Synthesis of Organic-Inorganic Hybrid Aluminum Hypophosphite Microspheres Flame Retardant and Its Flame Retardant Research on Thermoplastic Polyurethane
LIU Shengpeng, XU Zhi, ZHANG Xinyuan, WEI Huan, XIONG Yun, DING Yigang,HUANG Wenbo, XU Lili,3*
(1.Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education, Key Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430205,China; 2.Hubei Three Gorges Laboratory,Yichang 443007, China; 3.School of Technology Materials Science and Engineering, Wuhan Institute of Technology, Wuhan 430205, China)
Abstract: Aluminum hypophosphite microspheres (AHP) were synthesized by hydrothermal method using NaH2PO2∙H2O and AlCl3∙6H2O as raw materials, and then the AHP microspheres were polymerized by surface polymerization of micro-nanospheres with cyclic cross-linked poly(cyclotriphosphazene-co-4,4'-sulfonyldiphenol) (PZS).A new organic-inorganic poly(phosphonitrile)-modified aluminum hypophosphite microspheres (PZS-AHP) were synthesized by encapsulation and applied to flame retardant thermoplastic polyurethane (TPU).The microstructure and chemical composition of the PZS-AHP microsphere were characterized by scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectroscopy and X-ray spectroscopy.The thermal stability of PZS-AHP microsphere was explored with thermogravimetric analysis.Thermogravimetric data indicate that the PZS-AHP microspheres have excellent thermal stability.The thermal and flame-retarding properties of the TPU composites were evaluated by thermogravimetric (TG), limited oxygen index tests (LOI), and cone calorimeter test (CCT).The TPU composite achieved vertical burning (UL-94) V-0 grade and LOI value reached 29.2% when 10 wt% PZS-AHP was incorporated.Compared with those of pure TPU, the peak heat release rate (pHRR) and total heat release(THR) of TPU/10%PZS-AHP decreased by 82.2% and 42.5%, respectively.The results of CCT indicated that PZS-AHP microsphere could improve the flame retardancy of TPU composites.
Key words: polyphosphazene; thermoplastic polyurethane; flame retardancy; aluminum hypophosphite;surface polymerization
1 Introduction
Thermoplastic polyurethane (TPU), a material with excellent mechanical properties, abrasion resistance, tear resistance, acid and alkali resistance,oil resistance, and prominent processability, has been widely used industrially[1,2].However, high flammability, heavy melt-dripping and toxic gases release during combustion restrict its application[3,4].Therefore, it is of great significance to decrease the combustibility and improve anti-dripping performance of TPU effectively.
Halogenated flame retardants (FRs) as an efficient strategy in the past have been prohibited in Europe due to their potential hazards to human health and the environment[5].Therefore, environmentally halogenfree flame retardants have attracted a lot of interest.Compounds based on phosphorus, nitrogen, and silicon have gradually replaced the use of halogenated flame retardants[6].Moreover, phosphorus and aluminum can play a synergistic role in flame retardant polymers[7].Aluminum hypophosphite (AHP) is a high-efficiency phosphorus-based flame retardant.Due to its good thermal stability, high flame-retarding efficiency and cost-effectiveness[8-10], it has been widely utilized in various polymers, such as PET, PBT, and PA[11-13].However, a phosphorus-only based additive needs high loading to achieve good fire resistant effect, which will greatly affect the mechanical properties of the materials.Moreover, in the case of heating or impact,AHP will decompose and release phosphine gas[11,14-16].Phosphine is spontaneously flammable in air, which is not conducive to storage and processing[17].Therefore, AHP is always modified mainly through microencapsulation and synergistic compounding to solve the above mentioned problems[18-20].Wuet al[21]prepared microencapsulated ammonium polyphosphate(EPAPP) with an epoxy resin shell.The results showed that EPAPP has better compatibility with polypropylene(PP) in the composite compared with ammonium polyphosphate (APP).Inspired by the above research,the capsule material should provide a protective layer for AHP, and it could also play a synergistic effect with AHP to improve flame retardancy effectively.
As a well-known organic-inorganic hybrid material, polyphosphazene has tremendous structural flexibility, excellent biocompatibility and outstanding thermal stability, and it has been applied in the fields of biomaterials, electrical and optical materials, hybrid materials,etc[22,23].Cross-linked polyphosphazenes are a typical polyphosphazene polymer, which is synthesized by nucleophilic substitution of active chlorine in hexachlorocyclotriphosphazene (HCCP)with amines, phenols, alcohols or thiol compounds[24-26].Due to their high phosphorus and nitrogen contents and high thermal stability, cross-linked polyphosphazenes are widely used to reinforce flame retarding performance of composites[27-29].Moreover, the cross-linked polyphosphazene not only has outstanding flame retarding performance in the polymer matrix, but is also well compatible with polymer matrix[30].In addition,with the structural flexibility of the -P=N- units and the existence of hydroxyl or amino active groups,cross-linked polyphosphazenes are a better choice as a surface functionalization and microcapsule material[31].For instance, a novel organic-inorganic hybrid polyphosphazene modified manganese hypophosphite(PZS-MnHP) shuttle was synthesized to enhance the flame retardance and anti-dripping behavior of the PET system[32].
In our previous study, we propose a feasible route to prepare a novel structure based on AHP microsphere encapsulated within a layer of PZS coating.In this work, a novel organic-inorganic polyphosphazene modified aluminum hypophosphite microsphere (PZSAHP) was synthesized via a hydrothermal strategy and layer-by-layer assembly methods.PZS is combined with AHP microsphere through covalent grafting or electrostatic adsorption.The flame retardant and mechanical performances of TPU composites were investigated.In addition, the mechanisms for improved flame-retardancy were also discussed.
2 Experimental
2.1 Materials
Hexachlorocyclotriphosphazene (HCCP)(N3P3Cl6) and 4,4’-sulfonydiphenol (BPS) were purchased from Wuhan Geao Chemical Company.The HCCP was recrystallized twice from hot n-hexane.Sodium hypophosphite, aluminum chloride,polyvinylpyrrolidone (PVP K-30), trimethylamine(TEA, AR, 99.0%), acetonitrile (AR, 99.0%) and ethanol (99.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China.Thermoplastic polyurethane (TPU, Desmopan 9380A) was supplied from Bayer Material Science (Germany).
2.2 Synthesis of microsphere aluminum hypophosphite (AHP)
In a typical experiment, AlCl3•6H2O (1.0 g)was dissolved in 35 mL of deionized water with 30 min continuous stirring at room temperature.Then,NaH2PO2•H2O (2.3 g), PVP (1.0 g) and ethanol (35 mL) were added to the above system, followed by 30 min stirring.The obtained solution was transferred to a 100 mL Teflon autoclave and kept at 200 ℃ for 7 h.Al(H2PO2)3was synthesized, separated by filtration,washed with distilled water and ethanol, and dried at 80℃ in an electric oven for 24 h.
2.3 Fabrication of modified aluminum hypophosphite microspheres (PZSAHP)
Initially, the mixture of AHP (5 g, 0.023 mol),BPS (2.16 g, 0.008 6 mol), TEA (2.39 mL, 0.017 2 mol) and 400 mL acetonitrile was added to a three-neck flask equipped with a magnetic stirrer.Then, HCCP (1 g, 0.002 9 mol) dissolved in 100 mL acetonitrile was added dropwise into the three-neck flask.Afterwards,the mixture was maintained at 80 ℃ for an additional 7 h.Eventually, the obtained product was filtered and washed with acetonitrile and ethanol for three times,and dried in a vacuum oven at 80 ℃ for 24 h.The synthetic route was shown in Scheme 1.
