A review on surface coating strategies for anti-hygroscopic of high energy oxidizer ammonium dinitramide
Hongyu Yng , Fuyo Chen , Yiwen Hu , Qingqing Lu , Lei Xio , Yinglei Wng ,Fengqi Zho , Wei Jing , Gzi Ho ,*
a National Special Superfine Powder Engineering Research Center of China, School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
b Xi’an Modern Chemistry Research Institute, Xi’an 710065, China
Keywords:Ammonium dinitramide (ADN)Energetic materials Anti-hygroscopic Surface coating Compatibility analysis
ABSTRACT Ammonium dinitramide (ADN), which has the advantages of high energy density, no halogen and low characteristic signal,is not only considered as a new high-energy oxidizer that is expected to replace the traditional oxidizer ammonium perchlorate (AP) in solid propellants, but also a good performance explosive in itself.However, due to the strong hygroscopicity of ADN, its application in solid propellants and explosives is greatly limited.Solving the hygroscopicity of ADN is the key to realize the wide application of ADN.In this paper, we systematically review the research progress of anti-hygroscopic strategies of ADN coating.The surface coating methods are focusing on solvent volatilization, solventnon-solvent, melt crystallization and atomic layer deposition technology.The characteristics of the different methods are compared and analyzed, and the basis for the classification and selection of the coating materials are introduced in detail.In addition, the feasibility of material for surface coating of ADN is evaluated by several compatibility analysis methods.It is highly expected that the liquid phase method (solvent volatilization method, solvent-non-solvent method) would be the promising method for future ADN coating because of its effective, safety and facile operation.Furthermore, polymer materials, are the preferred coating materials due to their high viscosity, easy adhesion, good antihygroscopic effect, and heat resistance, which make ADN weak hygroscopicity, less sensitive, easier to preserve and good compatibility.
1.Introduction
ADN (NH4N(NO2)2), was first synthesized in the 1970s by the Zelinsky Institute of Organic Chemistry in the former Soviet Union[1],and has the advantages of high energy density,positive oxygen balance, good thermal stability, and environmental friendless.It is not only the most important component of solid propellant(highenergy oxidizer), but also an excellent performance of explosives.Oxidizer accounts for about 70% of the mass of solid propellant[2,3], and is one of the most important components of propellant.The traditional oxidizer-Ammonium perchlorate (AP) [4] emits chlorine byproducts during combustion[5],which can lead to acid rain and environmental pollution.Seriously,long-term exposure to ammonium perchlorate in humans can cause thyroid disorders[6].Replacing AP with ADN as an oxidizer in solid propellants can not only substantially increase propellant energy, but also reduce characteristic signal and environmental pollution.Zhu et al.[7]accessed ADN to replace 2/3 of AP in the systems of AP/Cyclotrimethylenetrinitramine(RDX)and AP/RDX/Al and found that the heat release of ADN/AP/RDX system increased by 1.6 times and the heat release of the ADN/AP/RDX/Al system increased by 1.68 times compared to the original system.In addition, ADN as a good performance explosive has a heat of explosion of 3.92 kJ/g and a detonation velocity of 5998 m/s at a charge diameter of 100 mm[8,9].Langlet A et al.[10]substituted ADN for Trinitrotoluene(TNT)as a substrate in TNT/RDX and TNT/Cyclotetramethylenetetranitramine (HMX) systems with a mass ratio of 30:70,compared to the original system,the combustion performance was improved by 18.75% and 18.82% respectively.
Abbreviations 18C6 1,4,7,10,13,16-Hexaoxacyclooctadecane A18 Octadecylamine AAOFCA Alcohol amine organic fluorine coupling agent ACOFCA Ammonium carboxylate organic fluorine coupling agent ADN Ammonium dinitramide ALD Atomic Layer Deposition AmGO Alkylated graphene oxide AMMO 3-azidomethyl-3-methyloxetane AN Ammonium nitrate AP Ammonium perchlorate BA-2 N-propynyl-2,2′-dihydroxyethyl amine BA-7 N-propynyl-3,3′-dipropionitrile amine BAMO 3,3-bis(azidomethyl)oxetane BDDA 1,4-butanediol diacrylate BPS Bis-propargyl-succinate Cab-O-Sil Amorphous fumed silica CL-20 Hexanitrohexaazaisowurtzitane CNTs Carbon nanotubes CTAB Cetyltrimethylammonium bromide DCM Dichloromethane DDI Dimeryl diisocyanate DSC Differential scanning calorimetry DVS Dynamic water vapor adsorption EC Ethyl cellulose ECOFCA Ester carboxylate organic fluorine coupling agent EDS Energy Dispersive X-ray Spectroscopy GAP Glycidyl azide polymer HEXA Hexamethylenetetramine HMX Cyclotetramethylenete-tranitramine HPA Heptadecanoic acid HTPB Hydroxyl-terminated polybutadiene HXA Hexadecanoic acid IPDI Isophorondiisocyanate IR Infrared KH550 γ-aminopropyltriethoxysilane MC Microcalorimetry MDX Molecular dynamics simulation MSF Mercaptan surfactants N100 Desmodur® N100 N2 Nitrogen NC Nitrocellulose NDZ-311 Bis(dioctyl pyrophosphate acyloxy)ethylene titanate NST Natural storage test PA Polyacrylate PBT Poly-BAMMO-THF PEG Polyethylene glycol PMMA Polymethyl methacrylate POSS Poligomeric silsesquioxane PS Polystyrene PTFE Polytetrafluoroethylene PU Polyurethane PVB Polyvinyl butyral PVDF Polyvinylidene fluoride RDX Cyclotrimethylenetrinitramine RH Relative Humidity SA Stearic acid TDI Toluene diisocyanate TGA Thermal Gravimetric Analysis THF Tetrahydrofuran TKX-50 Dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate TMOS Trimethoxysilane TNT Trinitrotoluene TOS Triethoxysilane TOVS Triethoxyvinylsilane VST Vacuum stability testing VTOS Vinyltrimethoxysilane
Although ADN has the advantages of high energy density, positive oxygen balance, good thermal stability, and environmental protection, its strong hygroscopicity makes its application in solid propellants and explosives is greatly limited[11].Therefore,solving the problem of hygroscopicity of ADN is the key to its the wide application.At present,there are three main methods to reduce the hygroscopicity of ADN.The first method is prilling technology which is used to change the morphology of ADN particles[12,13]to reduce the specific surface area of crystals, but prilling technology does not change the chemical structure of ADN surface or introduce other chemicals, so it cannot essentially solve the hygroscopicity problem.The second method is co-crystallization,in which ADN is formed into new single-phase crystals with other components at a certain stoichiometric ratio to change the physical and chemical properties in order to inhibit hygroscopicity[14].But introducing a large number of ligands for co-crystallization will lead to the loss of the excellent properties of ADN,such as the formation of co-crystal between ADN and 18C6 caused the oxygen balance to become negative [15], so that ADN lost its strong oxidation.In addition,most of the current researches on ADN co-crystal are theoretical simulation experiments, and the preparation of ADN co-crystal in practice is more challenging.The Third method is surface coating of ADN which makes the surface acquire new physical and chemical properties.Surface coating not only isolates the contact between ADN and water to reduce the hygroscopicity,but also improves the dispersion of ADN particles and the compatibility with other substances [16,17].
Among the above three methods,surface coating method is the most versatile modification method which has wide applications in energetic materials modification.Coating method can be used to reduce mechanical sensitivity [18], improve dispersibility [19,20],enhance reactivity [21], improve compatibility [22], and reduce hygroscopicity[23,24].Therefore,this paper systematically reviews the research progress of ADN anti-hygroscopic technology by surface coating.The emphasis of this review is focusing on the hygroscopic mechanism of ADN, the classification of ADN surface coating methods, the characteristics of different methods, and the basis of coating material classification and selection.In addition,several methods of material compatibility analysis are summarized for analyzing whether the coating materials can be applied to ADN systems.
2.The hygroscopic mechanism of ADN
ADN is an extremely hygroscopic energetic ionic salt with strong hygroscopicity (Fig.1), and studies have shown that ADN rapidly deliquesces when the relative humidity is higher than 70%[25].
The hygroscopic mechanism of ADN has been studied by many researchers before(Fig.2), and the main reasons for its strong hygroscopicity are as follows.
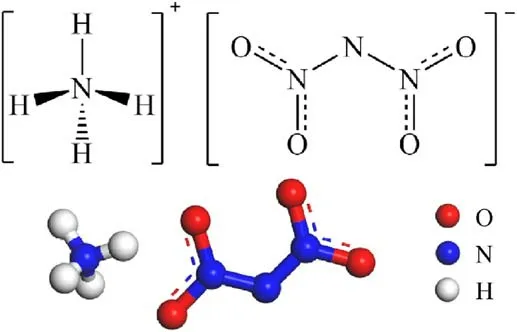
Fig.1.The structure of ADN.
(1) The high surface energy of ADN gives it a tendency to strongly adsorb moisture from the air to reduce its surface energy [26].
(2) ADN is an inorganic salt which has strong polar, and the strong electrostatic interaction between its solid surface and the water molecules in the air makes it easy to adsorb water molecules.
(3) NH4+and N(NO2)2-ions in ADN molecules tend to combine with water molecules in the form of hydrogen bonds,where the NH4+cation tends to hydrolyze with water molecules in the air, making the surface hydroxylated, and the N(NO2)2-anion also interacts with water in the air, attracting protons from water molecules thus weakening the intermolecular hydrogen bonds [27].
(4) AN as the photolysis product of ADN is extremely hygroscopic and covers the surface to enhance the hygroscopicity of the original material [28-30].
Wang et al.[31] analyzed the macroscopic hygroscopic process of spherical ADN by dynamic hygroscopic analysis method, which can be divided into three stages (Fig.3).
The first stage is gently moisture absorption process, and the main driving force of hygroscopicity in this stage is the adsorption of water vapor on the surface of ADN.
The second stage is dramatically enhanced moisture absorption process,which can be explained by a capillary condensation theory and the main factors affecting the hygroscopicity are the smooth degree of ADN surface.
The third stage is the gradual deliquescence process,this stage is mainly the role of water vapor and ADN liquefaction film surface.
In the theoretical study of the hygroscopic mechanism of ADN,it is generally believed that the strong hygroscopicity of ADN is mainly due to the electrostatic interaction between NH4+and H2O in ADN molecules and intermolecular hydrogen bonding.
Wang et al.[32] studied the hygroscopicity of ADN by density functional theory and found that the electrostatic and orbital interactions between ADN and H2O are the main interactions, and molecular dynamics simulations of the hygroscopicity of ADN crystal surfaces showed that the hygroscopicity of ADN intensified with the increase in the amount of H2O, which proved that the hygroscopicity of ADN was enhanced by the increase in ambient humidity.
In addition, Cui et al.[33] found that the structure of ADN molecules is a rare twofold three-dimensional network structure connected by hydrogen bonds through molecular simulations(Fig.4), and verified that the strong hygroscopicity of ADN is also due to the effect of this special hydrogen-bonded structure.
Hu et al.[34] explored the surface properties of ADN crystals and found that the hygroscopicity of the ADN crystal surface is related to the exposed anions and cations on the crystal surface and the smoothness of the surface(Fig.5).The NH4+cations are exposed on the crystal surface, which become adsorption sites attracting water molecules and forming hydrogen bonds.Thus,the more NH4+cations exposed on the crystal surface, the greater the hygroscopicity of the surface.According to the basic theory of wettability:the wettability properties of a solid substance are proportional to the roughness of the surface being wetted, and increasing the surface roughness enhances the wettability caused by surface chemistryinduced wettability [35].Therefore, the rougher the surface of ADN crystals, the greater their wettability.
3.Surface coating methods of ADN
The surface coating is a method which improves the physical and chemical properties of the material surface by attaching a small amount of coating material to the particle surface, and thus improves the dispersibility of the particles by reducing their surface energy to solve the agglomeration of powder particles [36].For coating ADN, firstly, the direct contact between ADN and environmental moisture is isolated to reduce the hygroscopicity of ADN[37].Secondly, the mechanical sensitivity of ADN can be reduced[38].Thirdly, the energy performance of ADN can’t be affected because of the low amount of coating agent[39].The disadvantage of the surface coating method is that there are more influencing variables in the coating process, including the selection of the coating method and material, the amount of coating material, the dispersion medium, the stirring speed, the equipment vessel, and the reaction temperature [40].
According to the principles of coating methods, this paper mainly refines the methods of coating ADN and summarizes the methods for coating ADN, mainly including solvent volatilization method, solvent-non-solvent method, melt crystallization method and atomic layer deposition method.
3.1.Solvent volatilization method
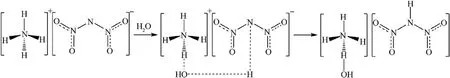
Fig.2.The hygroscopic mechanism of ADN.
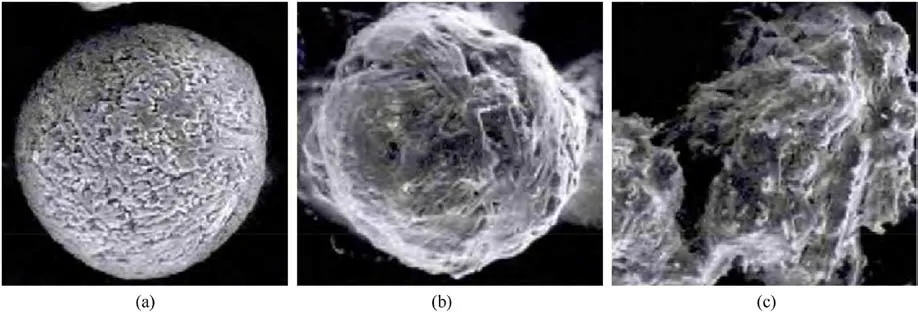
Fig.3.Macroscopic spherical ADN moisture absorption process: (a) Pre-experiment; (b) The first stage; (c) The second stage.
The solvent volatilization method involves dissolving the coating materials in a specified solvent(generally a non-solvent for ADN), adding ADN particles so that the coating material has adsorption sites, and stirring while heating and reducing pressure to supersaturate the solution so that the coating materials have sufficient driving force to precipitate and grow on the surface of ADN (Fig.6).The film produced by solvent volatilization method can reduce the contact between ADN and water molecules in the air, thereby reducing the hygroscopicity of ADN.
The solvent volatilization method was applied in the early 21st century for the anti-hygroscopic coating of ADN.Santhosh G [41]chose ethyl cellulose(EC)and polymethyl methacrylate(PMMA)to coat spherical ADN by solvent volatilization, in which the coating amount of was 0.5%-2%of ADN,Dichloromethane(DCM)was used as the dispersing solvent for the coating materials, and a small amount (0.05%)of amorphous fumed silica (Cab-O-Sil)to promote emulsification and reduce viscosity.The hygroscopicity of the coated samples was measured at 62% and 74% Relative Humidity(RH) (Fig.7).As shown in Table 1, coating of EC and PMMA both reduce the moisture absorption of ADN by about 50%.Comparing the moisture absorption of EC-coated ADN with PMMA-coated ADN, it can be found that PMMA-coated ADN had lower moisture absorption than EC-coated ADN, which may be due to the ester groups in PMMA which make the materials more hydrophobic.
The time comes to 2007,Kong[30]used Polyvinyl butyral(PVB),Polyethylene glycol (PEG), Hydroxyl-terminated polybutadiene(HTPB),Bis(dioctyl pyrophosphate acyloxy)ethylene titanate(NDZ-311), γ-aminopropyltriethoxysilane (KH550), Hexamethylenetetramine (HEXA), Octadecylamine (A18), Cetyltrimethylammonium bromide (CTAB) and liquid paraffin waxes surface coating sheet crystal ADN by solvent volatilization method, and the coating amounts were all 0.2%of the mass of ADN.The moisture absorption rates of different samples are shown in Table 2,which were tested under the experimental conditions of 30°C and 75%RH for 144 h.It can be seen that the anti-hygroscopic effect of ADN coated by PVB and HTPB was better than PEG,which may be attributed to the fact that the PVB molecule contains -OH and -C=O polar groups,while the HTPB molecule also contains -OH polar groups, which make the polymer molecules easily adsorbed on the surface of the particles and had good film forming property.In comparison with the moisture absorption rates of uncoated sheet crystal ADN and spherical ADN,that of ADN decreased after surface coating,but the anti-hygroscopic effect was not very obvious.It may be that the surface of sheet ADN is rough and angular,so the force between the coating material and ADN is weak,which makes the coating effect not obvious.
