laponite clay and the polymer chains form a tight three-dimensional network through hydrogen bondings and electrostatic interaction,so laponite NC hydrogels exhibit excellent mechanical properties.For example,Cuiet al[27]used laponite clay as a cooperative cross-linking agent to prepare a novel physically crosslinked hydrogels with unique self-reinforcing behavior.Gaharwaret al[28]reported a transparent,highly elastic(over 2000% strain) and tough hydrogel by adding laponite clay to the covalently cross-linkable PEG network.Interestingly,laponite clay also can promote the usage of rare earth ions by electrostatic adsorption of ionic complexes on laponite during the preparation of luminescent hybrid materials[29,30].
In this paper,we designed a double network structure to fabricate fluorescent hydrogels with high mechanical properties,ionic responsiveness and visual detection function by combining physically cross-linked hydrogels (laponite NC hydrogel and lanthanide ionic hydrogel).The first network,laponite-polyacrylamide(laponite-PAAM) network,was formed by hydrogen bonds between laponite clay and PAAM.The second network,terbium alginate (Tb-alginate) network,was ionically-crosslinked by coordination interactions between terbium ions and carboxylate ions of sodium alginate (Na-alginate) macromolecular chain.It can be expected that introduction of terbium ions endows the hydrogels with fluorescent characters.More importantly,the “antenna effect” of lanthanide ions provides this fluorescent double network hydrogel with a capacity to respond to multiple ionic stimuli,including aromatic carboxylic acids and heavy metal ions.The ionic responsiveness of the fluorescent hydrogels can be distinguished by naked eyes,which achieves the application of visual detection.At the same time,the characters of double networks and physically cross-linked networks ensure sufficient mechanical properties to the fluorescent hydrogels for the detection application.As far as we know,it is a simple and convenient method to prepare fluorescent hydrogels with the aim to access visual detection fields of application eventually.
2 Experimental
2.1 Materials
Sodium alginate (Na-alginate) was purchased from Aladdin Reagent Co.,Ltd.and used as received.Laponite (92.32wt% of Mg5.34Li0.66Si8O20(OH)4Na0.66,7.68wt% Na4P2O7) was provided by Aoyuan New Material Technology Co.,Ltd.and used after dried at 125℃ for 2 h.Acrylamide (AM),potassium persulfate(KPS),terbium chloride hexahydrate (TbCl3·6H2O) and sodium 4-bromomethyl benzoate were purchased from Sinopharm Chemical Reagent Co.,Ltd.and used as received.Deionized water produced by deionization and filtration using a millipore apparatus (resistivity=18.2 MΩ•cm),was used throughout the experiments.
2.2 Preparation of Laponite-polyacrylamide/terbium alginate hydrogels
To obtain laponite-polyacrylamide/terbium alginate (Laponite-PAAM/Tb-alginate) double network hydrogels,the first network was synthesized by free-radical polymerization of AM with laponite as the crosslinking agent,and the second network was formed by crosslinking Na-alginate with terbium ions.First,1.2 g laponite clay was added to 15 mL deionized water for ultrasonic treatment to form laponite dispersion until the dispersion became clear and transparent.Then,5 g AM,10 mL Na-alginate solution (0.05 g/mL) and 1 mL KPS solution (0.02 g/mL) were added into the laponite dispersion by slowly magnetic stirring to obtain uniformly dispersed hydrogel precursor of the first network.All the above procedures were carried out at room temperature.Next,the hydrogel precursor was vacuumized to remove air bubbles and then injected into a glass mold (60 mm×5 mm×5 mm).The hydrogel precursor was polymerized at 60 ℃ for 6 h to form the first network,Laponite-PAAM/Na-alginate hydrogel.Subsequently,the first network hydrogel was immersed in 0.1 mol/L TbCl3solution for 4 h to form the second network,followed by washing with deionized water to remove the uncoordinated terbium ions.Finally,the hydrogel was immersed in 0.013 mol/L 4-bromomethyl benzoate sodium solution with slow stirring for 6 h to obtain a double network hydrogel,coded as Laponite-PAAM/Tb-alginate hydrogel.
2.3 Material characterizations
2.3.1 Fluorescence analysis of hydrogels
Fluorescence of the Laponite-PAAM/Tb-alginate hydrogels was observed under 254 nm ultraviolet (UV)light (970CRT,Shanghai,China) at room temperature.The hydrogel samples were placed in a petri dish and the distance between the UV lamp and the hydrogel was fixed.Fluorescence of the hydrogels was recorded by a digital camera (D3200,Nikon,Japan).