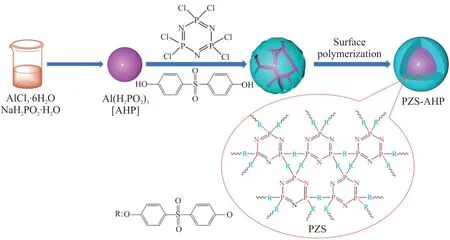
Scheme 1 The synthetic route of PZS-AHP microspheres
2.4 Preparation of TPU/PZS-AHP composites
The TPU/PZS-AHP composites were prepared by a melt-blending method.All materials were previously dried at 80 ℃ for 12 h to remove the moisture.The TPU/PZS-AHP composite samples were prepared using an internal mixer and a plate vulcanizing machine at 180±5 ℃, by changing the ratio of PZS-AHP and TPU to prepare different TPU composites.TPU with different concentrations of PZS-AHP microsphere,5 wt%, 10 wt% and 15 wt% corresponding to TPU/5%PZS-AHP, TPU/10%PZS-AHP and TPU/15%PZS-AHP composites were investigated in this work.For comparison, the similar process was utilized to prepare TPU/10%AHP.
2.5 Characterization
2.5.1 Structure characterization
The field-emission scanning electronic microscopy (FESEM), transmission electron microscopy (TEM), The Fourier transform infrared(FT-IR) and X-ray photoelectron spectroscopy (XPS)methods were used to analyze AHP, PZS-AHP.
2.5.2 Thermogravimetric analysis (TGA)
TGA test was performed using a Q-600 thermoanalyzer instrument under a nitrogen atmosphere with a 20 mL/min flow rate, and the sample(approximately 10 mg) was placed in a crucible.The heating rate is set to 10 ℃/min, and the test range is from 25 to 800 ℃.
2.5.3 Flame retardancy tests
The limiting oxygen index (LOI) was tested according to the standard oxygen index test ASTM D2863.The dimensions of all samples were 130 mm× 6.5 mm × 3 mm.The vertical burn test was carried out according to the ASTM D3801 test standard.The dimensions of specimens were 100 mm × 13 mm × 3 mm.The results presented were an average value of five replicates.
2.5.4 Cone calorimeter tests (CCT)
The cone calorimeter (Fire Testing Technology,UK) test were accomplished based on ISO 5660 standard programs with specimen dimension of 100 mm × 100 mm × 3 mm and were exposed horizontally to an external heat flux of 35 kW/m2for testing.
2.5.5 Thermogravimetric infrared (TG-IR)
Thermogravimetric analysis/infrared spectrometry(TG-IR) was conducted with a TG analyzer combined with a FTIR spectrometer.The measurements were carried out under nitrogen atmosphere with a heating rate of 20 °C/min.
2.5.6 X-ray diffraction (XRD) and Raman spectra
XRD was conducted by using an X-ray diffractometer, using Cu Kαradiation (λ= 0.154 18 nm).Raman spectra of the char residue were acquired using a DXR Raman spectrometer equipped with a 532 nm helium neon laser line.
2.5.7 Mechanical properties tests
The tensile strength data of TPU composites were obtained using an MTS CMT6104 universal testing machine based on GB 13022-91.
3 Results and discussion
3.1 Characterization of AHP and PZS-AHP microspheres
The preparation of PZS-AHP microspheres includes two steps.Firstly, nucleophilic substitution between HCCP and BPS occurs to form an oligomer.Secondly, these oligomers cross-link with each other to wrap the AHP microspheres.The morphology of the AHP and PZS-AHP particles was examined by SEM and TEM.The SEM and TEM images of the AHP and PZS-AHP microspheres are presented in Fig.1, which provide microstructure and morphology data of the AHP and PZS-AHP microspheres.It can be clearly seen from Figs.1(a) and 1(b) that the AHP has a spherical morphology, uniform dimensions and a particle size of about 5 µm with rough surface.Figs.1(c) and 1(d)exhibit the morphology of PZS-AHP microspheres.After the AHP microsphere was functionalized(Fig.1(c), 1(d)), the wrinkles disappeared.There are also smaller polyphosphazene microspheres that adsorb to the surface.As shown in Figs.1(e), 1(f), an extra layer of polymer phase was generated on the surface of AHP microsphere.It is expected that this polyphosphazene modified aluminum hypophosphite microsphere was successfully fabricated via surfacewrapped methods.

Fig.1 SEM images of the AHP (a, b) and PZS-AHP (c, d) microspheres and TEM images of PZS-AHP microspheres (e, f)
The FT-IR analysis provides essential structural information for the AHP and PZS-AHP hybrids(Fig.2).According to the information provided by the spectral line, the absorption peaks at 2 409 and 2 385 cm-1are attributed to the stretching vibration of –PH2, the peak located at 1 192 cm-1is ascribed to the bending vibration of –PH2, and the peak at 1 076 cm-1is assigned to the symmetric stretching vibration of P-O-P.The formation of the PZS-AHP is also confirmed by FT-IR.All the absorption peaks of AHP microspheres can be found in the PZS-AHP hybrid structure.For the PZS-AHP hybrids, there are some distinctive absorptions.The absorption peaks at 1 490 and 1 587 cm-1are in correspondence with the phenylene group of BPS.And the typical absorption at 1 291 cm-1belongs to the O=S=O group.The characteristic absorption of P-N group is centered at 886 cm-1, and the absorbance peak of Ar-O-P is at 944 cm-1, which indirectly indicates that the surface of the AHP has been successfully covered by a synthesized polyphosphazene.
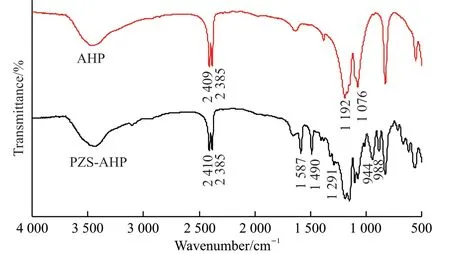
Fig.2 FT-IR spectra of AHP and PZS-AHP microspheres
In order to further verify the specific components of the surface coating, XPS analysis was used.In Fig.3(a), it can be observed that the original AHP microsphere mainly contains C, O, P and Al, with C being the part of the substrate material tested.Besides,the PZS-AHP exhibits additional N, S and Cl elements,which implies that the PZS is coated on the surface of AHP.Meanwhile, the high-resolution XPS spectrum of C 1s in Fig.3(b) is fitted into two typical peaks at 284.8 and 286.5 eV which are affiliated to the C-C and C-O bonds of PZS-AHP[30].The high-resolution P 2p XPS spectrum of PZS-AHP is shown in Fig.3(c) as well.The spectrum can be deconvoluted into two peaks at 133.5 and 134.3 eV corresponding to P-N and P-O bond of the polyphosphazene, respectively.Fig.3(d) shows the high-resolution Al 2p spectrum of PZS-AHP, and the characteristic peak of the Al element can be clearly seen.Table 1 shows the surface elemental composition of the AHP and PZS-AHP microspheres.The Al atom content of PZS-AHP is 0.82 wt%, which is much lower than that of AHP (10.96 wt%).Moreover, the N atom content of PZS-AHP is 6.29 wt%, while the AHP has no N atoms.The above results indicate that AHP is well coated.
