One-pot Synthesis of Hierarchical Flower-like WS2 Microspheres as Anode Materials for Lithium-ion Batteries
ZHANG Xianghua, TAN Hen, WANG Ze, XUE Maoquan
(1.School of Mechanical Engineering, Jiangsu University of Technology, Changzhou 213001, China; 2.School of Materials Engineering, Jiangsu University of Technology, Changzhou 213001, China; 3.Changzhou Vocational Institute of Industry Technology, Changzhou 213164, China)
Abstract: 3D hierarchical flowerlike WS2 microspheres were synthesized through a facile one-pot hydrothermal route.The as-synthesized samples were characterized by powder X-ray powder diffraction (XRD),energy-dispersive spectroscopy (EDS), scanning electron microscopy (SEM) and Raman.SEM images of the samples reveal that the hierarchical flowerlike WS2 microspheres with diameters of about 3-5 μm are composed of a number of curled nanosheets.Electrochemical tests such as charge/discharge, cyclic voltammetry, cycle life and rate performance were carried out on the WS2 sample.As an anode material for lithium-ion batteries,hierarchical flowerlike WS2 microspheres show excellent electrochemical performance.At a current density of 100 mA·g-1, a high specific capacity of 647.8 mA·h·g-1 was achieved after 120 discharge/charge cycles.The excellent electrochemical performance of WS2 as an anode material for lithium-ion batteries can be attributed to its special 3D hierarchical structure.
Key words: WS2; microspheres; lithium-ion batteries; electrochemical performance
1 Introduction
The development of new alternative energy sources and the reduction of greenhouse gas emissions have become the focus of attention in the world today[1-3].As a new type of green energy, rechargeable lithium-ion batteries (LIB) are recognized as one of the most ideal ways to alleviate energy shortages and global warming problems[4-6].At present, lithium-ion batteries are widely used in various portable electronic devices (such as notebook computers, mobile phones, digital cameras),and also considered as very promising power sources in electric vehicles, hybrid electric vehicles and stationary energy storage systems[7-10].Traditional lithium-ion batteries use graphite as the anode material, but the theoretical storage capacity of graphite is only 372 mAh·g-1,which limits the use of lithium-ion batteries[9-12].The high-capacity anode material is the key to meeting the future high energy density, longer cycle life and extreme safety requirements of lithium-ion batteries[4,13,14].Since traditional LIBs electrode materials are insufficient to meet these requirements, the development of new materials with excellent electrochemical performance is crucial for the next generation of LIBs[4].
Recently, transition metal dichalcogenides (TMD)have received widespread attention due to their special sandwich structure.The TMD materials can be represented by the molecular formula MX2, where M is a transition metal element, mainly including the IVB group (Ti, Zr, Hf), VB group (V, Nb, Ta) and VIB group ( Mo, W), and X represents the chalcogen (S,Se, Te).These materials form a layered structure of the form X-M-X with chalcogen atoms located in two hexagonal planes separated by a plane of metal atoms, as shown in Fig.1(a).The atoms in the layers are bonded through strong covalent bonds, and the layers are bonded through weak van der Waals interactions[15-18].This unique structure makes TMD exhibit good mechanical[19], physical[20], optical[21]and electrical[22]properties.And due to the high reversible capacity and unique layered structure, transition metal sulfides (TMDs) are considered to be the most promising alternative anodes for high-performance LIB.As a member of this family,WS2has demonstrated many unique structural features that can potentially be used in lithium-ion battery anodes[4,18,23].According to reports, it is calculated theoretically that 1 mol WS2can hold 4 mol electrons, and the corresponding lithium storage capacity is 433 mAh·g-1higher than that of graphite[23].In addition, the layer spacing of the WS2(002) plane is large (d= 0.62 nm),resulting in a small van der Waals interaction force between the layers, which is conducive to the insertion/extraction of Li+ions[24].However, due to the poor conductivity and the volume expansion caused by Li+ions insertion and desorption process, the specific capacity and rate stability of WS2will quickly decay.These shortcomings hinder its development for commercial LIB anode.Therefore, a variety of methods have been adopted to address these issues, such as constructing composite materials to enhance conductivity, and buff-ering volume changes by synthesizing nanostructured materials.
