Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
WU Yanxia ,LIANG Hailong* ,CHEN Yufeng ,HU Liming ,WANG Chunpeng
(Ceramics Science Institute,China Building Materials Academy,Beijing 10002,China)
Abstract: Zirconium-based metal-organic framework UiO-66 was successfully prepared by solvothermal method,and UiO-66 was modified by adding regulators such as formic acid,acetic acid,and hydrochloric acid.The NH3-SCR reactivity of the samples was evaluated by the denitration activity evaluation system,and the UiO-66 and the regulator-modified UiO-66 were characterized by XRD,SEM,BET,FTIR,TG,NH3-TPD,etc.,the effects of regulator types on the structure and properties of UiO-66 were investigated.The experimental results show that,after adding the modifier,the morphology of UiO-66 changes from irregular quadrilateral with serious agglomeration to particles with regular crystal shape and good dispersibility,and the crystal morphology of the catalyst is improved.In addition,after adding the modifier,UiO-66 has a larger specific surface area and stronger surface acidity,which optimizes the catalytic performance of UiO-66.The catalytic performance test results of NH3-SCR show that the low-temperature activity of UiO-66 is poor,and it only shows a certain catalytic activity at higher temperatures.The catalytic activity of UiO-66 was significantly improved after adding the regulator.Among them,the UiO-66-HCl modified with hydrochloric acid had the best catalytic activity,and the denitration rate reached 70% when the denitration temperature was 380 ℃.
Key words: UiO-66 catalyst;catalytic denitration;NH3-SCR;modified
1 Introduction
In recent years,with the development of industrialization,environmental problems such as acid rain and photochemical pollution continue to emerge.Nitrogen oxides (NOx),as one of the main culprits causing these problems,have attracted great attention from the material and environmental circles[1-3].Currently,the widely used commercial catalyst is V2O5-WO3(MoO3)/TiO2,which exhibits good NOxremoval at higher temperature range (300-400 ℃).However,this catalyst has many shortcomings,such as poor low temperature (<300 ℃) activity,narrow activity temperature window,toxic active components,and easy generation of N2O[4-6].Therefore,the search for catalysts with high catalytic activity,multiple active sites and non-toxicity at low temperature has become a research hotspot in the field of denitrification[7].
Metal-organic frameworks (MOFs),as a new class of materials[8-10],have been widely used in the field of catalysis due to their high porosity,multiple active sites and large specific surface area.However,the topological structure of MOFs is unstable,and there is a general disadvantage of poor heat resistance,which limits their further research and application.Zirconiumbased metal-organic framework materials (UiO-66)can be stable at 500 ℃,can withstand a mechanical pressure of 1.0 MPa,and also have strong acid and alkali resistance[11],with good potential for catalytic applications.Therefore,it has attracted extensive attention of researchers.UiO-66 is a rigid metalorganic framework material with metal zirconium as the inorganic ligand and 1,4 terephthalic acid as the organic ligand,which was synthesized by the Cavka research group of the University of Oslo in 2008[12].Li R[13]successfully synthesized zirconium-based metalorganic framework UiO-66 by solvothermal method,and explorably studied the catalytic activity of UiO-66 in CO-SCR reaction.The study found that when the denitration temperature was 170 ℃,the denitration rate of UiO-66-HCl reached 95%.
According to existing literature reports,the synthesis of UiO-66 is affected by many factors,and the regularity of the crystal morphology of the material is an important factor affecting its denitration performance.At present,people have begun to add regulators to nanomaterial precursors to reduce surface tension and surface energy,thereby reducing the degree of aggregation of solid or liquid particles in the dispersion system and keeping the dispersion system relatively stable.And then effectively control the size and morphology of nanoparticles[14-16].Therefore,this paper aimed to find a regulator that can improve the synthesis process of UiO-66 and assist in the synthesis of zirconium-based metal-organic frameworks with high crystallinity and regular morphology.