Fluorescence spectra of the Laponite-PAAM/Tb-alginate hydrogels were collected on an RF-5301PC fluorimeter (Kyoto,Japan) at room temperature.The excitation wavelength was maintained at 254 nm for all the measurements.Hydrogel samples were cut into sheets with a defined volume (30 mm×5 mm×1 mm).
2.3.2 Mechanical properties
The uniaxial tensile test was conducted on a WDW-L02 mechanical testing machine (Shandong,China) equipped with a 500 N load cell at room temperature.The strain rate for extension test was kept at 50 mm/min.The Laponite-PAAM/Tb-alginate hydrogel samples were cut into a dumbbell-shaped using a standard cutter.The dumbbell-shaped hydrogel samples had a total length of 60 mm,a total width of 12 mm and a thickness of 1 mm.The narrow section of the dumbbell-shaped hydrogel samples for testing had a length of 30 mm and a width of 4 mm.
2.3.3 Scanning electron microscopy
The microstructure of Laponite-PAAM/Tb-alginate hydrogel was analyzed by field emission scanning electron microscopy (FE-SEM,JEOL JSM7500F,Japan) at voltages of 5.0 kV.After frozen by using liquid nitrogen,the hydrogel sample was immediately transferred into a freeze dryer (FD-1C-50,Beijing,China) to be lyophilized at -20 ℃ for 36 h.The freshly fractured surface of the freeze-dried hydrogel sample was coated with gold by a sputter coater (EM SCD005,LEICA,Germany) under an argon atmosphere before SEM visualization.
3 Results and discussion
3.1 Fabrication and characterization of Laponite-PAAM/Tb-alginate hydrogels
In order to obtain fluorescent hydrogels with ionic responsiveness and excellent mechanical property,a double network structure of the hydrogels is fabricated.NC hydrogel is used as the first network to provide mechanical properties.Ionically-crosslinked hydrogel is worked as the second network to endow the hydrogels with ionic responsiveness.In addition,the double network structure benefits enhancing the mechanical properties.Fig.1 shows the preparation process of Laponite-PAAM/Tb-alginate hydrogels.

Fig.1 Preparation process,microstructure diagram and appearance photos of Laponite-PAAM/Tb-alginate hydrogels
Laponite clay as a reinforcing nanomaterial has octahedral layered structure with negative surface charge and positive edge charge[31].PAAM is a popular hydrogel polymer with a cheap price.They are chosen to fabricate the NC hydrogel (named as Laponite-PAAM/Na-alginate hydrogel).Laponite-PAAM/Na-alginate hydrogel is a pale-yellow opaque hydrogel,synthesized by radical polymerization of AM monomers in the precursor containing Na-alginate polymers,cross-linker laponite and initiator KPS.Hydrogen bonding interaction between oxygen-containing groups on the surface of laponite and amino groups on PAAM makes PAAM molecules cross-link to form the first network.Long molecular chains of noncross-linked Na-alginate are inserted into the first network.The cross-linked mechanism,microstructure diagram and appearance photo of the first network are shown in Fig.1.
Alginate is a polysaccharide composed of α-L-culronate andβ-d-mannuronic acid ester linked by linear arrangement of 1-4 glycosidic bonds[32],which can highly coordinate with polyvalent cations (such as Ca2+,Hg2+,and Tb3+).In order to fabricate the second network,the Laponite-PAAM/Na-alginate hydrogel is immersed in 0.1 mol/L TbCl3solution for 4 h to allow terbium (Tb3+) ions enter into the first network hydrogel through an ion-exchange approach between Tb3+and Na+.Based on coordination interaction between Tb3+ions and carboxylate ions of alginate,alginate molecules dispersing in the first network hydrogel are ionically-crosslinked,and then the double network hydrogel is generated,coded as Laponite-PAAM/Tb-alginate hydrogels.Compared with Laponite-PAAM/Na-alginate hydrogel,Laponite-PAAM/Tb-alginate hydrogel exhibits hardly swelling but no color change.The cross-linking mechanism and microstructure diagram of the second network are shown in Fig.1.Moreover,Tb3+ions are not only used as physically cross-linking points in the second network,but also as green emitters due to their high color purity and luminescent efficiency[33].As shown in Fig.1,the Laponite-PAAM/Tb-alginate hydrogel just exhibits dark green fluorescence under 254 nm UV light,which is caused by the coordination of water molecules with part of Tb3+ions[34].After sensitized in 0.013 mol/L 4-bromomethyl benzoate sodium solution for 6 h,the fluorescent intensity of the Laponite-PAAM/Tb-alginate hydrogel enhances from dark green to bright green,due to the “antenna effect”between terbium ions and sodium 4-bromomethyl benzoate[33].