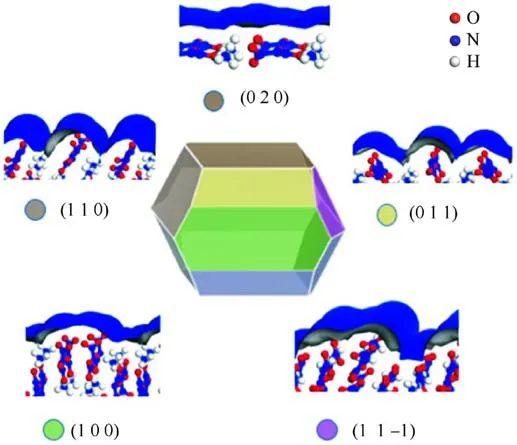
Fig.5.Major crystal surfaces of ADN.

Fig.6.Diagram of solvent volatilization method.
Second year,Xu et al.[42]dissolved Polyurethane(PU)binder in Tetrahydrofuran (THF) until saturated, and added ADN to the saturated solution.And through the solvent evaporation to make the PU coating on the surface of ADN.It can be seen from the SEM image(Fig.8)that the surface grooves of coated ADN particles were not obvious,and there was a flocculent coating film formed by PU.The content of the coating layer was calculated to be about 1.675%based on the change of element content.The moisture absorption of the coated ADN was only 0.136%when exposed to air for 30 days at 10°C and 50%-60% RH.The reduction of moisture absorption may be due to the fact that the PU coating layer consists of many soft segments of hydrocarbon chains with small polarity,which can form a good hydrophobic layer.The coating layer blocked the contact between ADN and moisture in the air, and also filled the gaps on the surface of the particles, which makes the surface of ADN smooth and reduced the specific surface area.In addition,the polyurethane coating reduced the mechanical sensitivity of the ADN.
In the same year,Wang[43]selected acetone as the solvent for the coating materials and prepared spherical ADN particles by solvent volatilization method.The ADN particles were coated with Stearic acid(SA),HEXA,PVB and TNT at coating amount of 0.2%.The hygroscopicity of ADN was measured at about 60%RH(Table 3).The results show that the best effect of ADN coated by SA was achieved at 25°C and 30°C,but the anti-hygroscopic effect of ADN coated by other materials was less obvious, which is due to the fact that the internal ADN is loose and porous.And SA not only can form an antihydrophobic layer on the surface of the particles,but also can plug the micro-pores inside ADN, thus reducing its hygroscopicity.However,at temperatures below 20°C,SA became less ductile and could not fully coat the surface and plug the internal micropores,so the anti-hydrophobic effect of SA coated ADN was not obvious.
After many materials have been tried to be applied to the coating of ADN, in 2016, Lu et al.[44], in order to overcome the incompatible problem of ADN and the isocyanates curing agents,used Glycidyl azide polymer (GAP)/Bis-propargyl-succinate (BPS)crosslinked polymers as coating materials to coat spherical ADN particles via 1,3 dipolar cycloaddition reaction.It can be found that when the mass ratio of GAP:BPS was 5:1 and the total coating ratio was 3%, the saturated moisture absorption rate of GAP/BPS crosslinked coating ADN was 0.78%, and it had good moisture resistance.The VST results showed that the system was moderately compatible and could improve the safety of the ADN coating process [45].The reason for the lower hygroscopicity of GAP/BPScoated ADN is that the system of coating material changed from the one-dimension linear to the two-dimension mesh through the 1,3-dipole cyclization reaction (Fig.9), which effectively improved the coverage of the coating layer.From the SEM images(Fig.10),it can be seen that the surface of spherical ADN was smoother after coating,the gaps on the surface of original particle were obviously smaller, and the coating of ADN was more complete.
Soon after, Rahman A [46] used Polystyrene (PS) and HTPB to coat ADN by ultrasonic-assisted solvent volatilization, and compared with direct coating of spherical ADN by solvent volatilization method.As shown in Figs.11 and 12, the size of spherical ADN coated directly by 5% PS ranged from 173 μm to 250 μm, the ADN particles prepared by ultrasound-assisted solvent volatilization were spherical with a particle size of 150-295 μm.The above phenomena indicated that ultrasound assistance could optimize the particle size.From the images of ADN moisture absorption rate in Fig.13, it can be seen that the moisture absorption rate was reduced after coating, and the moisture absorption rate of HTPBcoated ADN prepared by ultrasound-assisted solvent volatilization was 18%after 240 min,which was a significant reduction.The sample with graphene showed better anti-hygroscopic effect at the longer time (240 min) due to the fact that graphene is a hydrophobic material and its high aspect ratio plays the role of a physical barrier to reduce the moisture absorption of ADN [47-49].
Starting in the 1920s, a solution for ADN coating against moisture absorption was shown in a study [50] that the researchers produced three macromolecular prepolymers(consisting of Poly-3-azidomethyl-3-methyloxetane (PAMMO), GAP, and Poly-BAMOTHF (PBT) which crosslinked by 3,3-bis(azidomethyl)oxetane(BAMO) and THF) to crosslink with 1,4-butanediol diacrylate(BDDA) by 1,3-dipole addition cyclization reaction, and used the products to coat ADN particles by solvent volatilization method.The moisture absorption of the coated ADN was measured at 35°C and 50% RH (Table 4), and three coating systems were able to significantly reduce the saturation moisture absorption of ADN.And compared with the conventional GAP/N-100 system, all of coated systems had better compatibility with ADN.
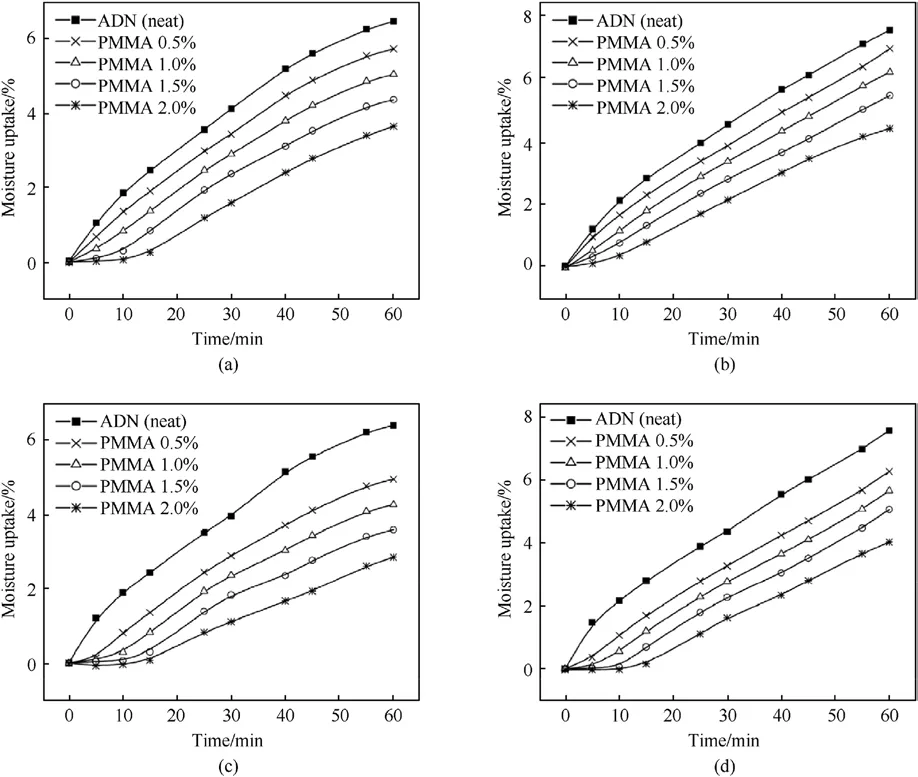
Fig.7.(a)Moisture absorption rate of ADN coated by EC at 62%RH;(b)Moisture absorption rate of EC-coated ADN at 74%RH;(c)Moisture absorption rate of ADN coated by PMMA at 62% RH; (d) Moisture absorption rate of ADN coated by PMMA at 74% RH.
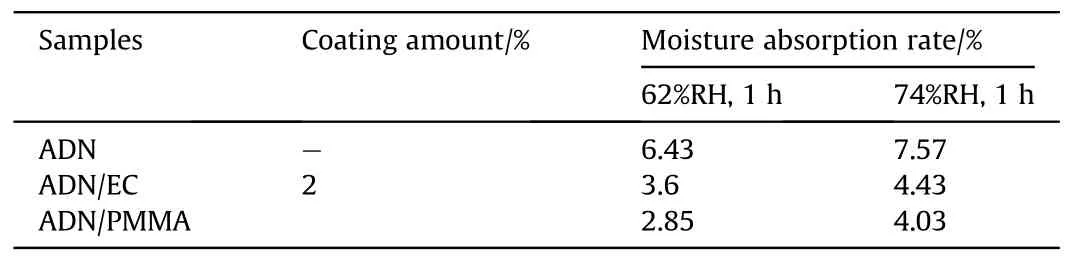
Table 1 Moisture absorption rate of ADN coated by EC and PMMA.
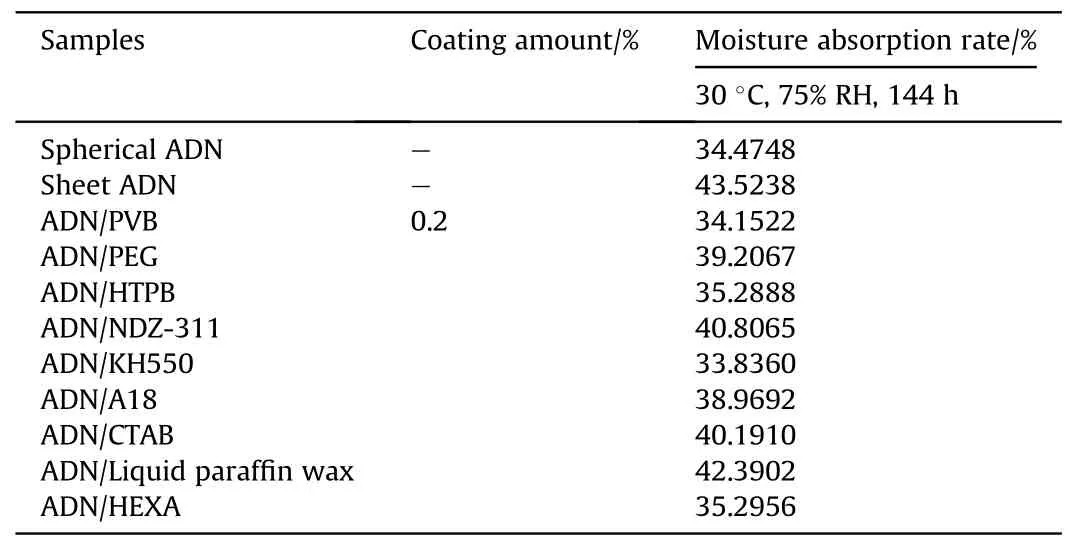
Table 2 Moisture absorption rates of ADN with different shapes and different coating materials.
Very Recently, Li et al.[51] prepared ADN@polyhedral oligomeric silsesquioxane (POSS)-NH2core-shell energetic microspheres by solvent evaporation method.The specific process flow is shown in Fig.14.
The SEM image in Fig.15(a)shows the original ADN@POSS-NH2.Fig.15(b) shows the sample after electron beam irradiation,revealing small cracks in the film covering the ADN surface (red dashed ellipse),confirming that the ADN surface was coated with a thin film.The Si elemental mapping in Fig.15(e) and the Energy Dispersive X-ray Spectroscopy (EDS) spectrum in Fig.15(f) also prove the presence of POSS-NH2on the ADN surface.After removing the internal ADN,the hollow shell POSS-NH2maintained its spherical shape,forming a uniform film about 350 nm thick on the ADN surface (Fig.16).These results suggest that POSS-NH2formed a relatively uniform coating on the ADN surface.
Fig.17 shows the DVS curves of ADN,POSS-NH2and ADN@POSSNH2at different relative humidity.The critical relative humidity of ADN@POSS-NH2was delayed from 51% to 53%, indicating better moisture absorption inhibition compared to uncoated ADN.

Fig.8.SEM images of ADN: (a) Before and (b) after coated by PU.
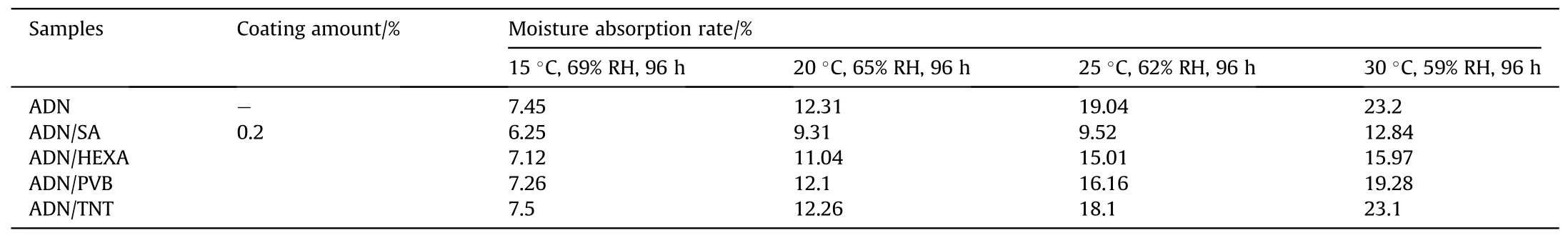
Table 3 Moisture absorption rates of ADN at different temperatures.
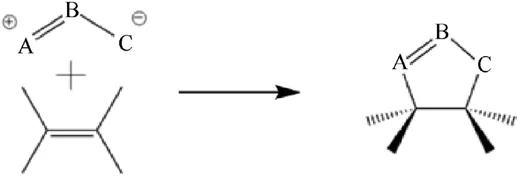
Fig.9.Mechanism of 1,3-dipole cyclization reaction.
Fig.18 shows the static adsorption processes of ADN and ADN@POSSNH2over 7 days at 20°C and 75% RH.Uncoated ADN became completely hygroscopic and deliquescent within 24 h,while ADN@POSS-NH2particles managed to remain solid for 6 days, demonstrating significantly reduced hygroscopicity after coating with POSS-NH2.POSS-NH2itself is insoluble in water and have excellent hydrophobicity, contributing to this effect.Thermal analysis results also show that POSS-NH2can promote the decomposition of ADN.
Shortly after the same year, in another study, Zhang et al.[52]prepared a homogeneous precursor solution by dissolving a certain amount of ADN and nitrocellulose (NC) in acetone at different concentrations (2.5, 5, 7.5 and 10 mg/mL), for a total mass ratio of 97:3.The solutions were sonicated for 30 min and stirred for 10 h.Using an electrostatic spray device,the samples were sprayed onto receiving tin foil and dried to produce ADN/NC samples.The preparation process is illustrated in Fig.19.
Fig.20 displays SEM images of ADN/NC samples prepared at four different concentrations and the original ADN.Notably, at a moderate concentration of 5 mg/mL as shown in Fig.20(c),particles of regular shape with some sphericity can be observed,with a size of approximately 5-10 μm,and small particles were stacked in layers.
The moisture absorption curves of the raw materials ADN and ADN/NC are shown in Fig.21 shown.The results indicate that hygroscopicity decreased significantly with the addition of NC, from 32.12%to 3.75%at 20°C and 50%RH.NC has a reticulated structure,rapid film formation, and insolubility in water, so that film was formed which effectively blocks contact between ADN and water molecules to reduce hygroscopicity when NC coated on the surface of ADN crystals by evaporation of acetone solvent.Furthermore,NC reduced the apparent activation energy and thermal explosion critical temperature of the sample,catalyzing the decomposition of ADN.