2 Experimental
2.1 Preparation of catalysts
2.1.1 Preparation of UiO-66
5.83 g zirconium tetrachloride and 4.15 g terephthalic acid were successively added into a beaker containing 375 mL DMF,stirred until dissolved and put into a teflon sealed autoclave,heated at 120 ℃ for 24 h.The products were washed with DMF and methanol for 3 times and collected by centrifugation.Finally,the product was placed in a vacuum drying oven at 120 ℃for 8 h to obtain the metal-organic skeleton material UiO-66 crystals,labeled as UiO-66.
2.1.2 Preparation of modified UiO-66
5.83 g zirconium tetrachloride and 4.15 g terephthalic acid are added successively in the beaker that 375 mL DMF is housed,respectively add the regulator as shown in Table 1,so that the mol ratio of terephthalic acid and regulator is 1: 4.Stir until dissolved and put into a Teflon sealed autoclave and heat at 120 ℃ for 24 h.The product was washed three times with DMF and methanol,respectively,and collected by centrifugation.Finally,the product was dried in a vacuum drying oven at 120 ℃ for 8 h to obtain metal organic framework material UiO-66 crystals,which were labeled as UiO-66-HCOOH,UiO-66-CH3COOH,and UiO-66-HCl.

Table 1 Types and dosages of regulators
2.2 Characterization of UiO-66
The catalyst was characterized by XRD using D8 Advance X-ray diffractometer produced by Brock Company in Germany.The test conditions are shown as follows: the current in the tube was 40 mA,the voltage in the tube was 40 kV,Kα was used as the radiation source,Cu was used as the target,the scanning range was 2θ=10°-80°,and the step was 0.02°.
The surface morphology of the catalyst was characterized by HITACHI S-4800 cold field emission scanning electron microscope (SEM).
The Autosorb-IQ physical adsorption apparatus of Quantachrome was used to test N2physical adsorption of the catalyst,and the specific surface area and pore structure characteristics of the catalyst were investigated.The samples with a certain mass (0.25-0.3 g) were heated and vacuumized at 240 ℃ for 2 h,and then tested in liquid nitrogen (-196 ℃).The specific surface area was calculated by multi-point BET equation,and the pore size and volume were calculated by BJH method.
FTIR was carried out on the FTIR Frontier spectrometer of PE Company.The test background was air,potassium bromide (KBr) tablet method was used to sample,the scanning wave number range was 400-4 000 cm-1,the resolution was 4 cm-1,and the collection times were 64.
Japan Seiko thermogravimetric differential thermal analyzer (6300 TG-DTA) was used for weight loss analysis of the catalyst.The temperature range was 50-800 ℃,and the heating rate was 10℃/min.
The NH3-TPD of the catalyst was characterized by Auto Chem II 2920 chemisorption apparatus.First,50 mg catalyst with particle size of 0.3-0.45 mm was pretreated at 500 ℃ in pure He atmosphere for 1 h,then the temperature was lowered to 50 ℃,and the NH3/He mixture with 2% volume fraction of NH3and flow rate of 30 mL/min was introduced for 30 min,and then the temperature was programmed to 550 ℃ at a heating rate of 10 ℃/min.TCD records signal values.
2.3 Activity evaluation of catalyst
The catalyst activity was evaluated in a stainless steel SCR fixed reactor with external heating in a tubular furnace.The experimental device is shown in Fig.1.The simulated flue gas consists of 0.05%(volume fraction) NO,0.05% (volume fraction) NH3,6% (volume fraction) O2,and N2as the equilibrium carrier gas.The amount of catalyst was 5 mL,the total gas flow rate was 1000 mL/min,and the space velocity was 12 000 h-1.The activity was evaluated in the temperature range from 80 to 360 ℃.NO concentration before and after the reaction was detected by German Testo 350 flue gas analyzer,and NO removal rate was calculated according to Eq.(1).