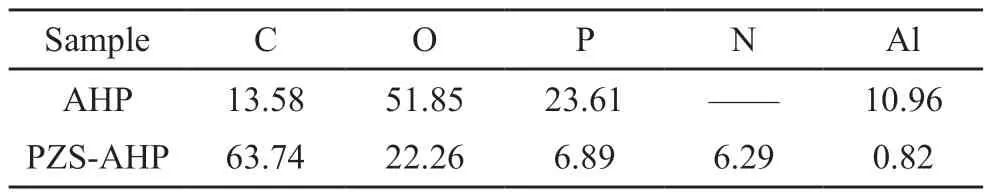
Table 1 Surface elemental composition of AHP and PZS-AHP/wt%
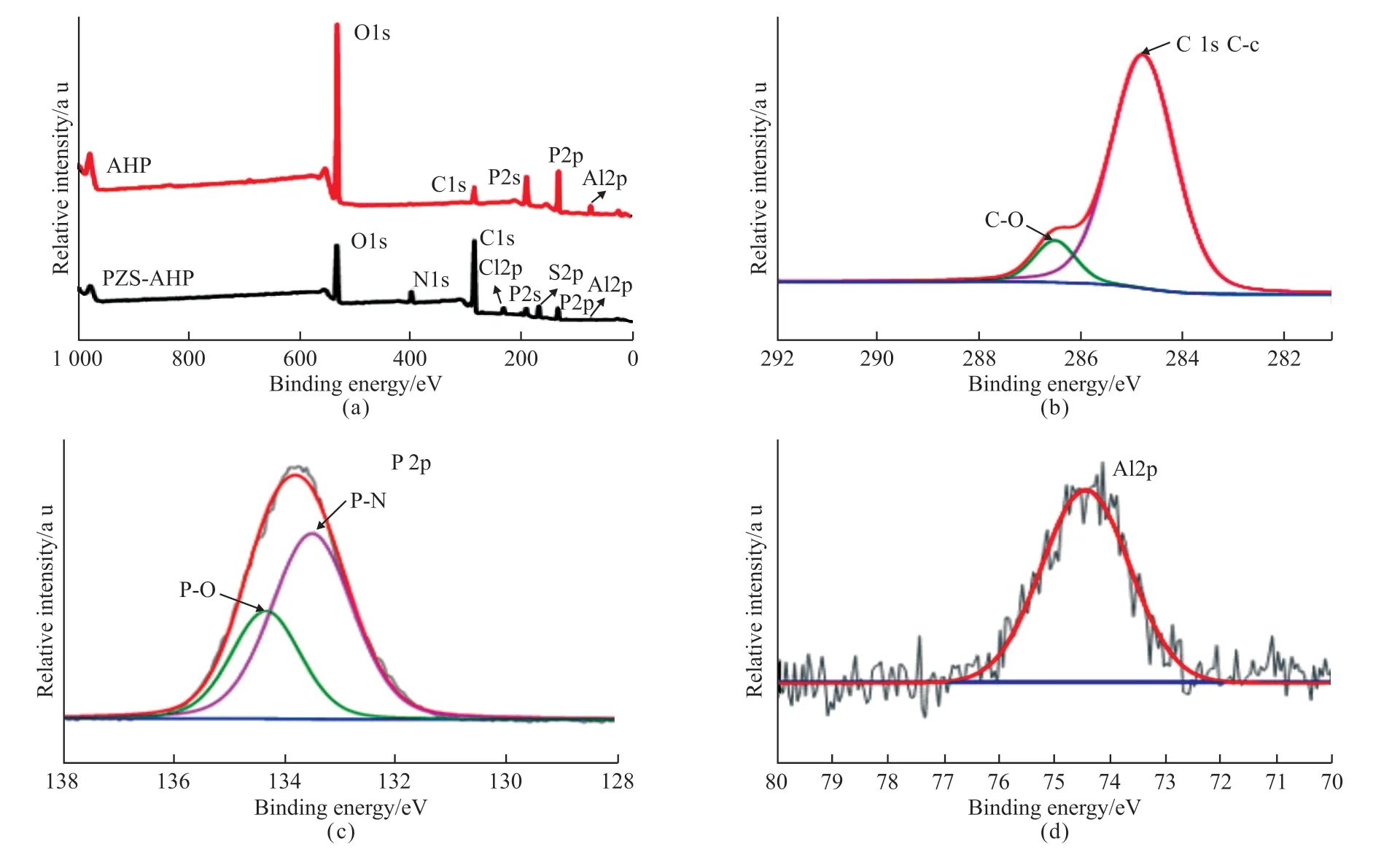
Fig.3 (a) XPS survey spectra of AHP, PZS-AHP; High-resolution XPS spectra of PZS-AHP in the (b) C 1s, (c) P 2p and (d) Al 2p regions
The thermal stability of the PZS-AHP microspheres was investigated with TGA under nitrogen atmosphere.The temperature at which 5%mass loss occurs is defined as the initial degradation temperature (T-5%).TGA and DTG curves of PZSAHP were shown in Fig.4.It was seen that PZSAHP microspheres possessed aT-5%value at about 343.6 ℃, indicating that the PZS-AHP microsphere possessed outstanding thermal stability.The PZS-AHP microsphere displayed a two-step degradation profile in the range of 100-700 ℃.The first step was attributed to unstable defects in the oligomer and preliminary degradation of the AHP microsphere.At this stage,the AHP microsphere would degrade into phosphine and pyrophosphates[11].The second degradation is due to the decomposition of PZS cross-linked polymers and pyrophosphate.Cross-linked polymers with P=N structures may undergo ring-opening polymerization reaction during pyrolysis[33].In addition, a high char residue of 71.89 wt% was achieved at 700 ℃.
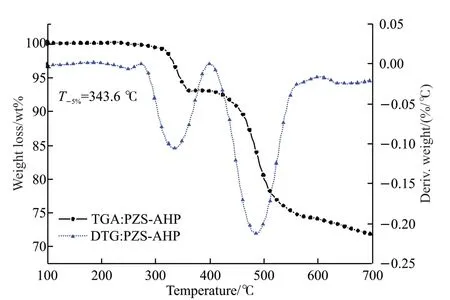
Fig.4 TGA and DTG curves of PZS-AHP microspheres under nitrogen atmosphere
3.2 Thermal stability and fractured surface characteristic of TPU composites
To explore the effect of PZS-AHP microspheres on the thermal properties of TPU, thermal stability of TPU/PZS-AHP composites was characterized with TGA under nitrogen, and the corresponding TGA and DTG curves are displayed in Fig.5.Detailed data of TGA curves are shown in Table 2.All TPU/PZSAHP composites present a one-stage main degradation process (Fig.5(a)), and exhibit similar decomposition behaviors to bare TPU.As can be observed from Fig.5(a), when PZS-AHP microspheres were incorporated, the initial decomposition temperature(T-5%) of the PZS-AHP sample increased slightly.The char residues of PZS-AHP sample at 700 ℃ obviously increased compared to that of pure TPU, which are ascribed to the high thermal stability and catalytic carbonization effect of the PZS-AHP microspheres.Moreover, the initial decomposition temperature of TPU/10%AHP sample is close to pure TPU, with a char yield of 20.02% at 700 ℃, lower than the char yield (23.06%) of TPU/10%PZS-AHP after combustion with the same loading.From the derivative thermogravimetric analysis (DTG) curves (Fig.5(b)),the maximum mass loss rates of TPU/AHP and TPU/PZS-AHP composites are much lower than that of pure TPU, revealing an enhanced thermal resistance of the TPU composites.AHP microspheres are the inorganic part as a physical barrier to inhibit oxygen exchange and diffusion of pyrolysis products, and PZS are the organic part which promotes the char formation, thus improving the thermal resistance of the composites against degradation.