To verify the double-network microstructure of Laponite-PAAM/Tb-alginate hydrogels described in Fig.1,Laponite-PAAM/Na-alginate hydrogel and Laponite-PAAM/Tb-alginate hydrogel are tested by SEM.It is obvious from Fig.2(a) that the Laponite-PAAM/Na-alginate hydrogel has loose networks with large holes.After Tb3+ions are introduced,Laponite-PAAM/Tb-alginate hydrogel presents dense networks with small holes,as shown in Fig.2(b).The more compact microstructure indicates that Laponite-PAAM/Tb-alginate hydrogel has higher cross-linking density,proving that alginate molecules interspersed in the first network are cross-linked by Tb3+ions to form the second network.
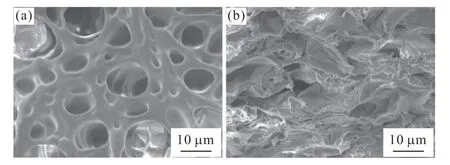
Fig.2 SEM images of Laponite-PAAM/Na-alginate hydrogel (a)and Laponite-PAAM/Tb-alginate hydrogel (b)
3.2 Mechanical properties of Laponite-PAAM/Tb-alginate hydrogels
The Laponite-PAAM/Tb-alginate hydrogel can be stretched to several times of their initial length as shown in Fig.3(a),indicating that the hydrogels have excellent mechanical properties.The fracture strain and fracture stress of the hydrogels containing different content of laponite are shown in Fig.4.With the amount of laponite increasing from 0.6 g to 1.4 g,the fracture strain of the hydrogels continues to increase.However,the fracture stress of the hydrogels increases from 119% to 279% first,and then decreases to 228%when the amount of laponite is above 1.2 g.The Laponite-PAAM/Tb-alginate hydrogel exhibits the best mechanical properties at 1.2 g laponite.The fracture strain reaches the maximum 228% and the fracture strength reaches the maximum 0.6 MPa,which is very close to the mechanical strength of the reported hydrogels with high mechanical properties[35,36].The good mechanical properties of Laponite-PAAM/Tb-alginate hydrogels are attributed to double-network structure and physically cross-linked interactions.For double-network structure,when one network is broken,the other network can keep the hydrogels in one piece until it is destroyed.Even if the hydrogen bond interactions of PAAM and laponite in the first network[37]and the ionically cross-linking interactions of Tb3+ions and alginate in the second network are broken under external forces,it is easy and quick for these two physically cross-linked networks to reform due to the natures of physically cross-linking interactions (see Fig.3(b)).After the content of laponite excesses 1.2 g,the viscosity of the precursor obviously increases,which results in uniform dispersion of laponite in the hydrogels.Stress concentration always occurs in the hydrogels with non-uniform structure under external force,so the breaking strength of the hydrogels decreases when more than 1.2 g laponite is used.At this time,the weight ratio of laponite,AM,Na-alginate and deionized water is settled at 2:10:1:50.This preparation formulation is used to prepare the hydrogels for the following ionic-responsiveness studies.Good mechanical properties make the hydrogels satisfy the requirements of stability and reliability for detection,so the Laponite-PAAM/Tb-alginate hydrogels have potential application in the field of ionic detection sensor.