Fig.1 Schematic diagram of catalyst activity evaluation device
3 Results and discussion
3.1 Analysis of components
The crystal structures of UiO-66 and UiO-66 samples modified with different modifiers were characterized by XRD,as shown in Fig.2.In the XRD spectrum of UiO-66 prepared in this paper,there are five main diffraction peaks at 2θ=7.4°,8.5°,14.8°,17.1°,and 25.8°,corresponding to (111),(200),(222),(400),and (442) planes.This is a typical characteristic diffraction peak of UiO-66.Compared with other peaks,the diffraction peak at 2θ=7.4° is the strongest.It is consistent with the spectrum reported in the Ref.[17],which proves the successful synthesis of UIO-66.After adding different kinds of modifiers,the samples all showed the same characteristic diffraction peaks as UiO-66,indicating that they have the same FCU topology.The use of formic acid,acetic acid,and hydrochloric acid as regulators during the synthesis did not change the crystal structure of UiO-66.
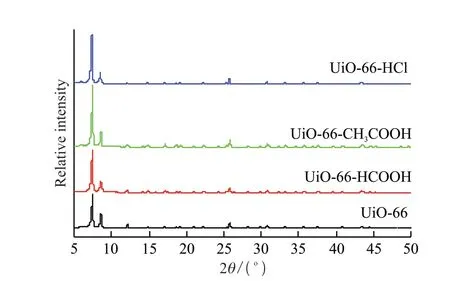
Fig.2 XRD patterns of UiO-66 and UiO-66 samples modified with different regulators
3.2 Micromorphology analysis
Fig.3 shows the SEM images of UiO-66 synthesized by adding different regulators.It can be seen from the figure that the addition of regulators in the process of preparation and synthesis will have a certain effect on the crystal morphology of UiO-66.This is mainly due to the competitive coordination of Zr6 clusters between the regulator and the ligand terephthalic acid after the addition of the regulator.This hinders the coordination linkage between the ligand terephthalic acid and the Zr6 cluster,thereby affecting the growth of UiO-66 crystals[18-20].As shown in Fig.3(a),when no modifier is added,the morphology of UiO-66 is an irregular quadrilateral with poor crystal morphology,low crystallinity,small cubic particles and serious agglomeration.It can be seen from Figs.3(b)-3(d) that after adding the modifier,the surface morphology of UiO-66 is significantly improved,the crystal structure tends to be regular,and the particle dispersion becomes better.When formic acid is added as a regulator,the morphology of UiO-66 is a regular intersecting quadrilateral,as shown in Fig.3(b).As shown in Fig.3(c),when acetic acid was added as the regulator,the morphology of UiO-66 was regular spherical particles.When hydrochloric acid is used as the regulator,as shown in Fig.3(d),the morphology of UiO-66 is between quadrilateral and spherical,showing a quadrilateral shape of vacancy angle.
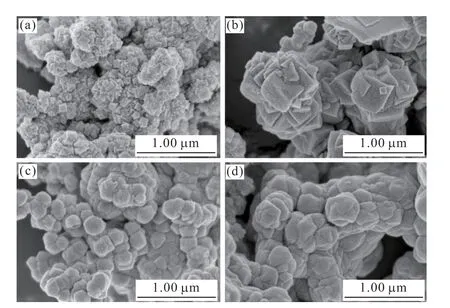
Fig.3 SEM images of UiO-66 and UiO-66 modified with different modifiers: (a)UiO-66;(b)UiO-66-HCOOH;(c)UiO-66-CH3COOH;(d)UiO-66-HCl
3.3 Fourier transform infrared spectroscopic analysis
In order to determine that the synthesized substance is UiO-66,the structure was characterized by Fourier transform infrared spectroscopy in this experiment,and the results are shown in Fig.4.It can be seen from Fig.4 that there is an obvious broad peak near the wavenumber of 3 400 cm-1,which corresponds to the characteristic peak of stretching vibration of water molecules or hydroxyl groups.This is mainly due to the fact that UiO-66 is a porous adsorbent material,and the physically adsorbed water or methanol exists inside the material[21].The peak at the wavenumber of 1 597 cm-1is the absorption peak caused by the vibration of the carbon-oxygen double bond C=O in the carboxyl group.The peak at the wavenumber of 1 390 cm-1is assigned to the stretching vibration of the carboxyl group.This carboxyl group is derived from terephthalic acid in the UiO-66 material[12].The peaks around the wavenumber of 796 cm-1are the characteristic absorption peaks of bending vibration of OH and CH[21].The vibrational peaks at wavenumbers 680 and 573 cm-1are assigned to the Zr-Ou3-Ostretching vibration and the asymmetric stretching vibration peaks of Zr-(OC) in the Zr6 cluster,respectively[21].The peak at wavenumber 476 cm-1is attributed to the vibrational peaks of OH and CH bending and Zr-O mixing[12].UiO-66 modified by adding formic acid,acetic acid and hydrochloric acid did not appear new vibration peaks,which were consistent with the characteristic peaks of UiO-66.It shows that UiO-66 can be successfully synthesized after adding the modifier,which is consistent with the results of XRD crystal phase analysis.