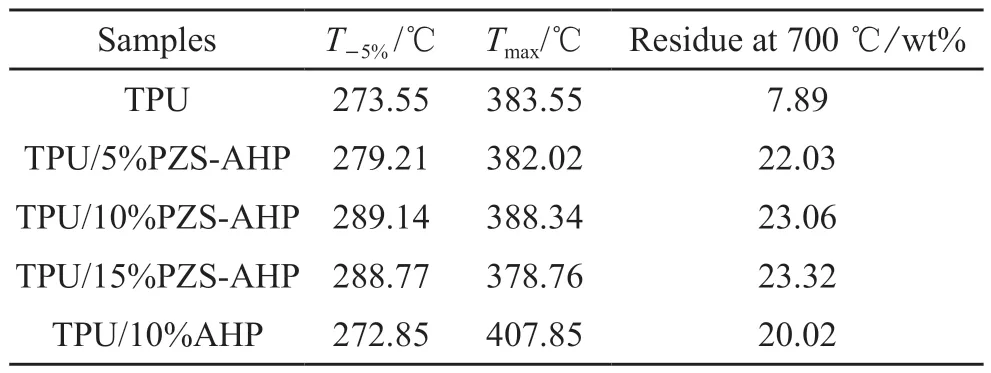
Table 2 TGA data of TPU and TPU composites under nitrogen
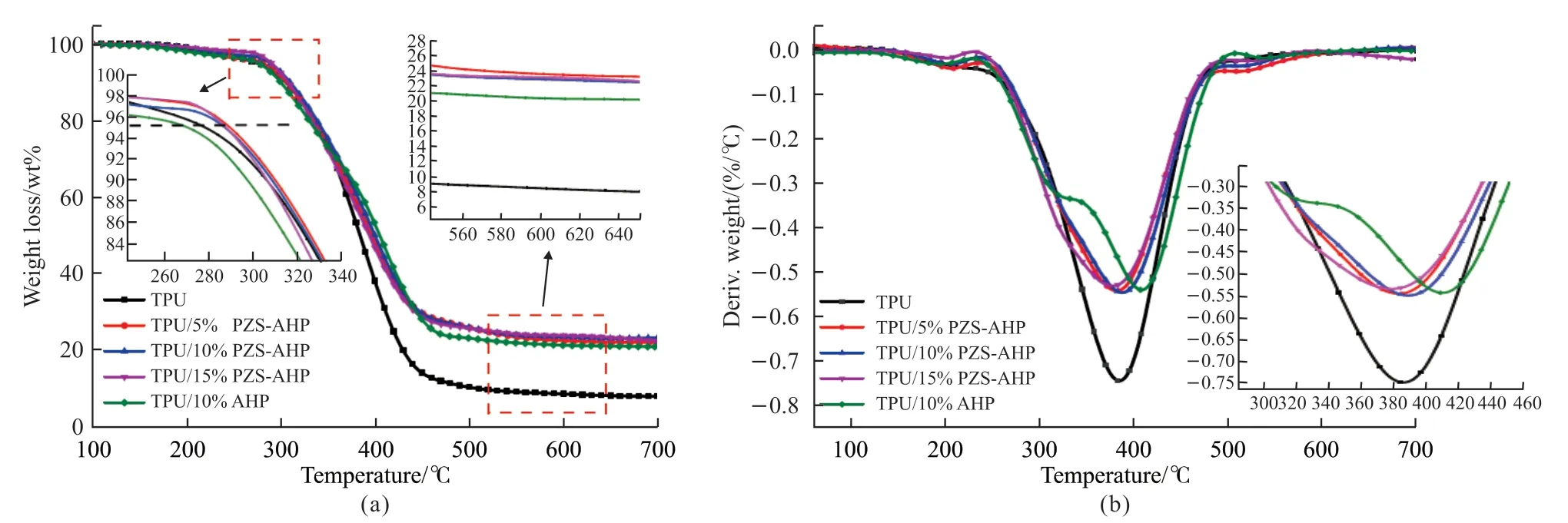
Fig.5 (a) TGA and (b) DTG thermograms of TPU and its composites under nitrogen
As well known, the interfacial interactions between polymeric matrices and additives play a crucial role in enhancing the mechanical and flame retarding performances of polymer composites.The microstructures of freeze-fractured surface for TPU, TPU/AHP and TPU/PZS-AHP samples were investigated by SEM.As can be observed from Fig.6(a), the fractured surface of the neat TPU is quite smooth with no-crinkled morphology.But for TPU/AHP composites (Fig.6(c)), there are cracked particles protruding obviously onto the fracture surface,indicating weak interface adhesion between TPU and neat AHP.As can be observed from Fig.6(b), it was evident that no large area agglomeration of PZS-AHP microspheres was found, indicating that PZS-AHP microspheres had good compatibility with the TPU matrix and were tightly embedded in the TPU matrix.This may be because that PZS wraps AHP greatly to reduces the interfacial tension and causes the enhanced interfacial adhesion.

Fig.6 SEM images of fractured sections of the TPU (a), TPU/10%PZS-AHP (b) and TPU/10%AHP (c) composites
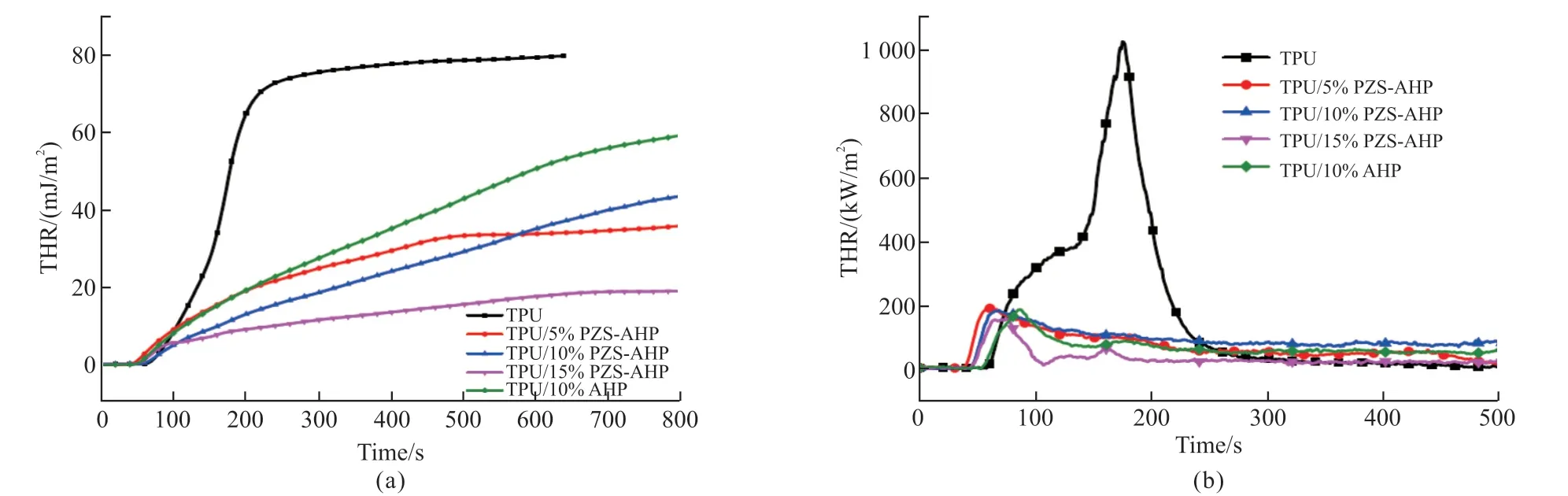
Fig.7 THR (a) and HRR (b) vs time curves of TPU and its composites
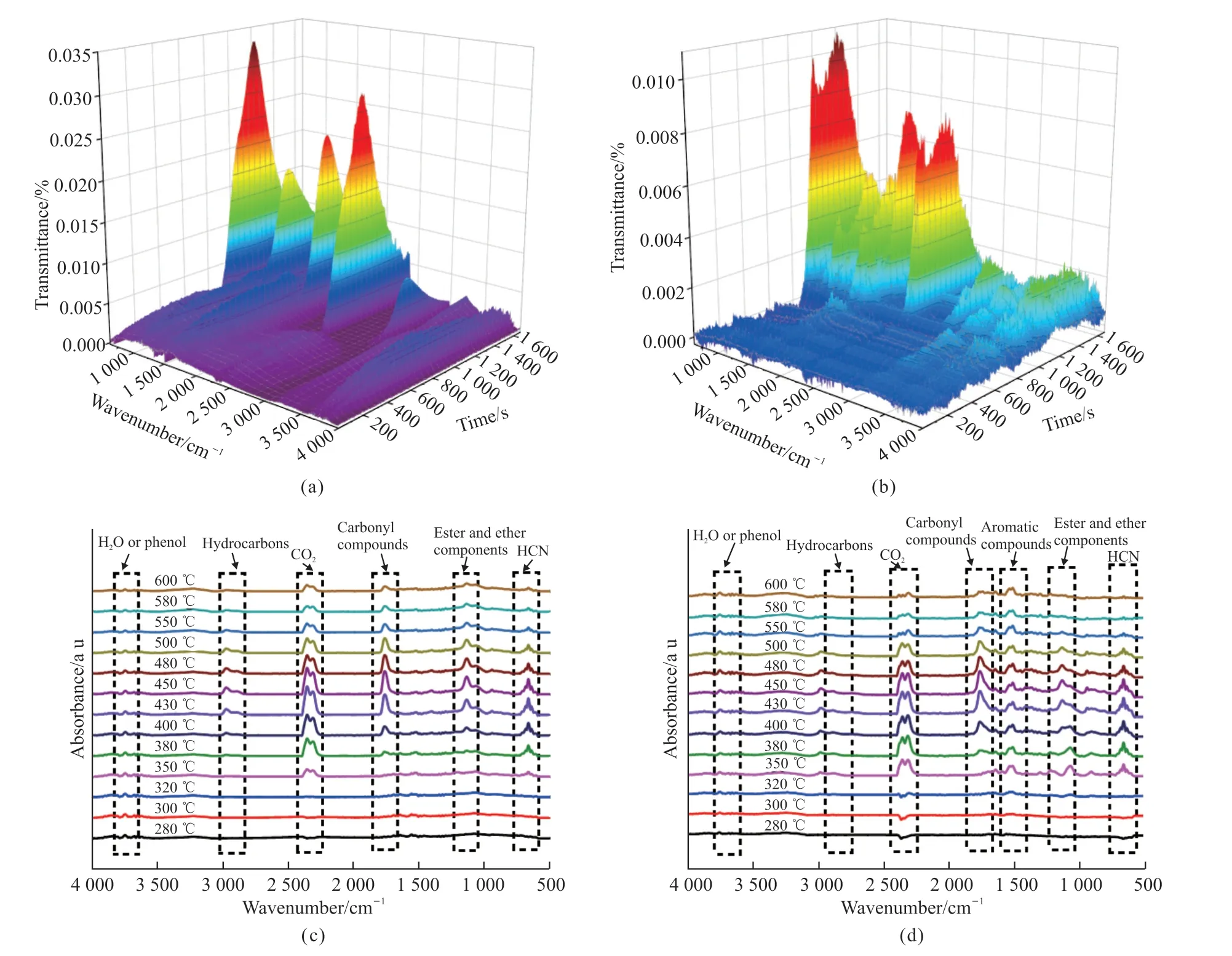
Fig.8 Three-dimensional (3D) TG-FTIR spectra of gasified pyrolysis products for (a) TPU and (b) TPU/15%PZS-AHP; FTIR spectra of the pyrolysis products for (c) TPU and (d) TPU/15%PZS-AHP at different temperatures
3.3 Flammability performance of TPU and its composites.
LOI and UL-94 vertical burning tests were performed to evaluate the flame retardancy of TPU and its composites.The LOI values and UL-94 testing results of the TPU composites were listed in Table 3.TPU had a very fast combustion rate and presented no rating in the UL-94 test accompanied with heavy melt dripping.It was noted that the LOI values increased with the increase in the AHP and PZS-AHP microspheres.The LOI values of the TPU/10%PZSAHP and TPU/15%PZS-AHP composites were increased to 29.4% and 30.7%, respectively.Moreover,both samples passed the V-0 rating in the UL-94 rating test, and no droplets were observed.Compared with AHP, a higher LOI value was obtained with the addition of PZS-AHP microspheres with the same loading.