The fluidized bed is produced by introducing pressurized gas,which causes a large amount of solid particulate matter to be forced to behave as a fluid in the process vessel as the pressurized gas flows upward through the perforated bottom plate of the process vessel[53].Fluidized bed has various applications such as particles prilling,drying and coating.In the coating process of fluidized bed,the particles behaving as fluids are the core materials, and the coating materials are sprayed onto the surface of the fluidized particles by atomizing the solution through nozzles.Then, the solvent is evaporated by airflow,so that the coating material forms a thin film on the surface of the core material.
In 2009, Heintz T et al.[54] applied Polyacrylate (PA), GAP and HTPB to coat ADN by fluidized beds(Fig.22).The particle size and specific surface area of the coated particles were characterized(Table 5), and the compatibility of coated ADN with Isophorondiisocyanate (IPDI) was analyzed (Table 6).The average particle size and distribution of ADN were almost unchanged as seen in the SEM image(Fig.23), indicating that the particles were basically free from agglomeration and fragmentation [55].Microcalorimetric data in Fig.24 shows that the compatibility of each
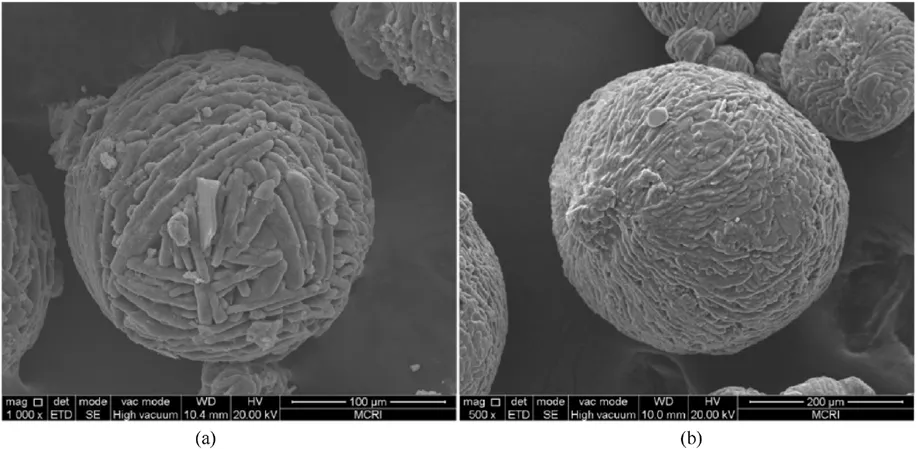
Fig.10.(a) SEM image of uncoated ADN particles; (b) SEM image of GAP/BPS-coated ADN particles.

Fig.11.SEM image of ADN produced by melt prilling and coating with 5 wt% PS.
(1) Gas flow rate: the particles are transformed from dispersed in the liquid phase into a high-speed gas.Increasing the airflow speed can improve the production intensity of the equipment, but it should not exceed the minimum fluidization velocity[56]to ensure that most particles are not carried out by the airflow, allowing sufficient contact time between the solution and the solid particles to ensure complete particle encapsulation.
(2) Instrument size: the design of the fluidized bed size should consider the impact on the particle size and yield of the particles.The size of the atomizer nozzles has an impact on the degree of uniformity of particle coating[57],the aperture of nozzles needs to be as small as possible to ensure that the sprayed droplets are small enough to be uniformly dispersed on the particle surface.The conveying machinery should be adapted to fluid resistance to ensure the safety of the use of the equipment.coating material(PA,GAP,and HTPB)was improved compared with the uncoated ADN.Especially for HTPB/N100, only 2.5% of the coating ratio was used,but the curve was very close to the reference curve for the reaction of pure HTPB cured with IPDI.
The antisolvent of ADN is used as the solvent of the coating material or the dispersant of the system in most of the solvent volatilization methods, thus there is a little influence on the morphology of ADN.Moreover, these methods are widely used in the process of sample preparation in the laboratory because of the simple operation and low requirements for instruments and equipment.
The fluidized beds can be used for large-scale production of coated ADN.By this method, it is not only possible to make the contact between the coating material and ADN more adequate and the coating more uniform,but also the solvent evaporation is more rapid and the drying is more efficient.The basic principle of this method is still solvent volatilization, but the factors to be considered in the preparation process are more complex, such as the following factors.
3.2.Solvent-non-solvent method
The principle of the difference in solubility of substances in different solvents is used the solvent-non-solvent method.When a solution of a substance is added to a non-solvent,the difference in solubility of the substance in different solvents is used to make the coating material precipitate on the surface of the substrate to form a coating layer (Fig.25).
The solvent and non-solvent are for the substance to be dissolved.The coating of ADN can be divided into two cases depending on the dissolved substance.In one case, the coating material is dissolved and added to the non-solvent of the coating material, so that the coating materials settle on the surface of the ADN particles and form a layer.In another case, ADN is dissolved and added to a non-solvent in which the coating material is dispersed,so that the ADN is precipitated inside the coating material (such as carbon nanotubes)to achieve a surface coating effect.These thin films can reduce the contact between ADN and environmental water molecules, thereby reducing its hygroscopicity.

Fig.12.(a) SEM image of ADN sonicated and coated with 5% of PS; (b) SEM image of ADN sonicated and coated with 5% of HTPB.
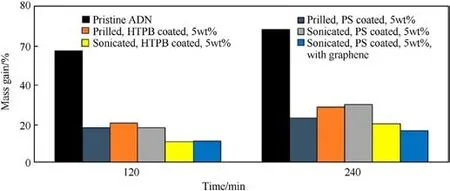
Fig.13.Mass gain in pristine ADN, melt prilled, and sonicated coating of the ADN particle.
In 2008,Yang[58]used AP and Carbon nanotubes(CNTs)to coat ADN by a solvent-non-solvent method.Two solvent systems were used for the study of AP-coated ADN.One system was acetone/DCM system and the other was ethanol/DCM system.Due to the different solubility of ADN in acetone and ethanol, the amount of AP in the particles prepared by the two systems was different.From the results of TG and XPS experiments (Figs.26 and 27), it could be calculated that the amount of AP in the particles prepared by the acetone/DCM system was 34.42%,and that in the particles prepared by the ethanol/DCM system was 50.08%.The moisture absorption of ADN was measured at 80%RH(Table 7).The results showed that the moisture absorption of ADN was reduced after coating, and the moisture absorption decrease of coated ADN prepared in the ethanol/DCM system was more obvious due to the coating amount in the ethanol/DCM system was larger than acetone/DCM system.
ADN was dissolved in the dispersion of CNTs, and then a nonsolvent of ADN was added to make ADN precipitate on the inner surface of CNTs.The moisture absorption rate was measured at 80%RH for 5 h (Table 8).The results showed that the treated CNTs contain fewer impurities,and the decrease of moisture absorption was better compared with the untreated CNTs.However,according to the SEM image(Fig.28),a considerable part of ADN was exposed to the outside of CNTs, which made the anti-hygroscopic effect of ADN not obvious.
After about 10 years,to study the coating of materials which are difficult to dissolve.Li et al.[59] added spherical modified ADN which recrystallized from ADN/ammonium nitrate type explosive including RDX, HMX and Hexanitrohexaazaisowurtzitane (CL-20)to a dispersion of graphene, and then added a non-polar solution into the graphene system to produce modified ADN particles coated by graphene.The moisture absorption was measured at 30°C and 75% RH for 12 h (Table 9).The results showed that the moisture absorption of modified ADN after coated was all about 6%,and the problem of energy performance degradation was improved after modification with other energetic materials.The reason for the decrease in moisture absorption was that graphene was a hydrophobic material,and its high aspect ratio made it to act as an antihumidity layer on the surface.
If the solvent and non-solvent are mutually soluble, the whole liquid phase system will be homogeneous and the material substances will be dispersed in the system.However, when the two liquid phases are insoluble, after mixed, the original system will form a new system named as the emulsion which usually composes of oil (esters, hydrocarbons, and silicones) and water and surfactants.Pickering emulsion is an emulsion in that surfactant molecules are replaced by solid particles,based on the theory that solidparticles irreversibly adsorb at the oil-water interface to form spatial barriers [60] (Fig.29).
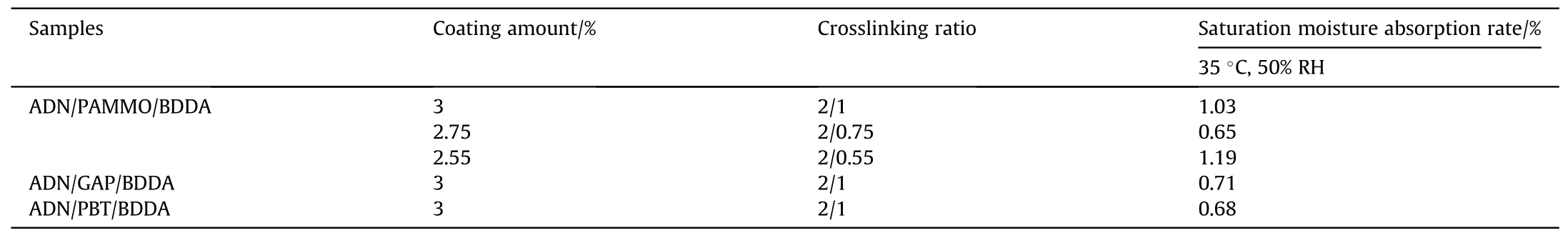
Table 4 Moisture absorption rate of cross-linked coated ADN particles.
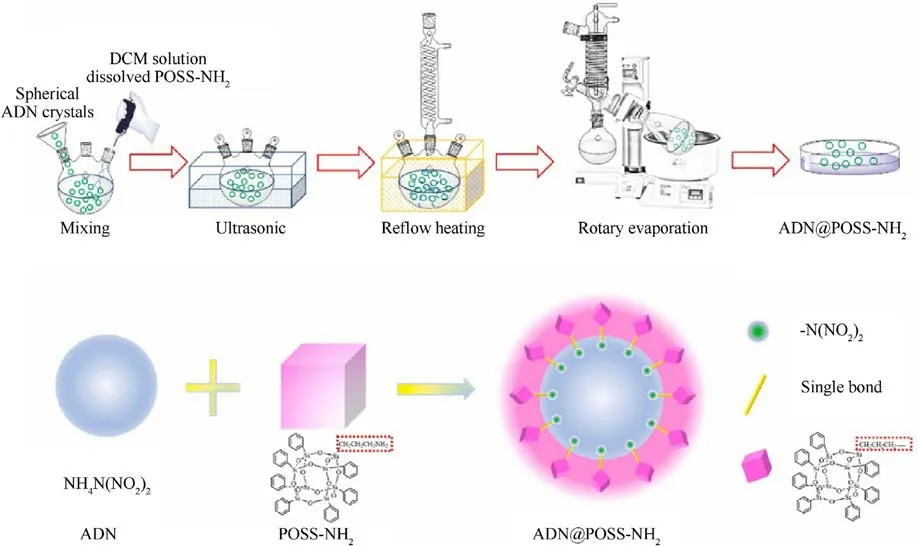
Fig.14.Detailed process schematic and interface chemical reaction illustration of ADN@POSS-NH2 core-shell energetic microsphere.
Soon after, a new idea was proposed.Yan et al.[61] reported a facile method to coat ADN by using Pickering emulsion as a soft template, and Alkylated graphene oxide (AmGO) nanosheets as particle surfactants.They emulsified an aqueous solution of ADN with a toluene suspension of AmGO and then cured the system by vacuum-assisted solvent evaporation to prepare AmGO-coated ADN core-shell structures after the emulsion was stabilized.
The moisture absorption rates were tested at 25°C and 50%RH for 300 min (Table 10).The results showed that the moisture absorption rates of ADN were reduced after coating, which was attributed to the fact that AmGO was an impermeable 2D sheet with a very high aspect ratio and specific surface area, and played the role of the moisture barrier.Moreover,when the proportion of toluene in the liquid phase increased, the moisture absorption of AmGO-coated ADN decreased.It was due to the increase of the proportion of toluene, the ratio of aqueous particles in the liquid phase became smaller (Fig.30), and more AmGO coated on the surface of water droplets.However,the amount of AmGO increased to 2.0 wt%,the inhibition of water adsorption was weakened,which may be due to increased agglomeration of AmGO sheets in the system when the solvent ratio is 2:1,resulting in a lower coverage of water droplets.
Although the moisture absorption rate of AmGO-coated ADN prepared by this method was reduced, the morphology of coated ADN after drying was non-spherical and the particles were not homogeneous (Fig.31).
Solvent-nonsolvent method is also a common preparation method for ADN coating in the laboratory.Compared with the solvent volatilization method, the solvent-nonsolvent method has a greater driving force for the material to precipitate, and adjusts the solution drop acceleration rate of the material can better ensure the uniformity of the coating layer.However,the effects of multiple solvents for different materials need to be considered in the solvent-nonsolvent method, thus the liquid phase system is more difficult to screen.In essence, the basic principle of solventnonsolvent method is used in the pricking emulsion method to form a soft template between the two phases,but the shape of the coated ADN is not spherical and the particles are not uniform,which is mainly due to the failure to control the crystal shape of ADN.
3.3.Melt crystallization method
The interfacial tension [62] of the recrystallization of molten ADN is used in the melt crystallization method to attach the coating material to the surface of ADN(Fig.32).In this process,the surface of ADN particles is covered with an outer layer, which can reduce the hygroscopicity of ADN.
In 2016,Ji et al.[63]mixed ADN and coating materials including trimethoxysilane (TMOS), triethoxysilane (TOS), triethoxyvinylsilane(TOVS),alcohol amine organic fluorine coupling agent(AAOFCA),ammonium carboxylate organic fluorine coupling agent(ACOFCA), ester carboxylate organic fluorine coupling agent(ECOFCA) and mercaptan surfactants (MSF) in the dispersant, stirred and heated to 92°C for 5 min to make ADN completely melted and dispersed, followed by rapid cooling to precipitate the solid particles, and filtered to obtain the coated ADN particles.
The saturated moisture absorption was measured at 35°C and 50% RH (Table 11).The results in Table 11 show that the moisture absorption of the coated ADN was basically less than 1%,except for the saturated moisture absorption of ADN coated with 0.5%TMOS,which was 2.22%, probably due to the small coating volume and slow mixing speed, the ADN was not completely coated.
In the last two years, Guo [64] used silane coupling agents including vinyltrimethoxysilane (VTOS), hexadecanoic acid (HXA),heptadecanoic acid (HPA) and fluoropolymers including polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF) to coat spherical ADN by the conventional melt crystallization method.ADN coated by low surface energy materials prepared from gravimetric clay(including#1,#2,#3 and#4)was produced through the use of an electrostatic spray device.Fig.33 [65] showed an electrostatic spray device which was used to coat energetic materials.
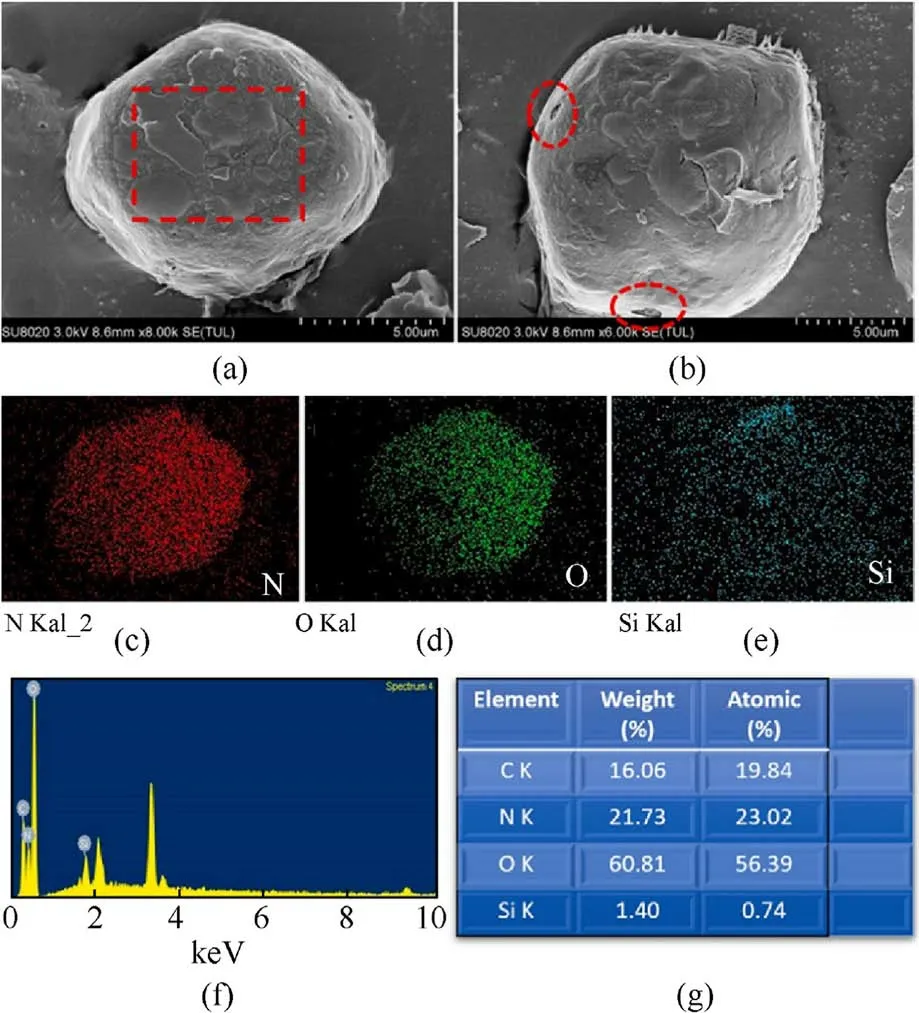
Fig.15.(a), (b) SEM images; (c)-(e) Element maps of N, O, Si; (f) EDS spectrum; (g) Corresponding EDS element content table of ADN@POSS-NH2.