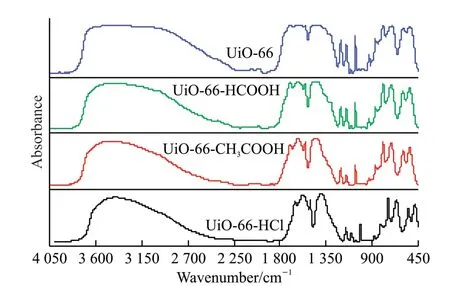
Fig.4 FTIR spectra of UiO-66 and UiO-66 samples modified with different regulators
3.4 Surface physical property analysis
It can be seen from Table 2 that the specific surface area of UiO-66 has changed after adding the regulator.The specific surface area of UiO-66-HCOOH is similar to that of UiO-66,which are 870.19 and 860.17 m2·g-1,respectively.However,the specific surface areas of UiO-66-CH3COOH and UiO-66-HCl increased significantly,which were 1 039.98 and 1 015.79 m2·g-1.This indicates that the addition of the regulator not only acts as an intermediate reactant and“regulates” the crystal growth,but also participates in the coordination of Zr6O4(OH)4,forming some Zr centers with the regulator group as the coordination terminal.The modifier group is decomposed by heating,so that more coordination defect sites appear,which increases the porosity and specific surface area of UiO-66 material[22].In general,the larger specific surface area is helpful for the adsorption and desorption of gas on the catalyst,and the increase of the specific surface area is one of the reasons for the higher NH3-SCR reaction activity of the catalyst after adding the modifier.

Table 2 Specific surface area of UiO-66 and UiO-66 samples modified with different regulators
3.5 Thermal stability analysis
The thermal stability and defect sites of metalorganic frameworks were tested by TG curve.Fig.5 is the TG curves of UiO-66 and UiO-66 modified by adding modifier.It can be seen from Fig.5 that the trend of the weight loss curves of UiO-66 and UiO-66 modified by adding modifiers is basically the same.When heated to 100 ℃,this part of the mass loss can be attributed to the water molecules adsorbed into the pores.When heated to 250 ℃,the loss of mass can be attributed to the residual DMF molecules in the pores.The hydroxyl groups on the Zr6 clusters in the UiO-66 structure were removed in the form of water in the range of 250-350 ℃.Non-ligand species coordinating with Zr6 clusters are also removed in this temperature range.In the range of 350-450 ℃,the mass loss tends to be stable.When the heating temperature exceeds 450 ℃,the crystal framework rapidly decomposes to form zirconia[12].It can be seen from the TG curve that the weight loss plateau of the samples with the added modifier is significantly lower than that of the pure phase UiO-66.This is because the regulator acid replaces part of the terephthalic acid in the crystal framework,resulting in a certain number of defect sites,thereby improving the porosity of the material and increasing the specific surface area.Therefore,the number of solvent molecules adsorbed in the pores of the material also increases.