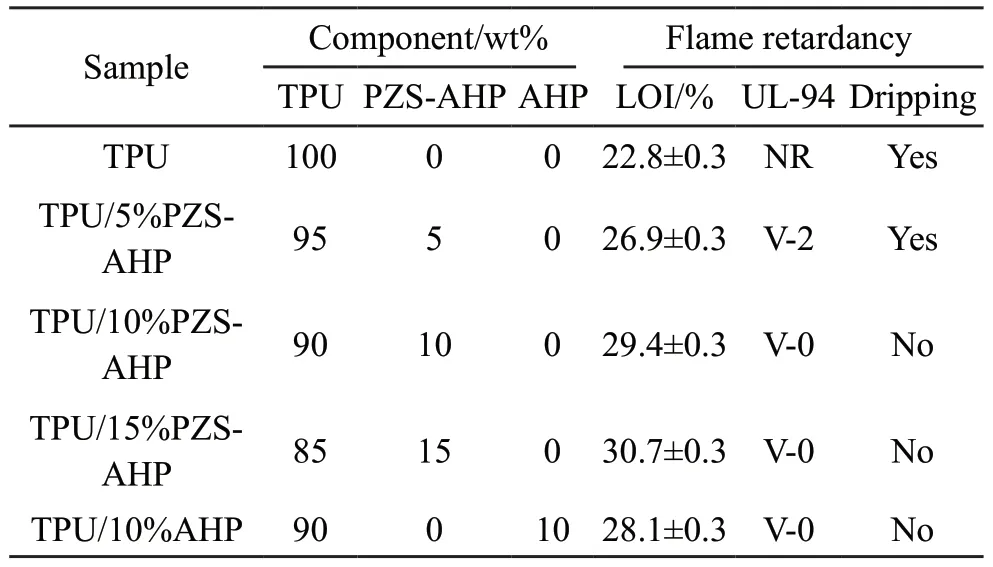
Table 3 LOI and vertical flame test of TPU and its composites materials
The fire retarding properties of TPU composites were investigated by cone calorimeter test which can imitate the real combustion environment of polymer materials.The heat release rate (HRR) and total heat release (THR)versustime curves of TPU composites are displayed in Figs.7(a), 7(b).Several crucial parameters, such as the time to ignition (TTI), pHRR,THR, the CO production (COP), the CO2production(CO2P), and maximum average rate of heat emission(MARHE) values obtained from cone calorimeter are listed in Table 4.It can be found in Table 4 that the TTI of TPU/10%PZS-AHP is 45 s.The TTI of TPU/PZSAHP composites was reduced compared to the TTI of pure TPU.Compared to pure TPU, the pHRR and THR values for the TPU containing 5% of PZS-AHP microspheres are significantly decreased by 81.6% and 12.5%, respectively.The incorporation of 15% PZSAHP microspheres brings about a 84.8% maximum decrease in pHRR, a 75.7% maximum decrease in THR, indicating the high flame-retarding efficiency of the filler.In particular, apparent decreases in the pHRR of 81.9% and THR of 31.9% were achieved with the introduction of 10% AHP into TPU.The addition of 10% PZS-AHP microspheres to the TPU further significantly decreases the pHRR and THR values.As a result, the pHRR and THR of the TPU/10%PZS-AHP are decreased by 82.2% and 42.5% compared to those of TPU, revealing a better flame-retarding performance among the same loading samples.In addition, to further evaluate the fire safety of TPU, MARHE (defined as the maximum value of THR (t)/twheretwas the testing time) was also calculated.Compared with pure TPU, the MARHE values of TPU/15%PZS-AHP was largely decreased, demonstrating that adding PZSAHP was able to slow down the combustion rate, and improve the flame retardancy of TPU[34].The significant reductions in the fire hazards of TPU composites are attributed to the physical barrier effect of AHP and cooperative catalytic charring effect between the AHP and PZS, thereby retarding the diffusion of pyrolysis products and forbidding the inner unburned materials exposed to fire during combustion.