The molten ADN was sent from the delivery tube to the guide tube of the nozzle by high-pressure air.As the guide tube was connected to a high-voltage negative pole, a dense charge was generated around it, so that the molten ADN took on a negative charge and flied toward the coating material under the action of electrostatic force and compressed air.The molten ADN cooled down and crystallized after contacting the coating materials, and the principle of interfacial tension[65]was used to form a layer on the surface of ADN.
The moisture absorption rate was tested after 400 h at 60°C and 80% RH (Tables 12 and 13).The results showed that the moisture absorption rate of coated ADN decreases significantly, which was due to the isolation of external moisture by the materials with low surface energy coated on the surface of ADN.The silane coupling agent could be dehydrated and polymerized with the water on the surface of ADN,thus the VTOS-coated ADN was more effective and had lower moisture absorption rate.In the coated ADN prepared by electrostatic spray device, the homemade material had the lower moisture absorption rate compared to other samples due to the larger amount of coating.
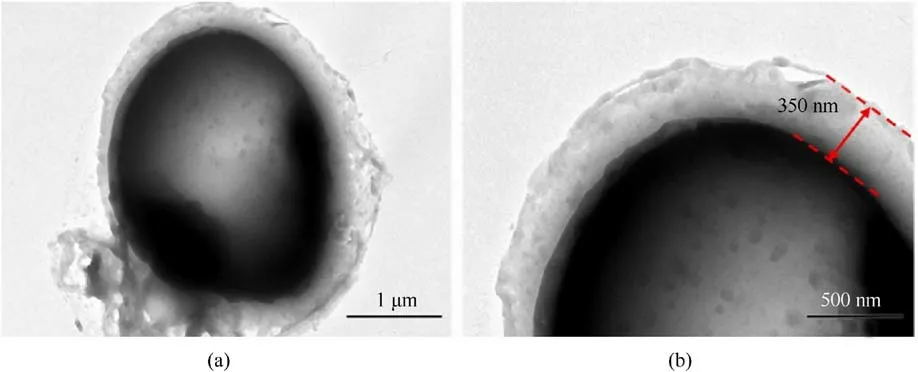
Fig.16.(a) TEM images and (b) partial enlarged detail of ADN@POSS-NH2.
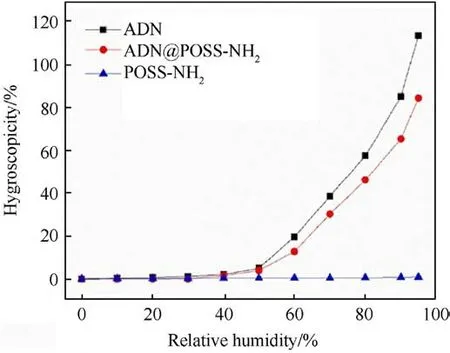
Fig.17.Dynamic vapor sorption traces of ADN, POSS-NH2 and ADN@POSS-NH2 at 20 °C.
Compared to the liquid phase coating method, the melt crystallization method combines prilling and coating techniques,minus the pre-spheroidization step.In addition, the use of electrostatic spray devices improves orientation and deposition efficiency of coating materials, which in turn reduces materials loss [67].However,it is of concern that the properties of ADN in the molten state differ from those of solid ADN.It has been shown that the sensitivity threshold of ADN powder to impact is 7.5 J.The sensitivity threshold of molten ADN to impact is below 0.25 J, which approximates the sensitivity level of nitrocellulose (0.2 J) [68],therefore, it is extremely hazardous to handle molten ADN.The researchers should take great care of performing any operation involving molten ADN which must be considered as a hazardous material.
3.4.Atomic layer deposition technology
Atomic Layer Deposition (ALD) is an advanced technique for nanostructure fabrication and surface modification [69,70](Fig.34).Thin film growth with atomic-level precision is achieved by chemical reaction of the gas-phase precursor with the substrate material in the reaction cell[71,72].The gas-phase precursors enter the reaction cell in the form of alternating pulses,so adjusting the period of the alternating pulses can control the growth thickness of the film.The thin film grown on the surface of ADN can effectively prevent water molecules in air from contacting the ADN surface,thus reducing the hygroscopicity of ADN.
With the development and innovation of experimental instruments, Gong et al.[73] synthesized alumina thin films coated with ADN by atomic layer deposition technique in 2014.The hygroscopicity was carried out at 25°C and 70% RH (Table 15).The results showed that the hygroscopicity of ADN samples increased slightly after ALD alumina coating,and the thickness of the coating film had basically no effect on the hygroscopicity.It was probably due to the existence of voids inside the ALD film which allowed water molecules to pass through.These voids provide gathering places and transport channels for moisture to penetrated rapidly throughout the whole film by the capillary cohesion effect,instead increasing the hygroscopicity of ADN.
Atomic layer deposition (ALD) allows the preparation of highquality and homogeneous films, its ability to operate at low temperatures,which is important for the coating of energetic materials.However, about 60% of the precursors are wasted during ALD because of its characteristics of inherent layer-by-layer which makes the deposition efficiency very low.Moreover, the available ALD materials only are inorganic metal oxides as an option,so that ALD cannot meet the requirements of organic polymer coatings currently.There are several problems that need to be addressed urgently for the future development of this technology[74].
3.5.Comparison and analysis of surface coating methods
In this chapter,ADN coating methods are classified into solvent volatilization method, solvent-nonsolvent method, melt crystallization method and atomic layer deposition method according to the basic principles of coating methods.
Solvent evaporation and solvent non-solvent methods are commonly used in laboratory research, with the advantages of safety and simple operation.They can form a uniform thin film layer with relatively easy control over its thickness,and are suitable for various types of ADN particles.The main difficulty lies in the fact that different liquid-phase systems have a significant impact on the coating effect, especially the solvent non-solvent method for dissolving ADN.Improper solvent selection may have negative effects on ADN.Almost all liquid-phase methods require the use of organic solvents, and the solvent evaporation process may release some harmful substances into the environment, causing pollution.
The melt crystallization method simplifies powder preparation and surface coating technology into one step, utilizing surface tension to achieve the coating of ADN particles during the crystallization process, thus simplifying the prilling process of ADN.However, production scale is relatively small, and the thickness of the encapsulation layer is not easily controlled.Melted ADN has high mechanical sensitivity which poses a certain safety risk during experimental operations.
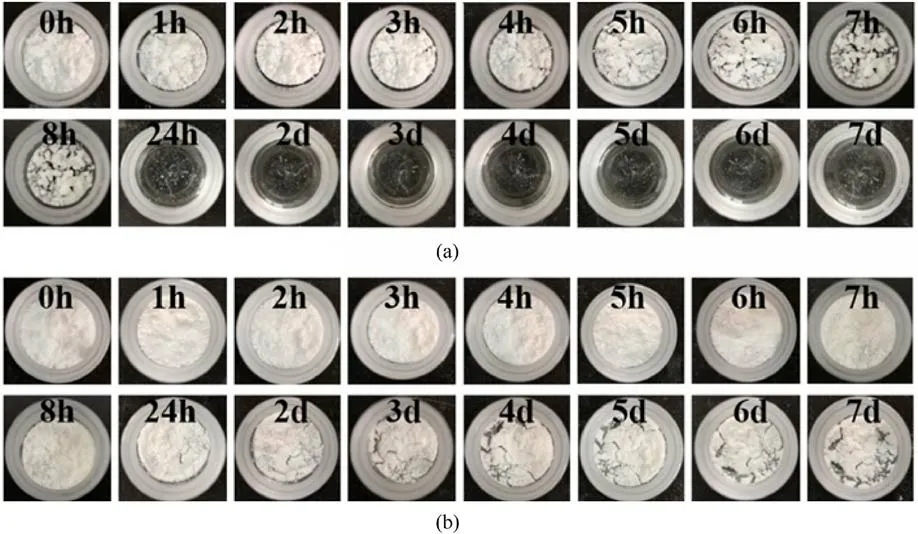
Fig.18.Macro-morphologies of (a) ADN and (b) ADN@POSS-NH2 after moisture absorption at different time (75% RH, 20 °C).
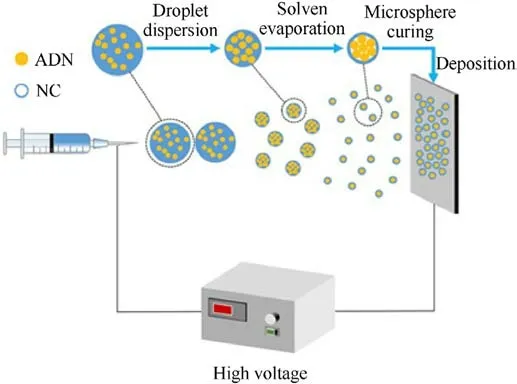
Fig.19.Schematic diagram of electrostatic spraying process.
Atomic layer deposition is a fine technology that can easily control the thickness of the encapsulation layer and obtain uniform and dense coatings.It can also avoid environmental pollution caused by some organic solvents.However, the choice of coating material is limited, and the production process is complex,requiring professional equipment and technology, resulting in higher costs.
Liquid phase method is safer and more economical than the other two methods, and is the preferred method for coating ADN.However,the influence of the coating process on the coating effect and the improvement of ADN recrystallization morphology in liquid-phase ADN still need further study.
4.Surface coating materials of ADN
The previous section reviews the coated ADN prepared by different methods and their hygroscopic status.It reveals that the coating materials chosen for each method are different which have a greater influence on the hygroscopicity of the coated ADN.
The following summarizes the coating materials used for ADN coating modification,which are mainly divided into six categories:energetic materials,surfactants,polymers,coupling agents,carbon materials and metal oxides (Table 16).
4.1.Energetic materials
The use of low-hygroscopic or non-hygroscopic energetic materials for the surface coating of ADN can isolate moisture from the air and achieve the anti-hygroscopic effect without affecting its energy performance.
AP, as a strong oxidizer with excellent overall performance at present, has a low hygroscopicity with only 1.11% for 72 h.The hygroscopicity of nano-AP is 3.01%[75],which is much lower than the hygroscopicity of ADN.Therefore, it is feasible to use AP as an anti-hygroscopic coating material for ADN on the theoretical basis.Yang [58] used AP as a coating material to prepare composite particles of ADN,but its coating amount reached 50%,which made the ADN loses its original advantages of low characteristic signal and environmental protection.
TNT is used as a safer explosive and has low hygroscopicity,and the co-crysta simulation reveals the existence of hydrogen bonding force between ADN and TNT[14].TNT was used as a surface coating material for ADN [43], and the hygroscopicity results showed that the coating of TNT had poor improvement on the hygroscopicity of ADN.It was probably because the ADN crystals were not deconstructed in the coating experiments, and TNT did not form an effective co-crystal with ADN to support favorable interfacial bonding.In the case of TNT,its oxygen balance is-74%,and using it as coating materials for ADN would reduce the oxygen balance.
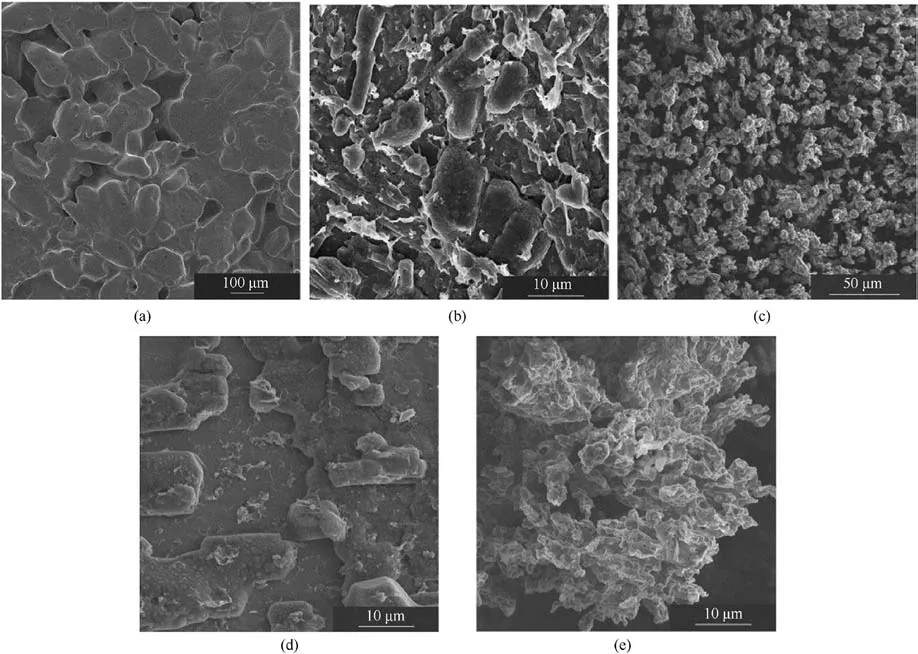
Fig.20.SEM images of raw ADN and ADN/NC obtained at different concentrations: (a) Raw ADN, (b) 2.5 mg/mL; (c) 5 mg/mL; (d) 7.5 mg/mL; (e) 10 mg/mL.
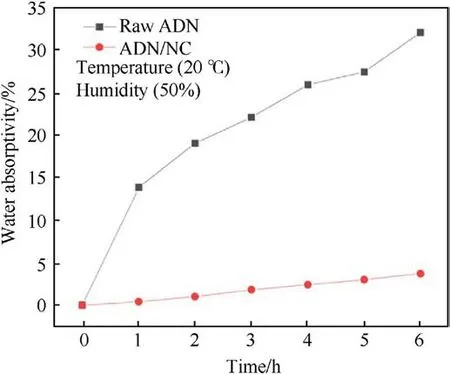
Fig.21.Water absorption curves of raw ADN and ADN/NC.
Lu et al.[76]doped ADN molecules with explosives(CL-20,RDX,HMX) molecules and found that doping a small amount of AN explosives molecules could improve the hygroscopicity of ADN.Therefore,energetic materials such as CL-20,RDX,and HMX can be considered as surface coating materials for ADN.
4.2.Surfactants
Surfactants are substances that contain fixed hydrophilic and lipophilic groups.When added in small quantities to solution systems, they can cause significant changes in the interfacial state of these systems.When ADN is coated by surfactants,the hydrophilic groups of surfactants interact with the hydrophilic groups of ADN,so the lipophilic groups of surfactants are exposed to the surface and play a hydrophobic role, thus the hygroscopicity of ADN is reducing [77].
Surfactants used as ADN surface coating materials mainly include cationic surfactants (CTAB), anionic surfactants (PA and HA),amphoteric surfactants(A18)and nonionic surfactants(HEXA,SA, EC and CAB).