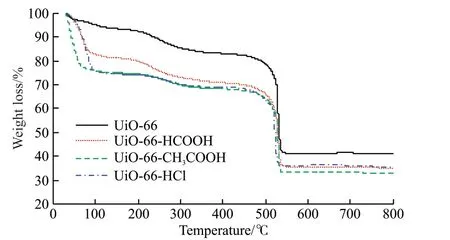
Fig.5 TG curves of UiO-66 and UiO-66 modified by adding modifier
3.6 Adsorption performance of catalysts for NH3
In the SCR reaction mechanism,it is generally believed that NH3is first adsorbed on the catalyst surface,and then undergoes a redox reaction with gaseous or adsorbed NO.The acidity of the catalyst surface is an important factor affecting the amount of NH3adsorption[23].Fig.6 are the NH3-TPD spectra of UiO-66 and UiO-66 samples modified with different regulators.According to the TG curve,the skeleton of UiO-66 begins to collapse at 450 ℃,so this paper only selects the adsorption-desorption performance of NH3before 400 ℃ to study.It can be seen from Fig.6 that at 200 ℃,the four samples began to desorb NH3.With the increase of temperature,the degree of NH3desorption intensified,until the amount of NH3desorption reached the maximum value around 275 ℃,and then,with the increase of temperature,the amount of NH3desorption gradually decreased.It can be seen from the literature that the desorption peak in this temperature range is derived from the desorption of NH3physically adsorbed on weakly acidic sites.It can be seen from Fig.7 that the size of the desorption peaks of NH3of the four samples is: UiO-66-HCl>UiO-66-CH3COOH>UiO-66-HCOOH>UiO-66.The surface acid content of UiO-66 increased after adding the modifier,and the increase of the surface acid content of UiO-66-HCl was the most obvious.After the regulator acid was added during the synthesis,the ligand terephthalic acid in the UiO-66 backbone structure was replaced by the regulator acid.During temperature-programmed desorption,not only the solvent molecules adsorbed in the catalyst pores and on the surface are removed,but also the regulator acid species are removed,resulting in more open Zr metal sites and more acid sites exposed[24,25].Therefore,the amount of surface acid of UiO-66 increases after adding the conditioner.A larger amount of surface acid can adsorb more NH3,which is beneficial to the increase of NO conversion rate,which is an important reason for the improvement of the catalytic performance of the catalyst after adding a modifier.

Fig.6 NH3-TPD spectra of UiO-66 and UiO-66 samples modified with different regulators
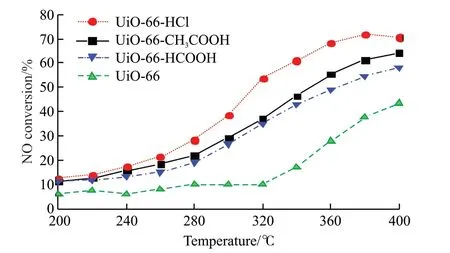
Fig.7 Activity curves of UiO-66 and UiO-66 samples modified with different regulators
3.7 Study on catalytic denitrification performance of UiO-66
Zr-containing MOFs have promising applications in gas catalysis due to their porosity and unique topology.In view of this,Zr-centered UiO-66 was synthesized and used as a catalyst for the NH3-SCR reaction.Figs.3-6 show the effects of different regulators on the denitration efficiency of UiO-66.It can be seen from the figure that the low-temperature activity of UiO-66 is poor;the activity of the catalyst increases significantly after 320 ℃;at 400 ℃,the NO conversion rate is the highest,reaching about 40%.It can also be seen from the figure that the denitration activities of the formic acid and acetic acid modified catalysts are close.With the increase of temperature,the NO conversion rate increases,reaching about 60%at 400 ℃.The NO conversion rate of UiO-66-HCl modified with hydrochloric acid is the highest,and the NO conversion rate can reach more than 70% at 380℃.In the UiO-66 material,one Zr6 regular octahedron is linked with 12 terephthalic acid ligands,forming a cage-like face-centered cubic crystal structure.Among them,Zr4+is connected by weak bonds,and the remaining axial coordination gaps are filled with weak water molecular bonds.At a suitable heat treatment temperature,water molecules are easily removed,resulting in a large number of Lewis acid sites and unsaturated Zr active sites exposed,thus enhancing the NH3-SCR reactivity.