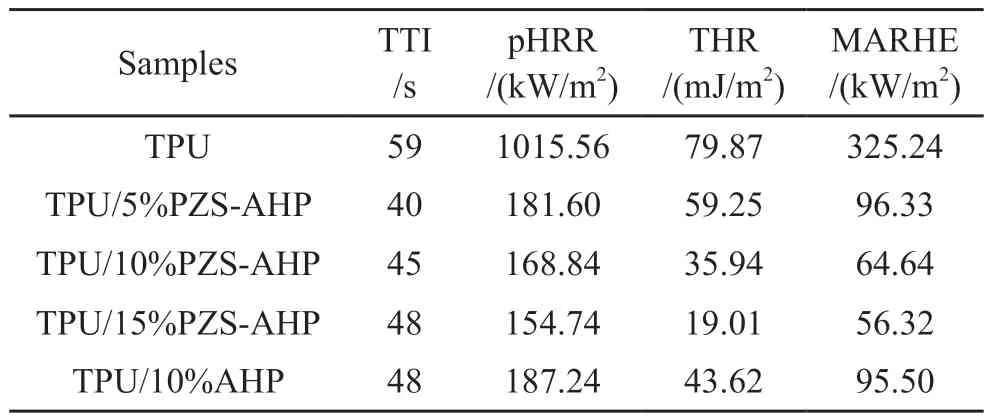
Table 4 Detailed combustion results of TPU and its composites obtained from cone calorimeter tests
3.4 Pyrolysis products analysis of TPU composites
In order to study the thermal decomposition behavior of TPU composites, TG-FTIR spectroscopy was employed to analyze the gases produced by TPU,TPU/15%PZS-AHP during their thermal degradation.In particular, the 3D TG-FTIR spectra which are obtained from the thermal decomposition process of TPU composites are shown in Figs.8(a), 8(b).Several toxic pyrolysis products are clearly identified by the typical FT-IR signals.The characteristic peaks of the gaseous decomposition products are located in the regions of 3 500-3 850, 2 800-3 200, 2 200-2 400,1 600-1 900, 1 200-1 500, and 600-1 000 cm-1.From Figs.8(a), 8(b), after the incorporation of TPU/15%PZS-AHP microspheres, significant diminutions in pronounced peaks of toxic gaseous volatiles can be observed.Figs.8(c), 8(d) depict the FTIR spectra of the pyrolysis gas products for TPU and TPU/15%PZS-AHP at different temperatures.Several characteristic peaks for the primary pyrolysis products of TPU composites located at 3 650 cm-1(H2O or phenol), 2 970 cm-1(hydrocarbons), 2 360 cm-1(CO2and CO), 1 760 cm-1(carbonyl compounds), 1 510 cm-1(aromatic compounds), 1 260 cm-1(ester and ether components) and 670 cm-1(HCN), respectively.It is noted that the volatile products of TPU/15%PZS-AHP turned up slightly earlier than that of pure TPU during the pyrolysis process, implying the catalytic effect of PZS-AHP in the TPU matrix.
3.5 Condensed phase flame-retardation analysis
To further assess the condensed phase flameretardation mechanism, the char residues of TPU composites obtained from cone calorimeter tests were evaluated.Fig.9 presents the digital photos of the external residues from the top view and the side view for TPU composites.It can be observed that the pure TPU was completely burned and there are few cracked residual chars remaining (Fig.9(a)).However,with the incorporation of PZS-AHP, the thickness and size of residual chars gradually increase (Figs.9(b)-9(d)).Moreover, a more continuous and compact char surface is formed after TPU/10%PZS-AHP combustion, and the quality of this char layer is better than that of TPU/10%AHP (Fig.9(e)).The phenomenon was attributed to the catalytic charring effect and the intumescent effect of PZS.
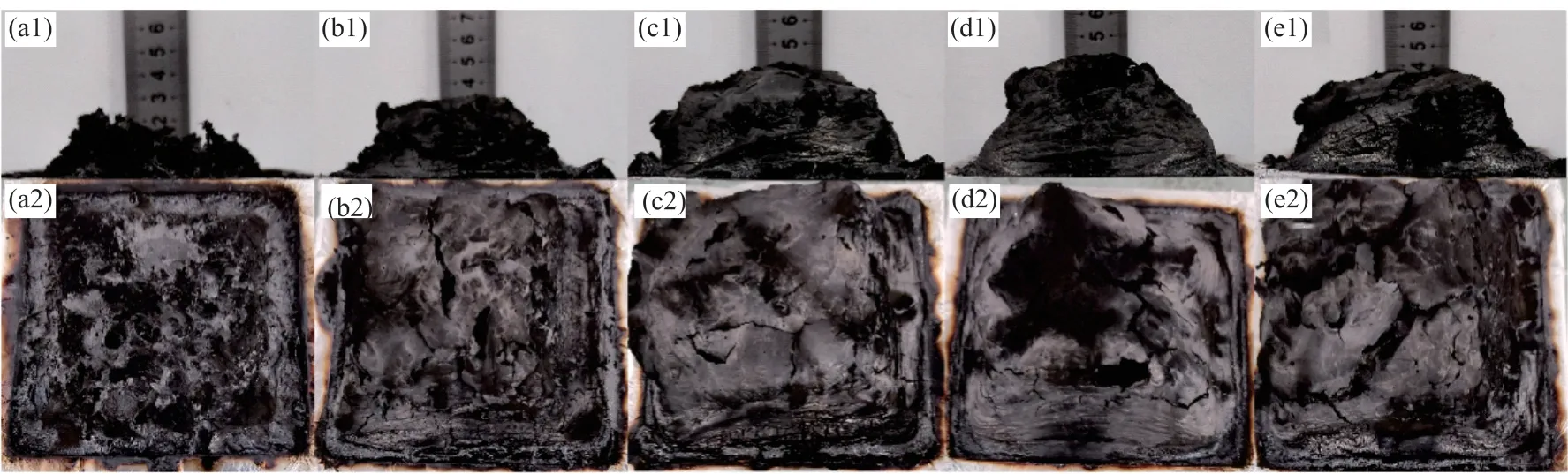
Fig.9 Digital photographs of the char residues from top view and side view for (a1, a2) TPU, (b1, b2) TPU/5%PZS-AHP, (c1, c2)TPU/10%PZS-AHP, (d1, d2) TPU/15%PZS-AHP and (e1, e2) TPU/10%AHP after cone calorimeter test
The microstructures of the char residues from TPU, TPU/10%PZS-AHP and TPU/10%AHP composites are shown in Fig.10(a-c).Many big cracks and holes in the char layer of pure TPU (Fig.10(a)) can be observed, which are due to the escape of volatile gases released from burning composites.As for the TPU/10%PZS-AHP (Fig.10(b)), the holes and cracks became smaller, more continuous and intumescent surfaces appeared in the char residues.It is reported that a more cohesive and compact char layer could act as an effective barrier to prevent further pyrolysis of the underlying substrate, to delay the heat and mass transfer, and meanwhile to isolate the oxygen needed in combustion[35,36].For TPU/10%AHP (Fig.10(c)), the number of holes in the char residue is quite lower.This is because PZS-AHP microspheres release additional gases such as N2, NH3and PH3during combustion.