EC is a polymeric nonionic surfactant which is insoluble in water.It is often used as a binder for tablets and film coating material in drug synthesis [78].It was used as coating materials of ADN,and the hygroscopicity of coated ADN decreased by more than 40% at the 64% and 72% RH [41], indicating that it had a good inhibiting effect on the hygroscopicity of ADN.
CTAB,PA,HA,and A18 all have long carbon chains,which make them hydrophobic.The moisture absorption rate of ADN coated by these materials showed that the moisture absorption rate of ADN was reduced to different degrees after coating,which proved their feasibility as the coating materials of ADN.
4.3.Polymers
Polymers have the advantages of low hygroscopicity,high elastic deformation and viscoelasticity,as well as abrasion resistance,heat resistance,and corrosion resistance.Using polymers as the coating materials of ADN can not only uniformly coat the surface and reduce the hygroscopicity of ADN,but also increase the tolerance of ADN to the environment [79,80].
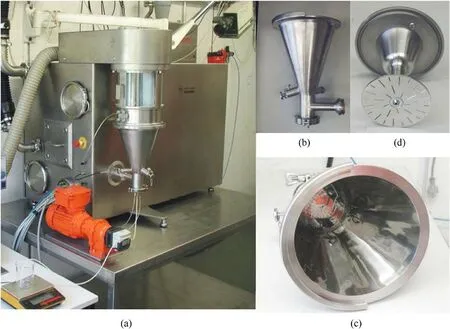
Fig.22.(a) Fluidized bed coating apparatus; (b), (c) Process vessel of the fluidized bed apparatus; (d) Perforated bottom plate.
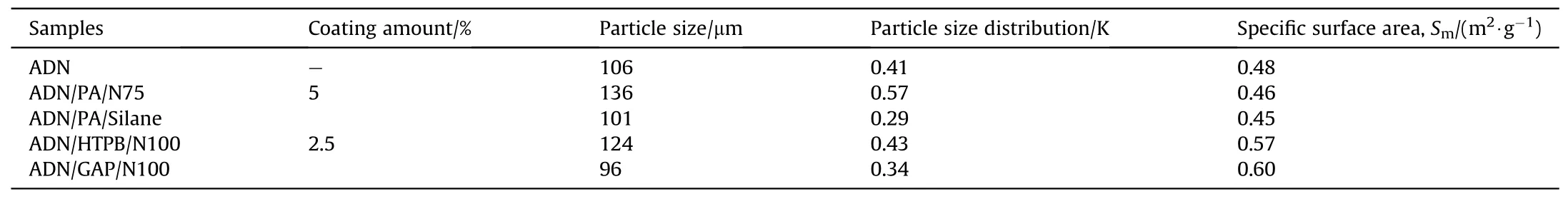
Table 5 Particle size and specific surface area analysis of ADN particles before and after coating.
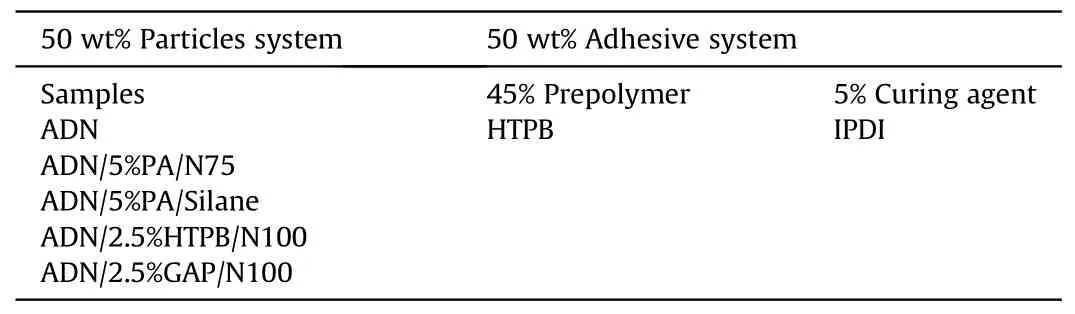
Table 6 Detailed composition of the samples used in the isocyanate IPDI curing tests.
The polymers used as the coating materials of ADN can be classified into two types according to their structures: linear polymers and network polymers.The network polymers have the larger coverage range than the linear polymers,so that ADN coated by network polymers has the better anti-hygroscopic effects at low coverage amounts which is proved by the lower moisture absorption of ADN coated by cross-linked polymers.In addition,different functional groups on the chains of molecules also affect the coating effect.Polymers containing polar groups such as hydroxyl and carbonyl groups can form hydrogen bonds with the surface of ADN,which are more easily attached on the surface of ADN.
HTPB has low glass transition temperature, excellent hydrophobicity,high level of active function,and low volatility[81],and one of its most important applications is as binders in solid propellants and pyrotechnics [82,83].Moreover, the -OH in the structure of HTPB easily forms hydrogen bonds with the surface of ADN, which makes HTPB as ideal coating materials of ADN.HTPBcoated ADN prepared by solvent volatilization under ultrasonicassisted conditions showed a reduction of 70% in hygroscopicity,which implied a good resistance to hygroscopicity[46].The HTPB/N-100-coated ADN which was prepared by fluidized beds has better compatibility in IPDI curing system [54].All the results showed the advantages of HTPB as the coating materials of ADN.
4.4.Coupling agents
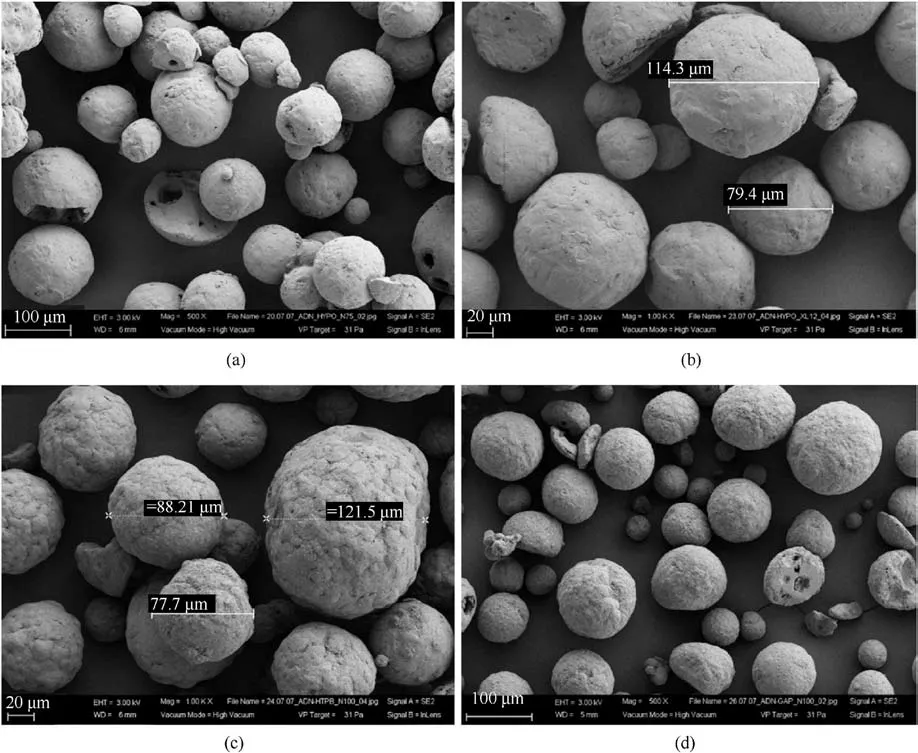
Fig.23.(a) ADN coated by 5% PA/N75; (b) ADN coated by 5% PA/silane; (c) ADN coated by 2.5% HTPB/N100; (d) ADN coated by 2.5% GAP/N100.
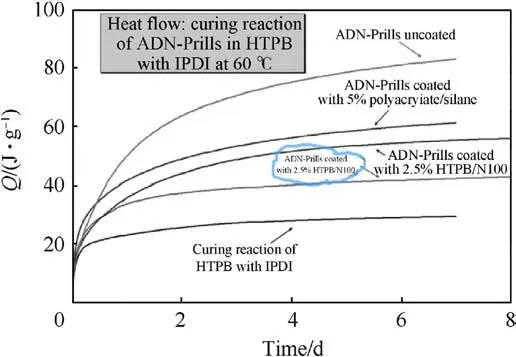
Fig.24.Microcalorimetry of ADN particles in HTPB/IPDI.
The coupling agent is a chemical substance with amphoteric structure that contains two groups with different chemical properties at each end of its molecular chain.At one end, it can hydrolyze with the water molecules on the surface of ADN and to form strong chemical bonds.At the other end are organic long chains,which acts as hydrophobic barrier on the outside.Coating ADN with coupling agents can reduce the surface energy of the ADN,which in turn decreases its hygroscopicity.In addition,coating ADN with coupling agents can significantly improve the mechanical properties of ADN-based propellants [84,85].
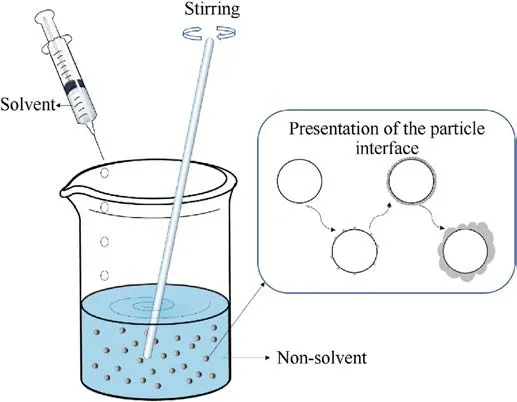
Fig.25.Diagram of the solvent-non-solvent method.
Fig.26.(a) TG of ADN coated in ethanol/DCM system; (b) TG of ADN coated in acetone/DCM system.
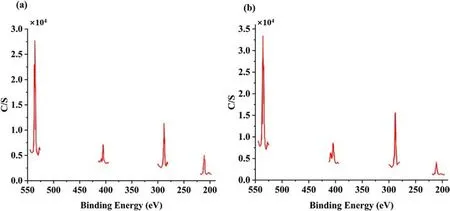
Fig.27.(a) XPS of ADN coated by AP in ethanol/DCM system; (b) XPS of ADN coated by AP in acetone/DCM system.
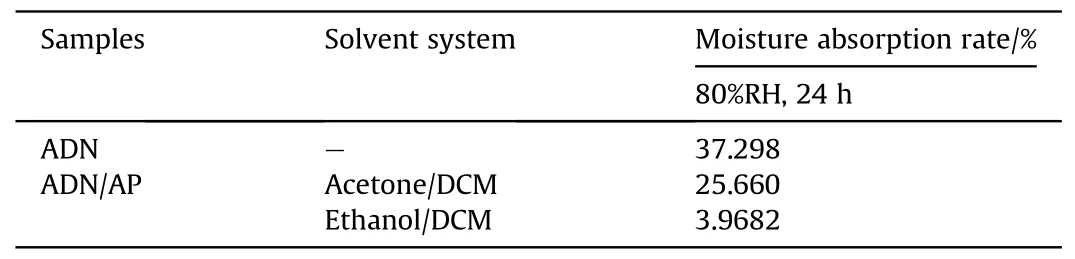
Table 7 Moisture absorption rate of ADN coated by AP in different solvent systems.
Depending on the molecular structure,the coupling agents used for the coating of ADN can be classified as phthalate coupling agents(NDZ-311),silane coupling agents(KH550,VTOS,TMOS,TOS and TOVS), and organic fluorine coupling agents (alcohol-amine salt type, carboxylate type, and ammonium carboxylate type).
Phthalate ester type coupling agent NDZ-311 is often used for surface modification of inorganic materials,which can improve the compatibility of inorganic fillers with organics [86], and the abrasion resistance of substrates is improved[87].When it was used as the coating material of ADN[30],the moisture absorption rate was reduced by 23% and ceased in the short time.
Organic fluorine coupling agents are coupling agents whose C substituted by F on the chains.Since fluorine is the most electronegative element with a strong ability to attract electrons and can shield the electron cloud of the C-C bond.Coupling agents containing F have lower free energy and show the properties of hydrophobia and oleophobia [88].When their branched chains are substituted by polar functional groups, they are easy to form hydrogen bonds with the ADN surface and are more easily bound to the ADN surface.Three organic fluorine coupling agents used to coat ADN [63], and the saturated moisture absorption of coatedADN was below 0.61%, which proved the feasibility of these materials as coating materials for ADN.
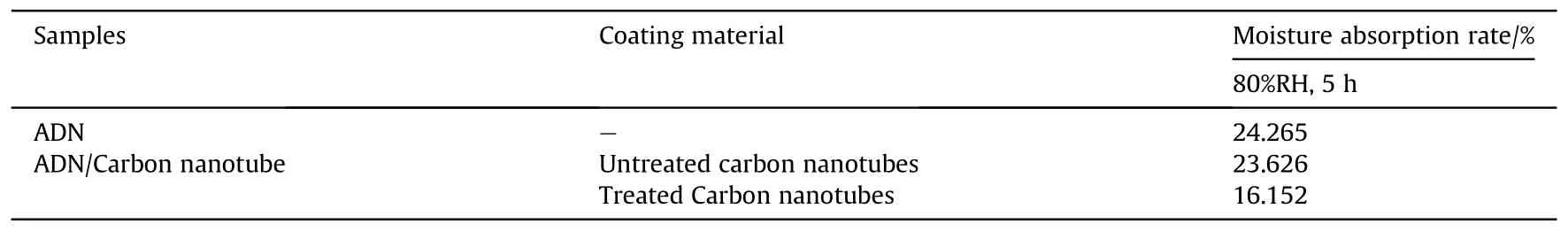
Table 8 Moisture absorption of ADN coated by carbon nanotube.
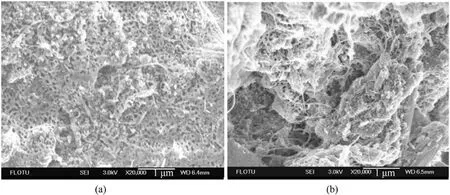
Fig.28.(a) SEM of ADN coated by untreated CNTs; (b) SEM of ADN coated by treated CNTs.
Fig.29.(a) Schematic of conventional emulsion; (b) schematic of Pricking emulsion.
4.5.Carbon materials
Carbon materials which are used to coat ADN include:graphene and its derivatives and carbon nanotubes(CNTs).Graphene and its derivatives have unique two-dimension nanostructures, superb specific surface area and ultra-high mechanical properties[89],and graphene has stronger hydrophobicity after amination.Carbon nanotubes (CNTs) are 3D hollow tubular structured carbon materials which have the advantages of huge aspect ratio,high modulus,and high strength.Because these materials are hydrophobic and have 2D or 3D structure, theoretically they can cover ADN and isolate external moisture, which can play the role of anti-hygroscopicity.
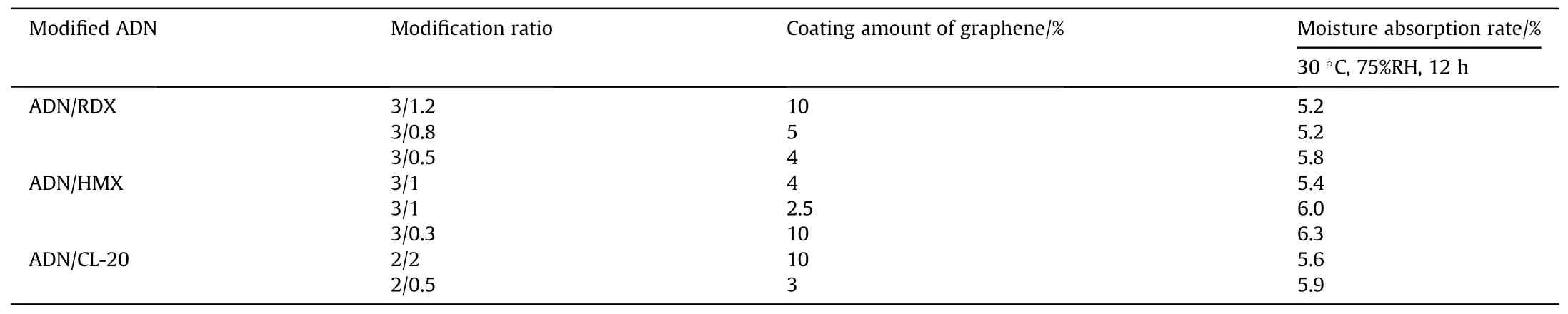
Table 9 Moisture absorption of modified ADN coated by graphene.