3.8 Analysis of adsorption catalysis mechanism
The chemical formula of UIO-66 is Zr6O4(OH)4(CO2)12,the structure is shown in Fig.8 .A Zr6 regular octahedron is linked with 12 terephthalic acid ligands to form a face-centered cubic crystal structure containing an octahedral central cage structure of 11 Å and a tetrahedral angular cage structure of 8 Å.These two types of pore cages are interconnected to form a triangular structure close to 6 Å that can only pass small molecules[26,27].The open pore structure and more open sites make the modified UiO-66 an excellent reaction catalyst,and the increase of the space around the cluster facilitates the simultaneous activation of the reactants.
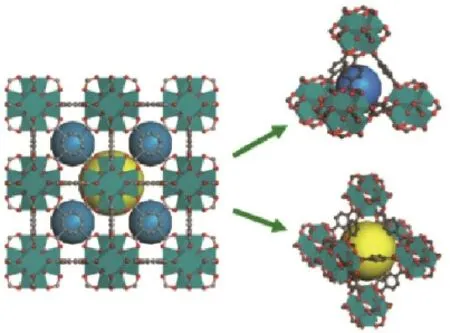
Fig.8 Schematic diagram of UiO-66 structure
UiO-66 has excellent chemical and hydrothermal stability,and its highly dispersed Zr sites,large specific surface area,and mesoporous structure are beneficial to the adsorption of catalytic reactants,and can provide more reactive sites.And the zirconium-based metalorganic framework is activated at high temperature,the small ligand molecules on its secondary structural unit are removed,and the Zr4+and Zr3+coordination unsaturated sites that can be used as Lewis acid sites are formed on the framework structure.In the process,it is beneficial to the adsorption of reactant NH3and promote the catalytic reaction[28].
UiO-66 utilizes the extremely large specific surface area and a large number of Lewis acid sites to adsorb NH3and NO on the unsaturated metal active sites,forming activated adsorbed species to promote the formation of H2O and N2from NH3and NOx.First,NH3,NO2,NO,and O2are adsorbed on the catalyst surface.Under the catalysis of Zr4+,O2oxidizes part of the adsorbed NH3and NO to N2and NO2.Zr4+is reduced to Zr3+.Zr3+then reacts with NO2and NO to reduce it to N2,and Zr3+changes back to Zr4+,and the process is cyclic.The reaction formulas are shown in Eqs.(2) to (6),wherein Eqs.(3),(5) and (6) are the main reactions[29],
4 Conclusions
In the process of solvothermal preparation,adding regulators formic acid,acetic acid and hydrochloric acid to the reaction solution can successfully obtain the crystal structure of UiO-66.After adding the modifier,UiO-66 changed from the irregular quadrilateral with serious agglomeration to the well-dispersed and regular morphology.The addition of the modifier makes more coordination defects appear in the UiO-66 crystal,which increases its porosity and specific surface area.The addition of modifier has little effect on the thermal stability of UiO-66.After adding the regulator acid to the synthetic raw material,more open Zr metal sites will be generated,thereby exposing more acid sites.The low temperature activity of UiO-66 is poor,and it only shows a certain catalytic activity at higher temperature.The catalytic activity of UiO-66 was significantly improved after adding the regulator.Among them,the UiO-66-HCl modified with hydrochloric acid had the best catalytic activity,and the denitration rate reached 70% when the denitration temperature was 380 ℃.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
Journal of Wuhan University of Technology(Materials Science Edition)2024年2期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
- Photocatalytic Activity Enhancement in Organic Dyes Degradation by Loading Ag Nanoparticles onto α-Fe2O3/ZnOs