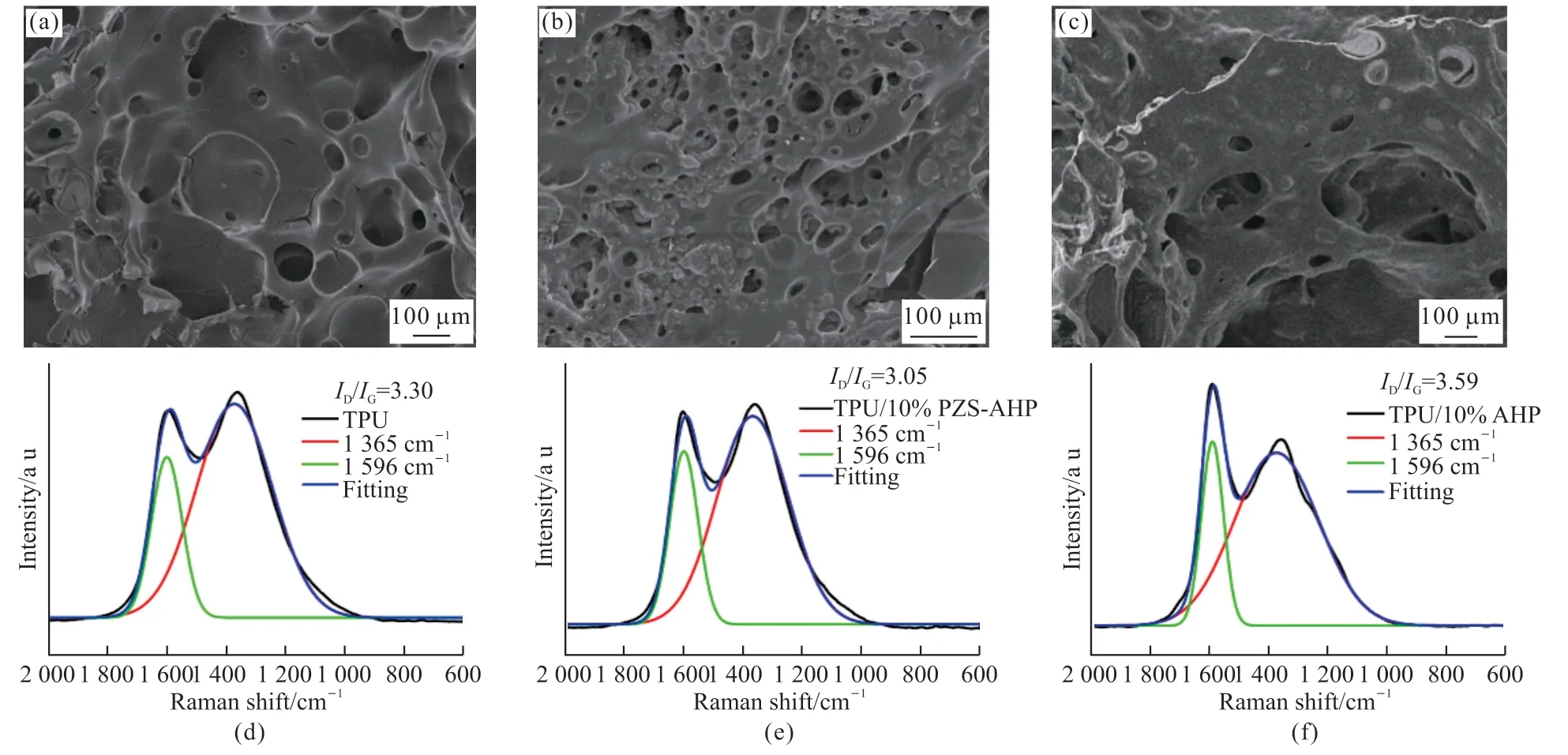
Fig.10 SEM images of the char residues from (a)TPU, (b)TPU/10%PZS-AHP, (c)TPU/AHP; Raman spectra of the char residues of (d) TPU,(e) TPU/10%PZS-AHP, (f) TPU/10%AHP
Raman spectroscopy was utilized to study the microstructure components and structure of the char residues.The spectrum for TPU (Fig.10(d)) depicts two bands at around 1 365 and 1 596 cm-1, which are denoted as D and G bands, respectively.In prior works, the area ratio of D to G band (ID/IG) reflects the graphitization degree of the char residue.A relatively lowerID/IGvalue equals to a higher graphitization degree[37].Apparently, the value ofID/IGfor neat TPU is 3.30, whereas TPU/10%AHP exhibits a higher value(3.59).As expected, the TPU/10%PZS-AHP composite exhibits the lowestID/IGvalue (3.05), revealing the highest graphitization degree.These results can be attributed to the catalyzing carbonization effect of PZS in the thermal decomposition process of TPU.
Fig.11 shows the FT-IR spectra and XRD patterns of the residual char for TPU and its composites after cone tests.In the corresponding FT-IR spectrum(Fig.11(a)), TPU composites exhibit analogous char structure to pure TPU.The broad peak detected at 3 440 cm-1is from stretching vibrations of O-H and N-H bonds, which come from phosphoric or polyphosphoric acid, amino compounds or water[38].The peak at 1 145 cm-1could be ascribed to the stretching vibrations of the P=N group in the polyphosphazene structure.The absorption around 970 cm-1is assigned to the vibrations of P-O-P bond,revealing the existence of cross-linked phosphorous oxides in the char layers[39-41].As shown in Fig.11(b),TPU/10%PZS-AHP and TPU/10%AHP exhibit similar diffraction peaks to that of pure TPU.It can be observed that the external char residue of pure TPU and its composites has a (002) typical graphitized carbon XRD pattern with a broad peak at around 25°.A smaller peak at about 42° is classified into diffraction of (100) or (101) peak of graphite carbon[42].The results illustrate that the charring process in the presence of PZS-AHP is not accompanied by the change of condensed structure after burning and the composition of char residue is mainly the carbon material.
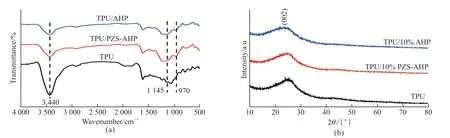
Fig.11 (a) FTIR spectra and (b) XRD patterns for char residue of TPU and its composites after cone calorimeter tests
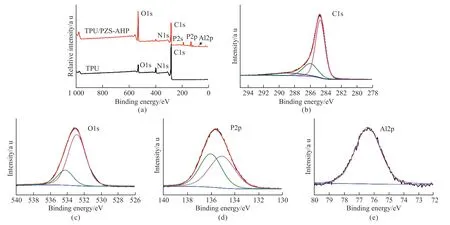
Fig.12 (a) XPS survey spectra for char residue of TPU and TPU/PZS-AHP after cone calorimeter tests; High-resolution XPS spectra for char residue of TPU/PZS-AHP in the (b) C 1s, (c) O1s, (d) P 2p and (e) Al 2p regions
The XPS analysis was utilized to provide more detailed information about elemental composition of the char residues.In the XPS survey spectra of TPU and TPU/10%PZS-AHP, the samples’ surface consists of C, N, and O elements, and the additional P and Al elements appear in the residual char of TPU/PZS-AHP,owing to the addition of PZS-AHP microsphere.For C 1s spectrum, the peak at 284.7 eV belongs to the C-C and C-H bonds in aliphatic and aromatic components,the peak at 286.0 eV is assigned to the C-O-P and C-O-C, and the peak at 288.7 eV is assigned to C═O or phosphate.In the O 1s spectrum, the peak at 532.8 eV is assigned to C=O or P=O in the carbonyl and phosphate groups.The peak at 534.2 eV corresponds to C–O–C, C–O–P or C–O–H.For P 2p spectrum,the peak at 135.1 eV is attributed to the P-O group in phosphate species[43].The peak at 136.1 eV is attributed to polyphosphates.For Al 2p spectrum, a peak at 76.3 eV is assigned to aluminum pyrophosphate[44].