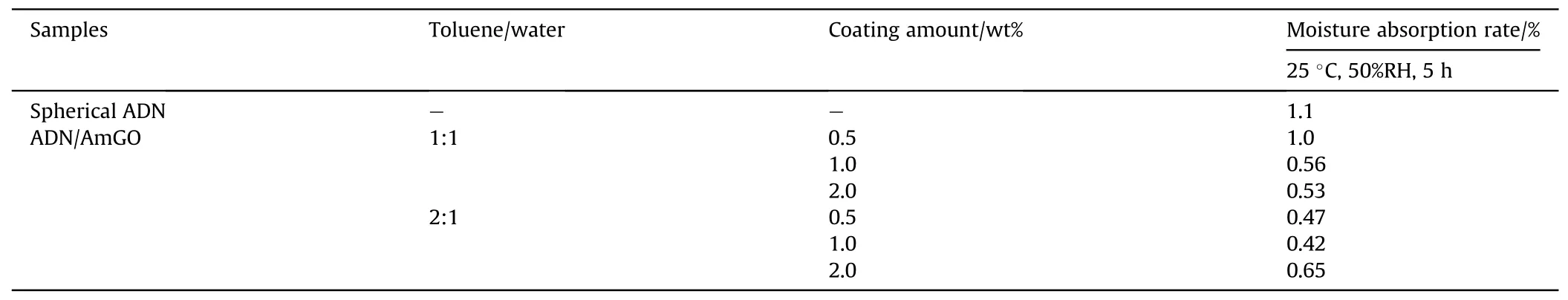
Table 10 Moisture absorption rate of ADN coated by AmGO.
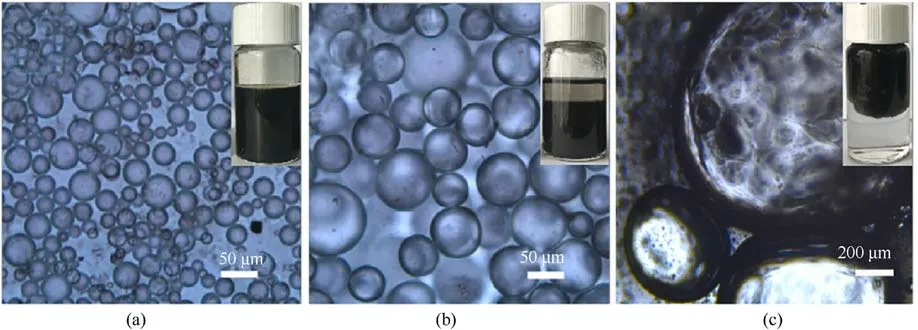
Fig.30.Optical micrographs of AmGO stabilized Pickering emulsion at different toluene/water ratios of (a) 2:1, (b) 1:1, (c) 1:2.
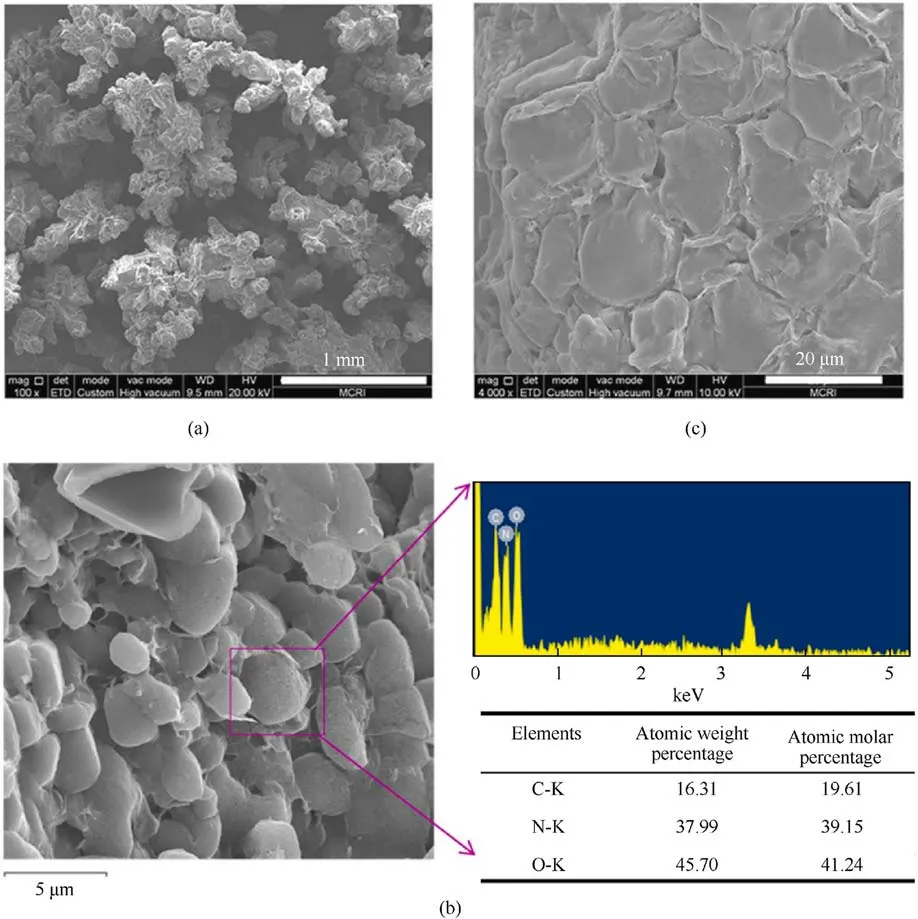
Fig.31.(a) ADN pellets, SEM images of ADN@(1.0 wt%).AmGO obtained by drying Pickering emulsion at different toluene to water ratios (b) 2:1 and (c) 1:1.
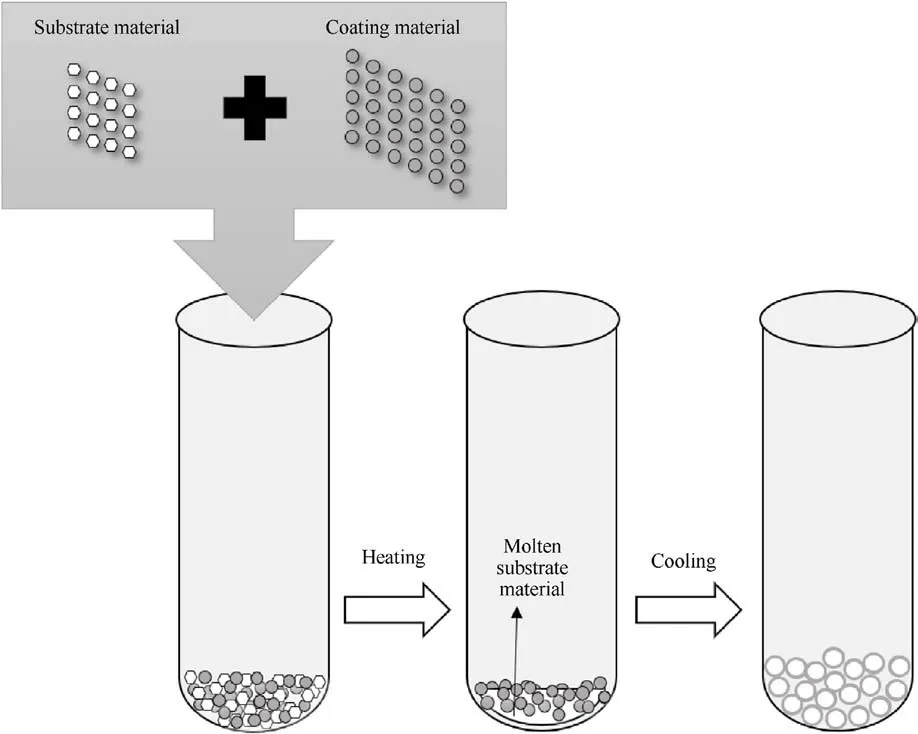
Fig.32.Diagram of melt crystallization method.
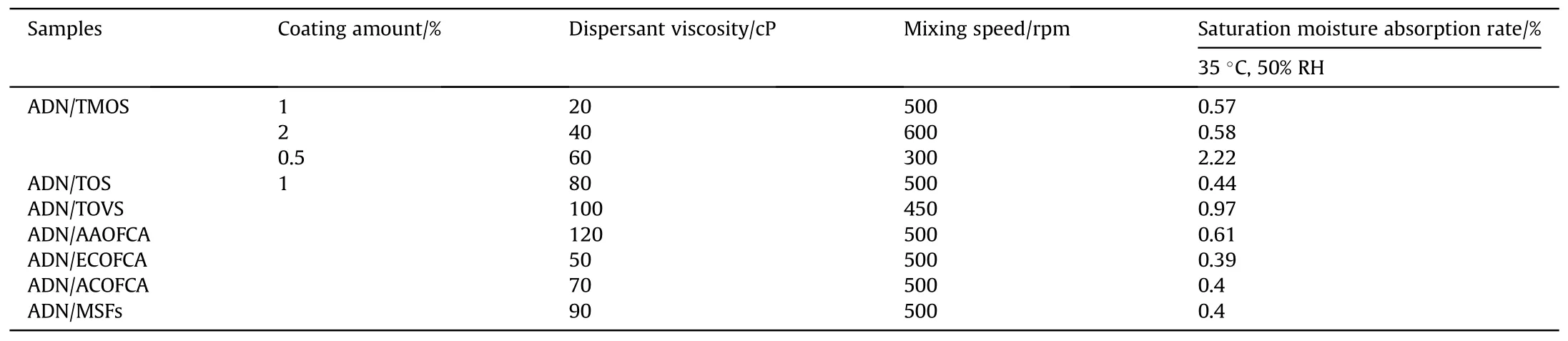
Table 11 Moisture absorption of ADN coated with different coating materials.
These materials are insoluble in conventional organic solvents,so it is only through the solvent-nonsolvent method that ADN can be recrystallized inside these materials to achieve the effect of coating against hygroscopicity.The hygroscopicity of AmGO-coated ADN by Pricking emulsion method was reduced up to 50%,but the morphology of ADN was not controlled in the recrystallization process[61].When ADN recrystallized in the CNTs system,some of the ADN precipitated outside the CNTs, which made the coating poorly resistant to moisture absorption[58].Therefore,the coating methods suitable for this type of materials still need to be explored in the future.
4.6.Metal oxides
The use of metal oxide materials to coat ADN is mainly limited by the coating method.Metal oxides can dissolve in acid or alkali solutions instead of conventional organic solvents,but using acid or alkali solutions as a dispersion system for ADN will cause ADN to dissolve in the water of the system,and it is very dangerous for ADN as an energetic material to contact with acid and alkali solutions.Therefore, it is difficult to coat ADN by liquid phase method using metal oxides as coating materials.
Fig.33.Schematic illustration of electrospray apparatus.

Table 12 Moisture absorption of ADN particles coated by melt crystallization method.
The conventional high-temperature resistant materials can be coated by mixing with the precursors of metal oxides and calcining at high temperature to generate metal oxides on the surface of substrate materials.While ADN as an energetic oxidizer cannot be calcined at high temperature.Alumina was chosen as the coating material because it is a commonly used material that can be synthesized by ALD method.Moreover,ALD alumina films can reduce the permeability of water molecules to 6 × 10-6g/(m2·d-1), so depositing alumina films on the surface of ADN is possible to isolate water molecules from the ADN particles thus reduce the hygroscopicity of ADN.Therefore, only Gong et al.[73] produced Al2O3film-coated ADN by atomic layer deposition method which can react at low temperatures.However, the moisture absorption of coated ADN increased.Therefore,the coating methods suitable for this type of materials still need to be explored in the future.
5.The principles of selecting surface coating materials
As an effective means to prevent ADN from moisture absorption,surface coating mainly can also reduce the mechanical sensitivity of ADN.However, due to the different types and properties of the coating materials, which have a great impact on the moisture absorption of ADN after coating.The selection of suitable coating materials according to the effect of moisture absorption to be achieved is the first principle to achieve the anti-hygroscopicity of coating.
5.1.Selection principles
Generally speaking,the following requirements are imposed on the coating materials of ADN.
(1) Coating materials should have low hygroscopicity or no hygroscopicity to ensure that the surface of ADN after coating is not hydrophilic to achieve resistance to hygroscopicity.Polymer materials such as PS with low surface energy[90],as well as surfactants and coupling agent materials which contain hydrophobic groups[63]reduced the hygroscopicity after coating ADN.
(2) Coating materials should have excellent ductility and plasticity, it can be uniformly dispersed and adsorbed on the surface of ADN.Otherwise, the ADN coating is incomplete and cannot achieve good anti-hygroscopic effect.The dense ADN particles coated by GAP/BPS crosslinked polymer had a saturation moisture absorption rate of only 0.78% [44].
(3) Coating materials should have good interfacial bonding with ADN, otherwise the coating layer is easy to fall off and the effect of reducing moisture absorption cannot be achieved.The Al2O3film obtained by ALD had gaps with the surface of ADN,so that external moisture penetrated into the interior of ADN through these gaps, which made the moisture absorption of the coated ADN increase [73].
(4) The surface-coated ADN should be suitable for the existing formulations of solid propellants and explosives.Compared to pure ADN, the compatibility of HTPB, PA and GAP-coated ADN with IPDI was all improved [54].Therefore, the compatibility of ADN, coating materials and other components in propellants and explosives needs to be considered.
5.2.Compatibility judgment method
The compatibility of energetic materials refers to the degree of change in the reactivity of the mixed system compared with the original single substance, when the energetic materials are in contact with each other or with other substances to form a mixed system [91,92].If the mixed components are incompatible, the system will occur precipitate crystals, generate gases, and accelerate heat release [93].Since energetic materials have destructive property as the main effect, the poorly compatible component system will interfere with use and endanger the personal safety of users in case of accidents.Therefore,the compatibility of energetic materials is an important index for evaluating their stabilities and reliabilities, and is also an important basis for evaluating their potential hazards in the design, production and transportation [94].
For the determination of the compatibility of mixed components, there are several common test methods, mainly including:VST, TGA, DSC, MC, MDS and IR.For these test methods, differentcountries or organizations have different experimental requirements and judgment standards.
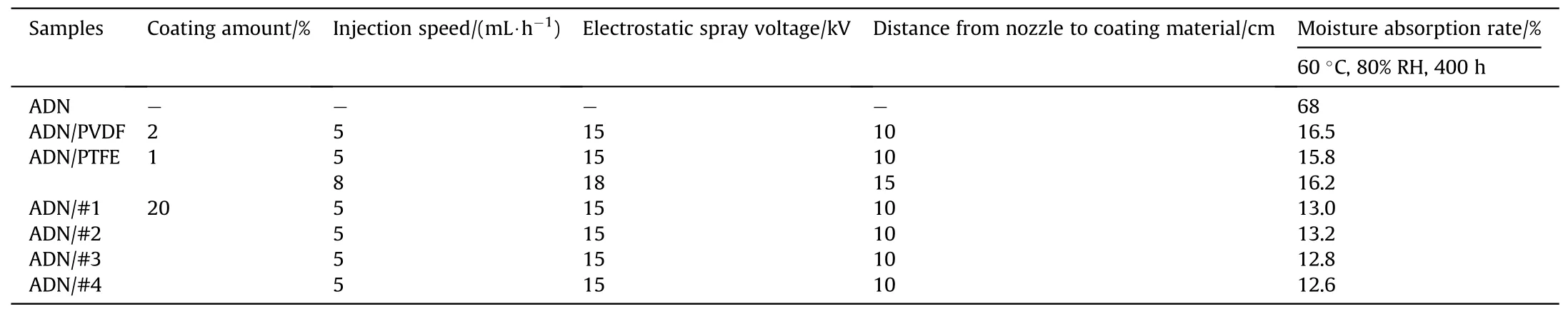
Table 13 Moisture absorption rate of ADN particles coated with electrostatic spray device.
5.2.1.Vacuum stability testing (VST)
VST can be used to test the compatibility of mixed components of energetic materials.It is based on the principle of assessing the compatibility of mixed systems by comparing the difference (R)between the gas evolution of the mixed component and the sum of two single components after heating [95].