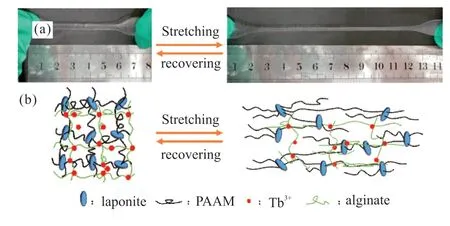
Fig.3 The Laponite-PAAM/Tb-alginate hydrogel was stretched(a);Schematics of the internal structure changes when the hydrogel was stretched (b)

Fig.4 Stress and strain curves of Laponite-PAAM/Tb-alginate hydrogels with different amount of laponite
3.3 Responsiveness of the hydrogels to organic acid radical ions
The unique photochemical property of lanthanum ions endows Laponite-PAAM/Tb-alginate hydrogels with fluorescent property,but the fluorescence is weak before sensitizing the hydrogels by organic acid salts,as shown in Fig.1.Interestingly,the types of sensitization reagents have great effects on the fluorescent property of the hydrogels,which is the source of the responsiveness of the hydrogels to organic acid radical ions.As shown in Fig.5(a),Laponite-PAAM/Tb-alginate hydrogels display different fluorescent colors and brightness after respectively immersed in sodium 4-bromomethyl benzoate solution,sodium salicylate solution and sodium terephthalate solution for 6 h.In sodium 4-bromomethyl benzoate solution,the fluorescent color of the hydrogels changes from dark green to bright green,and the fluorescent intensity increases from 190.65 a.u.to 596.64 a.u.(from Fig.5(b)).Because 4-bromomethyl benzoate ions can completely replace water molecules to coordinate with Tb3+ions in the hydrogels,“antenna effect” makes UV energy transfer from 4-bromomethyl benzoate ions to Tb3+ions,resulting in a fluorescence enhancement of the hydrogels.On the contrary,the fluorescent color of the hydrogel has little change in sodium terephthalate solution and the fluorescent intensity of the hydrogels is 98.32 a.u.,much lower than that in the sodium 4-bromomethyl benzoate solution.Due to high molecular symmetry of terephthalic acid radical ions,parity prohibition which is the electric dipole transition between the 4f-4f configuration of terbium is not eliminated.Energy from UV cannot be transferred from terephthalic acid radical ions to Tb3+ions[38],so the fluorescence of the hydrogels cannot be enhanced.However,the Laponite-PAAM/Tb-alginate hydrogels in the sodium salicylate solution emit bright blue fluorescence and the fluorescent intensity of the hydrogel is very low,5.72 a.u.Just as reported,the fluorescent emission efficiency of sodium salicylate is higher than that of Tb3+ions,so Laponite-PAAM/Tb-alginate hydrogels exhibit the blue characteristic fluorescent color of salicylic acid[39].All above results illustrate the fluorescence of Laponite-PAAM/Tb-alginate hydrogels has selective response to organic acid radical ions in solutions,which reveals their capability for chemical detecting application.

Fig.5 Fluorescence images (a) and fluorescent spectra (b) of Laponite-PAAM/Tb-alginate hydrogels in different organic acid salt solutions
Subsequently,the relationship between the fluorescent property of the hydrogels with the concentration of sodium 4-bromomethyl benzoate is investigated by analyzing the emission peak at 545 nm.As shown in the photos of Fig.6,the fluorescent color of the hydrogels becomes stronger first and then weaker with the concentration of sodium 4-bromomethyl benzoate increasing from 0.001 to 0.05 mol/L.The fluorescent intensity of the hydrogels shows the same trend,with the maximum fluorescent intensity at 0.013 mol/L (see Fig.6).When the concentration of sodium 4-bromomethyl benzoate rises in the solution,more and more 4-bromomethyl benzoate ions replace water molecules to form the coordination interactions with Tb3+ions,so the quenching effect of water gradually disappears and then the fluorescence of the hydrogels becomes stronger and stronger.At the same time,the alkalinity of the sodium 4-bromomethyl benzoate solution increases,which can inhibit the coordination interactions of 4-bromomethyl benzoate with Tb3+ions.Therefore,the hydrogel emits the strongest fluorescence in 0.013 mol/L sodium 4-bromomethyl benzoate solution.This fluorescence-concentration relationship is convenience for us to identify the concentration of sodium 4-bromomethylbenzoic acid by naked eyes or monitoring the fluorescent intensity.