3.6 Flame retardation mechanism of PZSAHP microsphere
Based on the analyses mentioned above, a flameretardation mechanism of PZS-AHP was proposed.On the one hand, during the early stage of combustion,thermal stable PZS acts as a physical barrier to inhibit the escape of pyrolysis volatiles with heat and mass transfer.The PZS can form phosphorus based compounds such as cross-linked phosphorous oxides, which work together with the nitrogenous carbon generated during the combustion to promote the formation of the compact and continuous char layers.Meanwhile, due to the barrier effect of the char layers, the diffusion of pyrolysis volatiles was delayed and resulted in a reduction in smoke emissions.On the other hand, the thermal decomposition of AHP produces phosphine and aluminum hydrogen phosphate.Phosphine is quickly oxidized to phosphoric acid.Furthermore, phosphoric acid dehydrates and releases water to generate polyphosphoric acid which could promote the degradation of polyurethane to form the compact char layer on the surface of TPU.At high temperature, aluminum hydrogen phosphate condenses to form aluminum pyrophosphate.This chemical substance exhibited high thermal stability and served as a good physical barrier, shielding the contact between the heat and TPU, and preventing the release of combustibles into the surrounding environment.
3.7 Mechanical properties of TPU composites
To investigate the effects of additives on the mechanical properties of TPU, the tensile tests of TPU and its composites are performed.It was observed from Fig.13 that the elongation at break and tensile strength for neat TPU is 1 239.9% and 19.94 MPa, respectively.Compared with neat TPU, the elongation at break and tensile strength of the TPU/PZS-AHP composites decreased gradually with the increase in the PZS-AHP microsphere.It can be seen that PZS-AHP microsphere had little influence on the elongation at break and tensile strength of TPU/PZS-AHP composites, indicating a good compatibility of PZS-AHP microsphere in the TPU matrix.The elongation at break and tensile strength of TPU/10%PZS-AHP is 1 064.8%and 13.7 MPa, and the corresponding values of TPU/10%AHP are 447.9% and 7.21 MPa, respectively.These results imply that the surface functionalization of PZS-AHP improves its interfacial interactions with the TPU matrix, compared to untreated AHP, thereby enhancing the mechanical and thermal performances of TPU composites.
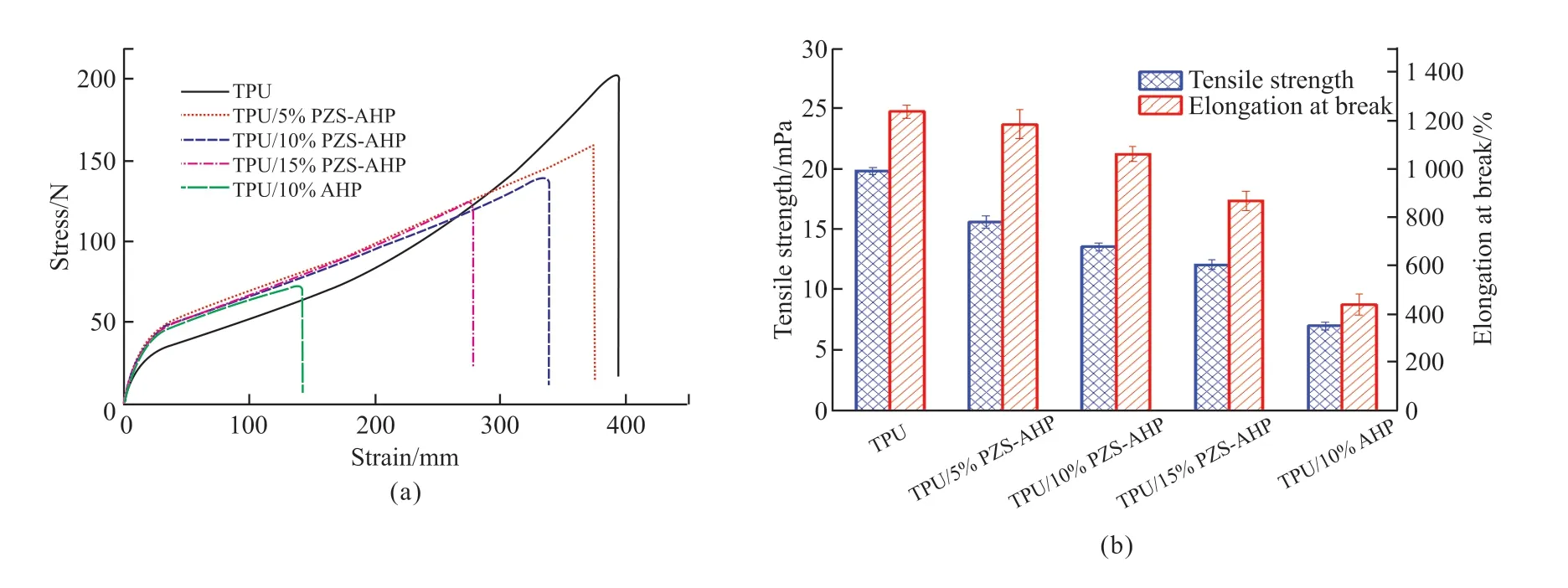
Fig.13 Mechanical properties of TPU and its composites; (a) stress-strain curves, (b) tensile strength and elongation at break
4 Conclusions
In this work, a novel organic-inorganic polyphosphazene modified aluminum hypophosphite microsphere (PZS-AHP) was synthesized via hydrothermal strategy and surface-wrapped methods,where, AHP served as a model to form a layer of PZS shell.This well characterized hybrid structure possessed high thermal stability and flame retardance.The incorporation of PZS-AHP microspheres leads to significant improvement in thermal stability, and when the content of PZS-AHP microsphere was increased to 10 wt%, and the composites could achieve a LOI value of 29.2% and the UL-94 V-0 rating.The TGA and cone tests were conducted to investigate the thermal stability and flame retarding properties of TPU composites.With the addition of PZS-AHP microsphere, the initial degradation temperature and char yield are increased,implying the enhancement of thermal stability.In addition, the THR, pHRR, TSP and SPR values for the TPU/PZS-AHP composites decreased significantly.The incorporation of 15 wt% PZS-AHP microsphere into TPU leads to a 84.8% and 75.7% reduction in pHRR and THR, respectively.Furthermore, TG-FTIR analysis indicated that the incorporation of PZS-AHP microsphere can reduce pyrolysis gas release during the thermal degradation process.It is noted that the PZS-AHP microsphere had little influence on the elongation at break and tensile strength of the TPU/PZS-AHP composites.The apparent enhancement in the fire safety performance was principally due to the cooperation between PZS and AHP.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- One-pot Synthesis of Hierarchical Flower-like WS2 Microspheres as Anode Materials for Lithium-ion Batteries
- Controllable Synthesis of Au NRs and Its Flexible SERS Optical Fiber Probe with High Sensitivity
- Effciient Direct Decomposition of NO over La0.8A0.2NiO3(A=K, Ba, Y) Catalysts under Microwave Irradiation
- Appreciable Enhancement of Photocatalytic Performance for N-doped SrMoO4via the Vapor-thermal Method
- Infulence of Current Density on the Photocatalytic Activity of Nd:TiO2Coatings
- The Negative Thermal Expansion Property of NdMnO3 Based on Pores Effect and Phase Transition