Chinese military standard GJB 5891.16-2006 [96] test has the following requirements: 0.5 g of explosives, 0.5 g of contact materials and 1.0 g of mixed samples with a mass of 1:1 heat at a constant temperature of 100°C for 40 h respectively to compare the net outgassing R.The standards for evaluating compatibility are as follows:
R <0.60 mL, the mixed system is compatible.
0.60 mL ≤R ≤1.00 mL, the mixed system is less compatible.
R >1.00 mL, the mixed system is incompatible.
Zhang et al.[97]heated the ADN and NC/NG components with a mass ratio of 1:1 at 90°C for 40 h as required by this standard and found R=11 mL,indicating that the mixed systems of ADN and NC/NG interacted violently with each other and were severely incompatible.
The NATO has different experimental requirements and judgment standards for VTS, namely STANAG 4147 - test 1 [98].It requires that 2.5 g of explosives, 2.5 g of contact materials and 5.0 g mixed samples with a mass ratio of 1:1 heat at 100°C constant temperature for 40 h respectively.For the materials which decompose too quickly below 100°C,the test can be conducted at 100°C, but the time of minimum constant temperature should be increased by 2.5 times whenever the heating temperature decreases by 10°C.The judgment standards are as follows:
R <5 mL, the mixed system is found to be compatible.
R >5 mL, the mixed system is determined to be incompatible.
R=5 mL,it is necessary to take other methods to determine the compatibility.
Dimić M [99].heated samples of propellants (NGB-051), polymeric materials(Nylon 12 and PMMA)and their mixtures at 90°C for 96 h.The results of VTS were shown in Table 17.It was found that NGB-051 was incompatible with Nylon 12 and compatible with PMMA.
The advantage of VST is that the operating temperature of test is close to actual application, and the data has high reference value.However, VST is conducted in a vacuum environment, so the evaluation cannot accurately determine the compatibility of mixed components in air.In addition,the VST has a long period and only shows the reaction results.Moreover, it is only applicable to reaction systems that emits gases.
5.2.2.Thermal gravimetric analysis (TGA)
TGA is used to test the compatibility of mixed components of energetic materials.The principle is to measure the compatibility of mixed components by comparing the difference between the change in mass of the mixed components and the sum of the two single components after heating.
The standard of NATO STANAG 4147-Test 3-Procedure 3 A-Dynamic thermogravimetry [98] requires that the single component and mixed components with a mass ratio of 1:1 heat at a rising rate of 2°C/min under the N2atmosphere to measure the mass change.The difference (Δ G) between the mass change of mixed components and the sum of two single components is calculated which can be used to measure the compatibility of mixed system.The judgment standards are as follows:
Δ G <4%,indicating that the mixed components are compatible.
Δ G in the range of 4%-20%, indicating that the mixed components are incompatible to some extent and need further testing.
Δ G > 20%, indicating that the mixed components are incompatible.
Santhosh G et al.[100] evaluated the compatibility of ADN and the binders GAP, GAP/PBAMO (80/20), PEG1500 and HTPB.The weight loss percentages were calculated from Figs.35(a)and 35(b).It was found that the Δ G of ADN with PEG1500 was 24.6%, indicating that their mixed components were incompatible,and the Δ G of the mixed components of ADN with the other three systems was less than the Δ G obtained from the single component calculation G,indicating that their mixed components were compatible.
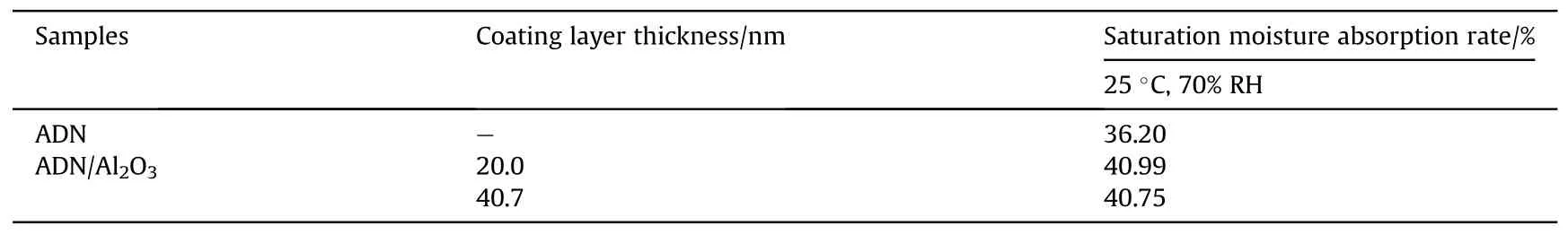
Table 15 Moisture absorption of ALD-deposited alumina-covered ADN.
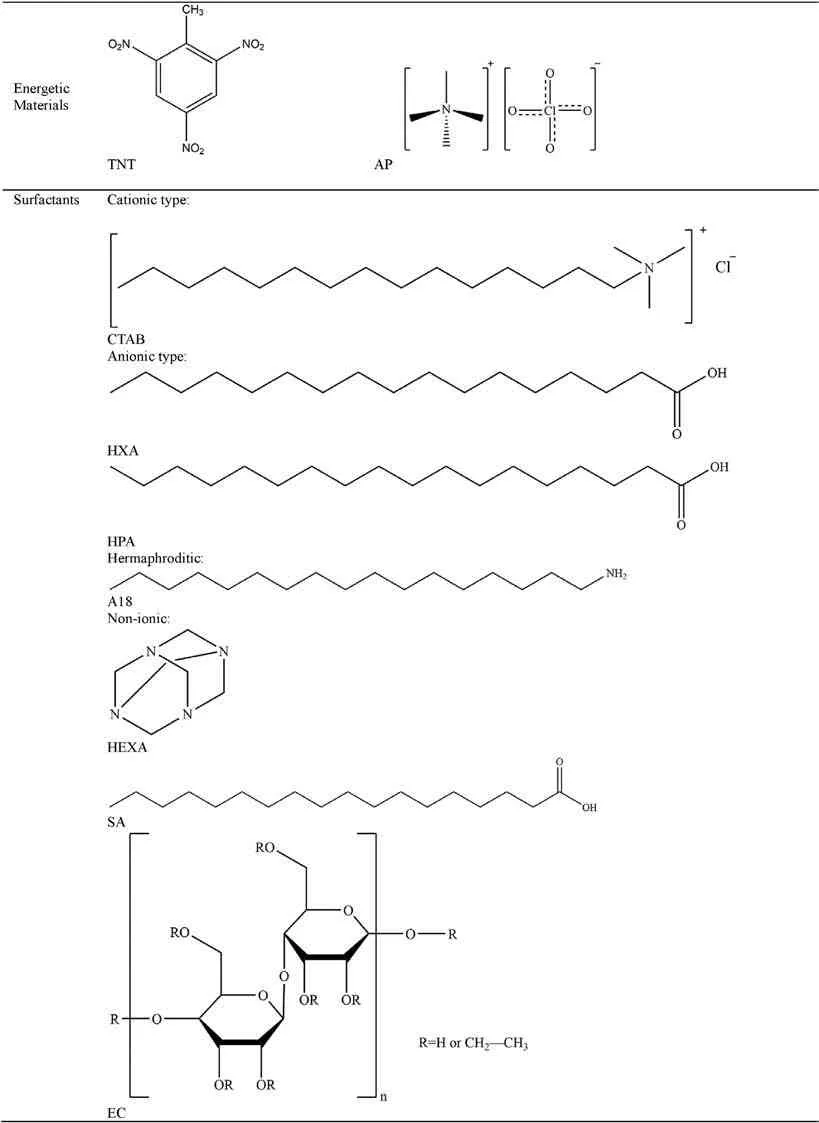
Table 16 Summary and classification of ADN coating materials.
?

?

?
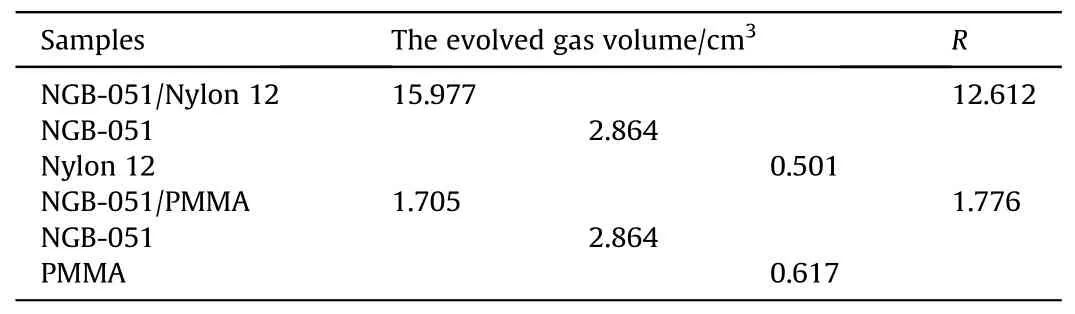
Table 17 Results of the VST.
Yang et al.[94] mentioned the NST method which analyze the weight loss under natural conditions.A certain proportion of a mixture, together with the blank samples of two single components,are placed in a constant temperature environment,and their weight loss are recorded periodically.This method evaluates the compatibility by comparing the percentage difference (Δ G%) between the weight loss of the mixed components and the sum of two single components,as well as the ratio(K)of the weight loss to the amount of decomposition.The judgment standards are as follows:
Δ G ≤0.1%, the mixed components are compatible.
0.1% <Δ G <0.2%, the mixed components are sub-compatible.
Δ G ≥0.2%,the mixed components are incompatible.
Meanwhile,
K ≤1, no chemical reaction between components, the mixed components are compatible.
1 <K <2,the amount of decomposition of the components is so greater than the amount of reaction, that the mixed components are sub-compatible.
K ≥2, the amount of decomposed component is less than the amount of reaction, and the chemical reactions occur mainly between two components, so mixed components are incompatible.
TGA has the advantages of simplicity, rapidity and low dosage,but it is only suitable for the compatibility determination of substances which are not volatilize or sublimate easily.NST takes a long time, while can better simulate the actual situation.So, it is generally used to evaluate the mixed components of energetic materials with more stringent compatibility requirements,providing an accurate basis for their safe storage and use in the natural environment.
5.2.3.Differential scanning calorimetry (DSC)
DSC is able to characterize physical or chemical processes by measuring changes in the thermodynamic properties of substances.The compatibility of the mixed components is determined by the change in peak temperature (Δ Tp) and apparent activation energy(Δ Ea)of decomposition between the mixed sample and two single components.
The Chinese military standard GJB 5891.17-2006 [101] describes that a very small amount of explosives, contact materials and mixed materials with a mass ratio of 1:1 (samples with large differences in density, change the ratio as appropriate) heat at a rising rate of 5°C/min respectively until the first decomposition peak appears on the DSC curve or the required experimental temperature is reached.
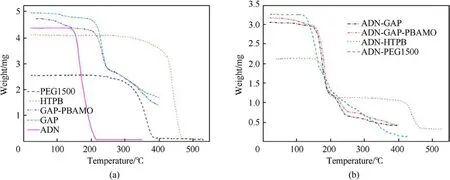
Fig.35.(a) TG of individual materials; (b) TG of ADN and its admixtures with polymeric binders.
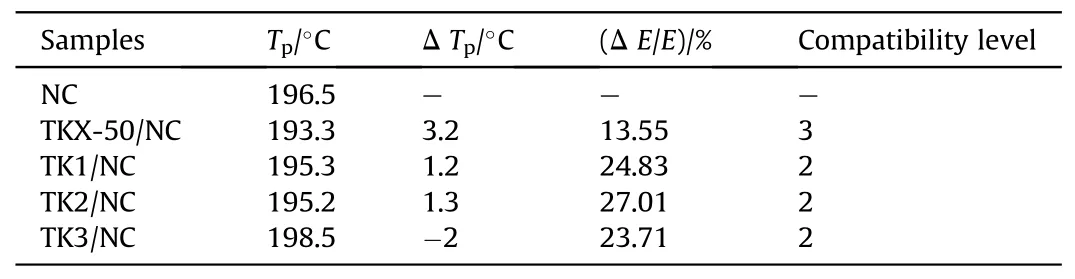
Table 18 Compatibility results of TKX-50, TK1, TK2 and TK3 with NC.
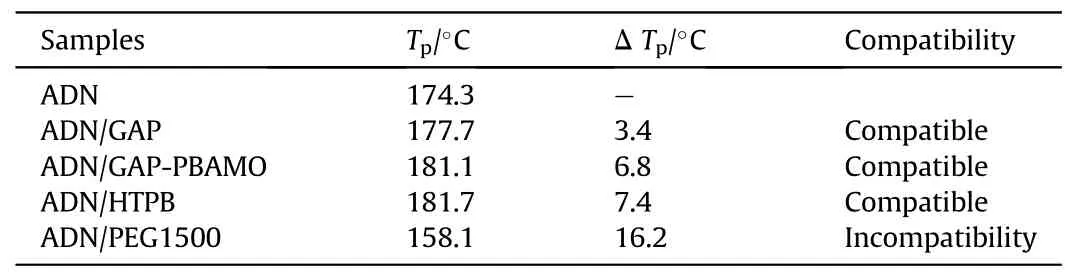
Table 19 Summary of STANAG DSC results.
According to Eq.(1),the change of the first decomposition peak temperature (Δ Tp) of the mixed sample relative to the reference sample is calculated.
where,
Δ Tp(°C): the change of the first decomposition peak temperature of the mixed sample relative to the reference sample.
Tp1(°C): the first decomposition peak temperature of the reference sample.
Tp2(°C):the first decomposition peak temperature of the mixed sample.
By calculating the apparent activation energy of the mixed sample and the reference sample, the variable fraction of the apparent activation energy of the mixed sample relative to the reference sample can be obtained according to Eq.(2).
where,
Δ E/Ea(%):variable fraction of the apparent activation energy of the mixed sample relative to the reference sample.
Ea1(kJ/mol):apparent activation energy of the reference sample.
Ea2(kJ/mol): apparent activation energy of the mixed sample.
The determination standards are as follows.
Δ Tp≤2.0°C,Δ E/Ea≤20%,compatibility is judged as good,with a compatibility grade of 1.
Δ Tp≤2.0°C,Δ E/Ea>20%,compatibility is judged as good,with a compatibility grade of 2.
2.0°C <Δ Tp<5.0°C,Δ E/Ea≤20%, compatibility is judged as poor, with a compatibility grade of 3.
2.0°C <Δ Tp<5.0°C, Δ E/Ea>20%, compatibility is judged as poor, with a compatibility grade of 4.
Δ Tp≥5.0°C, compatibility is judged as incompatible, with a compatibility grade of 5.
Ma et al.[102]tested the compatibility of their mixed system of Dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and NC modified with KH550 by DSC.The DSC curves were shown in Fig.36.In Table 18, it was found that the pure TKX-50/NC system with Δ Tp=3.2°C and Δ E/Ea=13.55%was less compatible.The Δ Tpof the KH550-modified TKX-50/NC systems (TK1, TK2, and TK3)were all ≤2.0°C,and the Δ E/Eawere all >20%.Compared with pure TKX-50/NC,the compatibility of these systems had been improved.
The standard of NATO STANAG 4147-Test 4 [98] is to judge the compatibility of mixed components by DSC, that the experiments are required to be conducted at rising rate of 2°C/min under N2environment.The temperature change(Δ Tp)of the decomposition exotherm peak of the mixed component is compared with that of the single component.The judgment standards are as follows:
Δ Tp<4°C, the mixed components are compatible.
Δ Tpis in the range of 4-20°C, the mixed components are incompatible to some extent.
Δ Tp>20°C, the mixed components are incompatible.
The compatibility of the mixed components is assessed by the activation energy Eacalculated by the Kissinger method in STANAG 4147-Test 3C [98].The standards are as follow.
(1) If the activation energy is lower and the frequency factor is larger for the admixture than the explosive, then the test material is considered incompatible.
(2) If only small differences in activation energy and frequency factors are observed but the rate constant of the admixture is greater than the explosive at room temperature,then the test material is considered incompatible.
(3) However small the difference in rate constants, if the value for the admixture at the temperature of interest is equal to or less than 10, then there is a strong indication that the test material is compatible.
(4) If the temperature of interest is elevated(i.e.>200°C)then the rate constant would be much lower at room temperature and would be compatible.