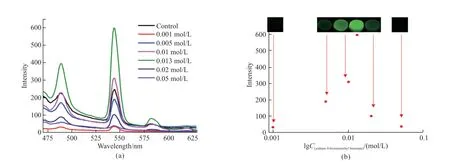
Fig.6 Photoluminescence spectra (a) and photoluminescence intensity and image (b) of Laponite-PAAM/Tb-alginate hydrogels in different concentrations of sodium 4-bromomethyl benzoate
3.4 Responsiveness of Laponite-PAAM/Tbalginate hydrogels to metal ions
Since the ionic bondings of Tb3+ions and carboxylate ions of alginate are a dynamic physically crosslinked interaction,their reversible character ensures that Tb3+ions in the Laponite-PAAM/Tb-alginate hydrogels can be ion-exchanged with other metal ions.As a result,the fluorescence of the hydrogels can make responsive changes after Tb3+ions in the hydrogels are exchanged by other metal ions.
The responsiveness of Laponite-PAAM/Tb-alginate hydrogels to various metal ions is investigated,from alkali to transition metal ions.Fig.7 shows the fluorescent color of the Laponite-PAAM/Tb-alginate hydrogels after immersed in aqueous solutions containing different metal ions for 5 or 60 min.In the solutions with alkali metal ions (Ca2+or Mg2+ions),the hydrogels still emit bright green fluorescence,the same as the control groups,no matter the immersion time is short or long and the ionic concentration of the solution is low or high.Comparing with Ca2+or Mg2+ions,the coordination interaction of carboxylate ions on alginate with Tb3+ions is stronger.Therefore,Tb3+ions in the hydrogels are not replaced by Ca2+or Mg2+ions,and the existence of alkali metal ions has no influence on the fluorescence property of the hydrogels.In contrast,the fluorescence of the hydrogels has changed dramatically after immersed in solutions with transition metal ions (Hg2+,Pb2+,Cu2+or Mn2+).At 1.0×10-2mol/L transition metal ions,the fluorescence becomes weak in 5 min,and quenches in 60 min.Even if the ionic concentration drops down to 1.0×10-4mol/L,the fluorescence weakening can be distinguished by naked eyes.Due to the larger coordination equilibrium constants of transition metal ions and carboxylic acid radical ions[40],transition metal ions effectively replace Tb3+ions to form more stable covalent-like coordination bondings with carboxylic groups of alginate in the hydrogels.Tb3+ions leave from the hydrogels,resulting in the fluorescence quenching.However,it is worth noting that the mechanical properties of the hydrogels have changed scarcely even though the fluorescence disappears.The reason is that the ionic hydrogel networks are maintained by the coordination interactions of carboxylic groups of alginate with transition metal ions.It can be expected that the different response of the fluorescence to transition metal ions and alkali metal ions endows the hydrogels with the application of detecting metal ions.
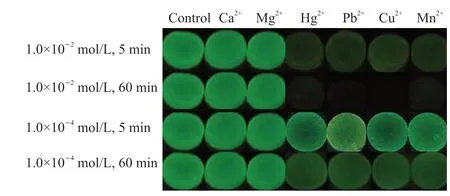
Fig.7 Fluorescence images of Laponite-PAAM/Tb-alginate hydrogels after immersed in aqueous solutions containing different metal ions for 5 or 60 min.The control group is the hydrogels without immersion
3.5 Application on detecting mercury ions
Based on the responsiveness of the fluorescence of the hydrogels to transition metal ions,a method is developed to detect Hg2+ions in solutions by the Laponite-PAAM/Tb-alginate hydrogels.As shown in Fig.8(a),it is found by naked eyes that the fluorescence of the hydrogel is attenuated sharply within 5 min at 1.0×10-2mol/L Hg2+ions.The quick response time benefits to the ionic detection application.5 min later,the fluorescence of the hydrogels continues to become weaker and weaker.Until 30 min,the fluorescence of the hydrogels nearly quenches and slightly changes from 30 to 60 min.The fluorescent spectra also record these changes.As shown in Fig.8(b),the fluorescent intensity (the emission peak at 545 nm) of the hydrogel decreases from 597.79 to 323.45 a.u.within 2 min.The fluorescent intensity reduces to 37.57 a.u.after 30 min,which is similar to the fluorescent intensity (25.03 a.u.)at 60 min.The changes of fluorescent intensity agree with the phenomena of naked eye observation.

Fig.8 Fluorescent image (a) and fluorescent spectra (b) of Laponite-PAAM/Tb-alginate hydrogels with different immersed time (λex=254 nm)
Subsequently,the fluorescent intensity of Laponite-PAAM/Tb-alginate hydrogels is investigated at different concentrations of Hg2+ions.Interestingly,the fluorescent intensity exhibits a downward trend when the concentration of Hg2+ions gradually changes from 1×10-5to 1×10-2mol/L (see Fig.9(a),the emission peak at 545 nm).With the increasing concentration of Hg2+ions,more and more Tb3+ions in the hydrogels are exchanged out by Hg2+ions,so the fluorescent intensity of the hydrogels gradually decreases.A linear relationship between the fluorescent intensity and the Hg2+concentration (see Fig.9(b)) is set up and expressed by a standard linear regression equation:y=-171.83x-315.90 (R2=0.9989).The linear relationship enables the quantitative detection of Hg2+ions in the concentration range of 1.0×10-2-1.0×10-5mol/L.The minimum detection concentration of this method is 1×10-5mol/L,which is lower than the maximum contaminant level for the safe drinking of water (4×10-4mol/L) recommended by the environmental protection agency (EPA),USA[41].
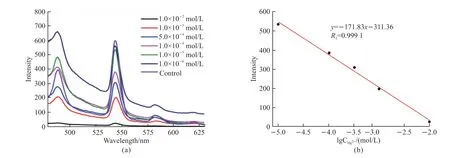
Fig.9 Fluorescent spectra (a) of Laponite-PAAM/Tb-alginate hydrogels immersed in Hg(NO3)2 solutions of 10-2-10-6 mol/L (λex=254 nm)for 60 min and linear diagram (b) of fluorescent intensity to lgCHg2+
Moreover,Hg2+ions are detected by the hydrogels in the presence of alkali metal ions and the results are shown in Fig.10.The fluorescence of the hydrogels is weakened when the hydrogels are immersed in the mixed solution of Hg2+and Ca2+ions,even if the concentration of Ca2+ions is 1.0×10-3or 1.0×10-2mol/L.The degree of fluorescence weakening depends on the Hg2+concentration and increases from 1.0×10-5to 1.0×10-2mol/L.The same result is found in the mixed solution of Hg2+and Mg2+ions.All these indicate that alkali ions have no effects on the response of the hydrogels to Hg2+ions.So,this detection method is reliable in mixed solutions.Therefore,it suggests that the Laponite-PAAM/Tb-alginate hydrogel can be applied as a quantified sensor to detect trace Hg2+ions in aqueous solutions.

Fig.10 Fluorescent images of Laponite-PAAM/Tb-alginate hydrogels immersed in Hg(NO3)2+Ca(NO3)2 mixed solution (a) and Hg(NO3)2+Mg(NO3)2 mixed solution (b)
4 Conclusions
In summary,we have proposed a simple and convenient strategy for fabrication of fluorescent double network hydrogels by facile incorporation of lanthanide ions and laponite nanoparticles.The hydrogels have excellent mechanical properties (breaking strength is 228% and the fracture stress is 0.6 MPa when the amount of laponite is 1.2 g),which provides a guarantee for the stability and reliability of ionic detection.The fluorescence of the hydrogels exhibits the selective responsiveness to different metal ions and organic acid radical ions.4-bromomethyl benzoic acid radical ions obviously causes the fluorescence enhancement,but fails for terephthalic acid radical ions and salicylic acid radical ions.Transition metal ions make the fluorescence weaken even quench,while alkali metal ions have no effects on the fluorescence of the hydrogels.The ionic responsiveness can be observed by naked eyes,so the hydrogels can realize visual detection to various ions.For Hg2+ions detection,the fluorescence intensity has a linear relationship with Hg2+concentration in the range of 1×10-2-1×10-5mol/L,and the limit detection is 1×10-5mol/L.Alkali metal ions do not interfere with the detection to Hg2+ions.The hydrogels still keep an intact shape after experiencing the detection process more than 20 times.Therefore,the Laponite-PAAM/Tb-alginate hydrogels with ionic responsiveness and high mechanical properties provide a new design solution for visual sensing detection materials.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
- Mussel-inspired Methacrylic Gelatin-dopamine/Ag Nanoparticles/Graphene Oxide Hydrogels with Improved Adhesive and Antibacterial Properties for Applications as Wound Dressings
- Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
- Synthesis and Characterization of Nonionic Waterborne Polyurethane and Application to Wool Fabric Finishing
- Damage Mechanism of Ultra-thin Asphalt Overlay (UTAO)based on Discrete Element Method
- Preparation of Laser Cladding Coating Undercooling Cu-based Alloy and Co on Non-equilibrium Solidification Structure