Santhosh G et al.[100] evaluated the compatibility analysis problem of ADN with binders GAP, GAP/poly-BAMO (PBAMO) (80/20),Polyethylene glycol-1500(PEG1500)and HTPB using DSC.The DSC curves were shown in Fig.37.In Table 19,it was found that the difference between the decomposition peak temperature of ADN/PEG1500 and ADN was 16.2°C (a value between 4°C and 20°C),indicating that the mixed component showed incompatibility.As seen in Table 20, The Eavalue of the ADN-PEG1500admixture was much lower than that of ADN, and the frequency factor (ln(A)) for the admixture was lower and the rate constant value (k) at 30°C was much higher than that of ADN,which concluded that PEG1500 was incompatible with ADN.
DSC has the advantages of rapidity, simple operation, small sample size and high safety.However, the method also has limitations.Firstly, DSC is used to determine the decomposition process of substances at a higher temperature, which is much higher than the actual use and storage temperature of mixed components.In the case of multiple decomposition peaks, it is necessary to determine whether the decomposition peak selected for evaluating the compatibility of substances is correct.Secondly,when the mixed components contain strong oxidizing or reducing agents, the DSC method may not be suitable for compatibility testing.Finally, the thermal stability of the sample under test is affected by various factors such as sample state, particle size,heating rate, and atmosphere, which may lead to errors in the results.Therefore, it is necessary to combine DSC with other compatibility testing methods to comprehensively evaluate the compatibility of mixed components.
5.2.4.Microcalorimetry (MC)
The MC method for determining the compatibility of mixed components of energetic materials is based on comparing the difference between heat flow curves (or heat) of two single components and mixed components at a certain temperature to evaluate their compatibility.
Chinese military standard GJB 5891.15-2006[103]uses the MC method for compatibility testing of energetic mixed components,and the experiment requires that 1 g tested agents, 1 g contact materials and 2 g mixed materials with a mass ratio of 1:1 heat at 100°C (the temperature can be reduced appropriately for faster decomposition samples)for 1 h in the microcalorimeter(sensitivity is not less than 50 mV/mW), and the heat flow is recorded continuously.To obtain the actual heat flow values of the three systems,their respective heat flow curves are recorded for a period of 40 h.The theoretical heat flow value of the mixed system is obtained by adding up the heat flow values of the measured samples and the contact materials.The standards are as follows.
(1) If the absolute value of the mixed sample heat flow value is
less than the theoretical heat flow value, or the absolutevalue of the mixed sample heat flow value is greater than the theoretical heat flow value,but the absolute value of the heat flow value is less than 0.1 mW/g, then the tested mixed sample is judged to be compatible.

Table 20 Activation energies, frequency factor and rate constant values for ADN and ADNPEG1500 mixture.
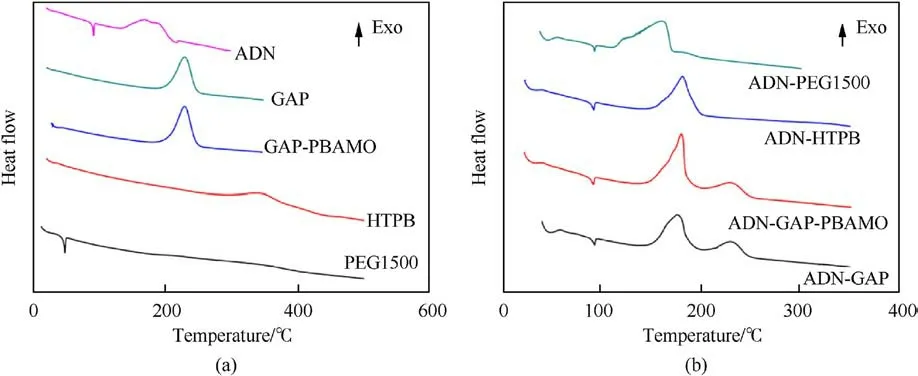
Fig.37.(a) DSC traces of individual materials; (b) DSC traces of ADN and its admixtures with polymers.
(2) If the absolute value of the heat flow value of the mixed sample is greater than the absolute value of the theoretical heat flow value,and the absolute value of the heat flow value is not less than 0.1 mW/g, then the tested mixed sample is considered to be less compatible (or incompatible).
According to NATO STANAG 4147-Test2 [98] standard, the heat production rate of a 1:1 mixed system weighing 10 g is measured over a period of 168 h.The experiments on the test explosives and test materials are repeated under the same conditions,respectively.
The relative increase(D)in heat flux of the mixture compared to the single component is calculated using Eq.(3).
where,M(J/g),E(J/g),and S(J/g)represent the time-integrated heat release values per unit mass of the mixture, explosive, and test material, respectively.
The standards are as follows:
D <2, the test material is compatible with the explosive.
2 = D ≤3, another method should be used to determine compatibility.
D >3, the test material is incompatible with the explosive.
Note:the formulas and standards only apply to 1:1 mixtures of explosives and test materials.
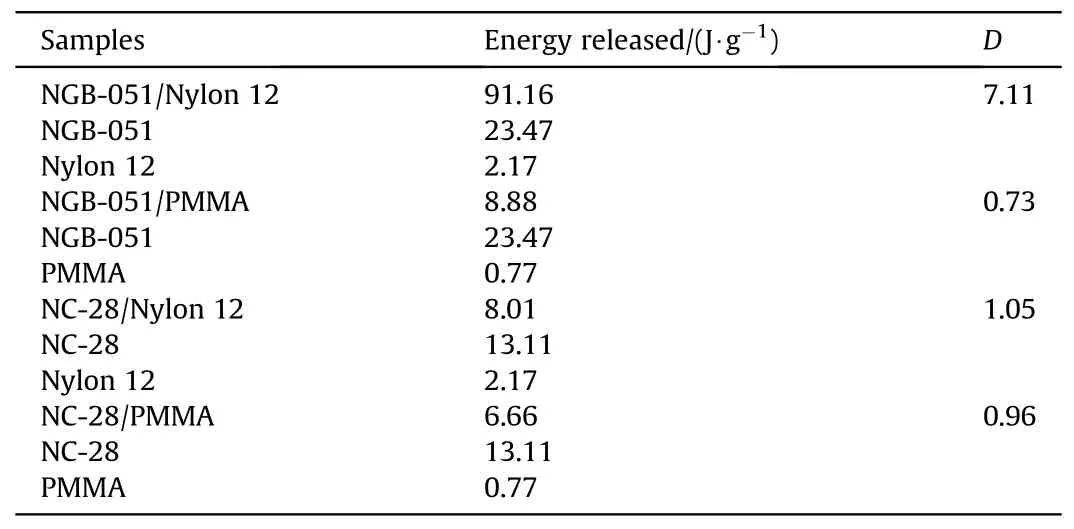
Table 21 Values of energy released and the relative compatibility.
Dimić M et al.[99]took the MC method of Test 2 in the STANAG 4147 standard, and compared the relative increase in heat flow of four mixed systems of two propellants (NGB-051 and NC-28) and two polymer materials(Nylon 12 and PMMA).The heat flow curves were shown in Fig.38.In Table 21, it was found that the relative increase in heat flow for the NGB-051/Nylon 12 system was D=7.11 >3,which was an incompatible system,and the rest of the systems with D ≤1.05, which all are compatible systems.
MC method has the advantage of high sensitivity,allowing it to detect even very small changes in heat.This makes it excellent for applications in the field of pyrolysis of energetic materials and highly effective for assessing the compatibility of components in energetic materials.
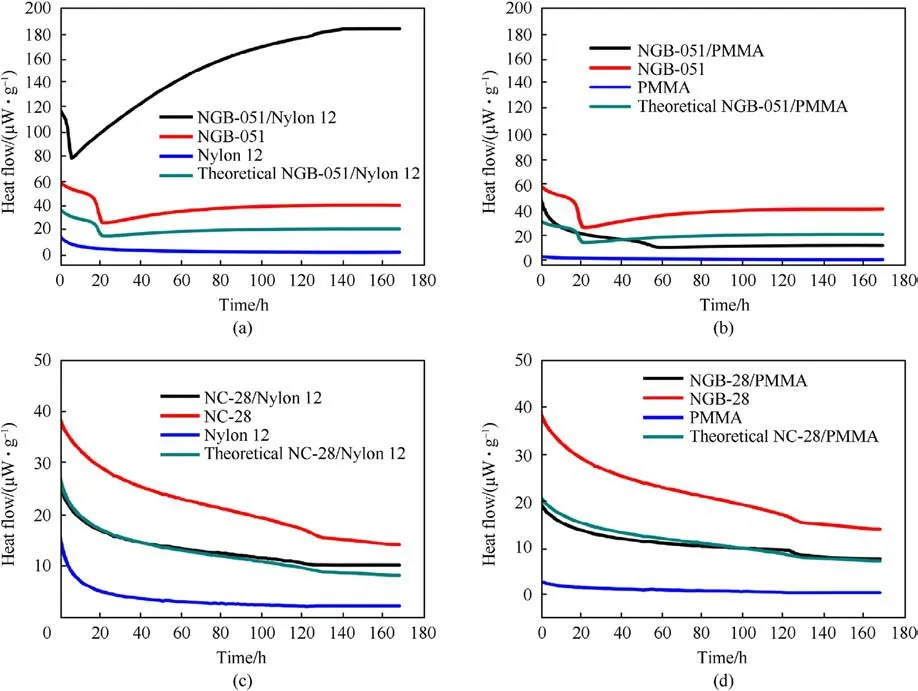
Fig.38.(a) Heat flow curves of components and mixtures NGB-051/Nylon 12; (b) Heat flow curves of components and mixtures NGB-051/PMMA; (c) Heat flow curves of components and mixtures NC-28/Nylon12; (d) Heat flow curves of components and mixtures NC-28/PMMA.
5.2.5.Molecular dynamics simulation (MDS)
The MDS method employs Material Studio (MS) to calculate relevant properties (such as solubility parameters and cohesion energy densities of single components,radial distribution function)or parameters (such as density variation of mixed components) to characterize the compatibility of mixed components in energetic materials.Up to now, there is no strict standard to judge the compatibility of materials using MDS,so it can only be evaluated by comparing the solubility parameters and binding energy of materials.Generally, the smaller the difference in solubility parameters of single components, the smaller the density change of mixed components, and the larger the change in intermolecular binding energy, the better the compatibility of the mixed components.
Sheibani N[104]evaluated the compatibility between ADN/GAP propellants and different isocyanate curing agents including N100,IPDI, toluene diisocyanate (TDI), dimeryl diisocyanate (DDI) and IPDI by studying the solubility parameters and interaction energy differences between ADN, GAP and different isocyanate curing agents using the concepts of molecular dynamics and solubility parameters.It was found that the interaction energy between IPDI/N100 curing system and GAP was 20.51 kcal/mol higher than that of ADN, and this energy difference was 18.67 kcal/mol when the curing system was DDI system,so IPDI/N100 and DDI were suitable curing systems.However, considering from the concept of solubility parameter,the solubility parameter of IPDI/N100 curing system differs from GAP by about 1.00 MPa0.5while DDI exceeds 5.00 MPa0.5indicating that there is no uniform miscibility between DDI and GAP.Therefore, in terms of interaction energy and solubility parameters, IPDI/N100 was finally screened as the most suitable curing system among the studied curing systems.
Chen et al.[105] used MDS to calculate the density, binding energy, radial distribution function, solubility parameters, trigger bond lengths and mechanical properties of the ADN/fluoropolymer hybrid system to explore the interaction and compatibility between ADN and fluoropolymers.The results showed that ADN had a stronger interaction and better compatibility with PTFE and F26 than PVDF, and the incorporation of fluoropolymers improved the mechanical and safety properties of ADN in the hybrid system.
It is worth noting that MDS can effectively assess the compatibility of mixed systems at the physical level but it does not prove that in the chemical level.Therefore, mixed systems that are expected to undergo chemical reactions are not suitable for compatibility assessment by this method alone.
5.2.6.Infrared spectroscopy (IR)
The principle of IR to assess the compatibility of mixed components in energetic materials is that the IR spectra produced by the mixed components show significant deviations compared to the spectra of the individual components.The degree of band deviation can be used to quantitatively characterize the compatibility between mixed systems of energetic materials.
Yan et al.[106]designed and synthesized the bonding agents Npropynyl-2,2′-dihydroxyethyl amine (BA-2) and N-propynyl-3,3′-dipropionitrile amine (BA-7) based on click chemistry to enhance the interfacial interaction between binder/solid filler of ADN-based triazole cross-linked curing system.The study characterized the reactivity of the bonding agent with the energetic binder represented by GAP using IR spectroscopy.As shown in Fig.39, the reaction between BA-2 and GAP was demonstrated the appearance of a vibrational absorption peak of the triazole ring skeleton at 1633 cm-1in the FL-IR spectrum.The weak absorption peaks of the triazole ring(H-C≡)gradually enhanced at 3144 cm-1,and the N=N and C=N skeletal vibrational peaks in the triazole ring appearing at 1636 cm-1and 1552 cm-1, respectively.These observations provided evidence of the reaction between GAP and BA-7.All of the above indicated that the bonding agent achieved bonding with the energetic binder through an addition reaction.
IR spectroscopy can quickly identify the occurrence of a chemical reaction in a system and the type and intensity of reaction from the positions of the bands.However, it is difficult to observe the band shifts of relevant functional groups in the non-bonded components.IR spectroscopy is generally used in combination with other methods to evaluate the compatibility of energetic polymer components.If the characteristic peak of the reactive groups is obscured by other strong absorption peaks,the peak change cannot be observed.
In summary,VST and DSC are the two commonly used methods to assess the compatibility of energetic materials.The two methods can complement each other to rapidly and accurately assess the compatibility of most energetic materials.TGA is also a kind of thermal analysis, which is typically used in combination with DSC to evaluate the compatibility of mixed materials while qualitatively and quantitatively analyzing their decomposition products.The MC method is suitable for determining the compatibility of mixed systems at relatively lower temperatures, mainly due to its higher sensitivity in detecting the heat release from reactions with lower exothermic rates at low temperatures.This method can be used to measure compatibility between mixed systems at lower temperatures,where other methods may not be as effective.
Fig.39.(a) FT-IR spectrum of the reaction of BA-2 and GAP; (b) FT-IR spectrum of the reaction of BA-7 and GAP.
Each method of judging compatibility has its own applicable scope and limitations.When evaluating the compatibility of different mixed components,relying on a single evaluation method may lead to misjudgment.Therefore, it is recommended that at least two or more methods be used in combination to determine compatibility.
6.Conclusions
Combined with the current research status, surface coating is widely used as an effective anti-hygroscopicity technology for ADN.Among above, the liquid phase methods such as solvent volatilization method and solvent-non-solvent method are likely to be the main methods for coating ADN in the future because of its safety and simple equipment requirements.But for the solvent-nonsolvent method using dissolved ADN, it is still necessary to control the morphology of ADN recrystallization to ensure the coating effect.
Compared with other materials, polymer materials are the preferred alternative coating materials due to their high viscosity,ease of adhesion and good anti-hygroscopic effect.Additionally,their wear resistance, heat resistance and corrosion resistance make ADN less sensitive and easier to preserve.However, considering the energy problem, the amount of coating needs to be controlled to reduce its impact on the performance of AND.Therefore, the coating process requires high precision to ensure that the coating layer is uniform and dense, thus achieving the desired anti-hygroscopicity effect.When screening ADN coating materials, various methods should be employed to assess the compatibility between components, while ensuring the effect of anti-hygroscopicity.This approach is necessary to ensure the safety of production and storage of ADN after coating, as well as its application in propellant and explosive formulations.
Up to now, research on ADN anti-hygroscopicity is not mature.However,surface coating of ADN can be combined with compound modification and spheroidization techniques to achieve the desired effect through the complement use of multiple methods.Compound modification and spheroidization of ADN can reduce moisture absorption, which can be further reduced after coating.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos.21805139,12102194 and 22005144), the Joint Funds of the National Natural Science Foundation of China (Grant No.U2141202), the Fundamental Research Funds for the Central Universities (Grant No.30921011203), the Young Elite Scientists Sponsorship Program by CAST (YESS Program,2021QNRC001).
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