According to reports, nano-structured electrode materials have the advantages of high surface area,high reactivity, high electrical conductivity and ionic conductivity, and reduced lithium-ion insertion size, so high electrochemical performance can be achieved[25].Therefore, there is an urgent need to find the desired structure and morphology of WS2with enhanced conductivity to improve electrochemical performance.And many efforts have been devoted to the preparation of WS2nanostructures with different morphologies to improve anode performance.For example, Liuet alsynthesized mesoporous WS2by a vacuum assisted impregnation route, and achieved discharge capacity of 805 mAh·g-1after 100 cycles at a current of 0.1 A·g-1[25].Yanget aldesigned and fabricated WS2nanosheets with preferential [001] orientation and perfect single crystalline structures, and the nanosheets exhibited a high specific capacity, good cycling performance and excellent rate capability[4].Yehet alsynthesized a few-layer WS2through a jet cavitation process,which provided a capacity of 581 mAh·g-1and 257 mAh·g-1at 0.1 and 10 C, respectively[26].Ansariet alreported that a porous WS2composed of a few layered nanosheets was synthesized by a hydrothermal method,and it showed a high specific capacity of 292 mA h·g-1at a current density of 0.2 C with excellent cycling stability over 100 cycles[18].Srinivaaset alprepared 1 T metallic phase few-layered WS2nanoflowers with an unusual zigzag chain type structure, and showed excellent electrochemical performance[27].Recent studies have found that three-dimensional hierarchical nanostructure not only has good structural stability, but also has the advantages of microstructure and nanostructure.It can improve the performance of anode materials by enlarging the contact area between the electrode and the electrolyte, improving the surface reactivity,and overcoming its poor electronic conductivity[28-30].However, most of the research on the use of WS2as anode materials for lithium-ion batteries has focused on two-dimensional sheet materials, and there are few studies on three-dimensional hierarchical nanostructures.
In this work, we fabricated the hierarchical flower-like WS2microspheres by a facile hydrothermal reaction method.The electrochemical performance of WS2microspheres were investigated by a variety of electrochemical testing techniques.
2 Experimental
2.1 Synthesis of hierarchical flower-like WS2 microspheres
The raw materials, thiourea (CH4N2S), sodium tungstate dihydrate (Na2WO4·2H2O) and oxalic acid(H2C2O4·2H2O), were purchased from Aladdin Chemical Reagent Company.All reagents were of analytical grade and were used without further purification.In the typical synthesis, 1.16 g of sodium tungstate and 1.33 g of thiourea were first dissolved in 30 mL of deionized water by magnetic stirring for 15 min.Then add 0.7 g of oxalic acid to the above solution and continue stirring for 15 minutes.Then the solution was transferred into a 50 mL stainless steel autoclave, sealed and maintained at 240 ℃ for 24 h.After naturally cooling to room temperature, the final black product was collected by centrifuged and washed three times with deionized water and ethanol each, and dried in an oven at 60 ℃for 12 hours.
2.2 Materials characterization
The phase structure of the as-prepared product was identified by X-ray diffraction (XRD) on a powder X-ray diffraction (PW1830, Philips).The morphologies images of the samples were captured by scanning electron microscope (SEM, JEOL, JSM-6390).Energy dispersive X-ray spectrometry (EDS) was used for qualitative chemical analyses.The Raman spectrum was collected on a LabRAM HR Raman microscope,and the laser excitation wavelength was 532 nm.
2.3 Electrochemical measurements
The electrochemical measurements were performed using a two-electrode cell.Lithium foil was used as the counter electrode and reference electrode.The poly-propylene film (Celgard-2300) was used as a separator, and electrolyte consisted of 1.0 mol·L-1LiPF6in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) in a volume ratio of 1:1.The working electrode was prepared as described below.Super P carbon black, polyvinylidene fluoride (PVDF)and the as-prepared WS2samples are dispersed in N-methyl-2-pyrrolidone (NMP) at a weight ratio of 10:10:80 to make a uniform slurry.Then the slurry was coated on the copper foil and dried at 100 ℃ for 12 hours, and finally the working electrode was obtained.The test half coin battery (CR-2032) was assembled in an argon-filled glove box.The galvanostatic charge/discharge tests were carried out on a LAND 2001A Battery Tester in a voltage range of 0.01-3.0 V at room temperature.Cyclic voltammetry (CV) measurements were performed on an electrochemical workstation(CHI660B) in the potential range of 0.01-3.00 VvsLi/Li+at a scan rate of 0.5 mV·s-1.
3 Results and discussion
3.1 Structure and morphological features of hierarchical flower-like WS2 microspheres
The XRD pattern of the obtained flower-like WS2microspheres is shown in Fig.2(a).The peaks at 2θ= 14.15°, 28.51°, 32.72°,39.32°, 49.61, 58.52°, and 69.71° were correlated to the (002), (004), (100), (103),(105), (110), and (200) planes of WS2, respectively,and all the diffraction peaks can be well indexed to PDF card No.08-0237.EDS spectrum is presented in Fig.2(b), which reveals that the sample consisted of element W and S.The Raman spectrum of the hierarchical flower-like WS2microspheres is shown in Fig.2(c).Similar to the description in Literature 30, the two characteristic peaks of the WS2phase are located at 350 and 420 cm-1, corresponding to the in-plane vibration mode (E12g) and the out-of-plane vibration mode (A1g),respectively[4,30,31].
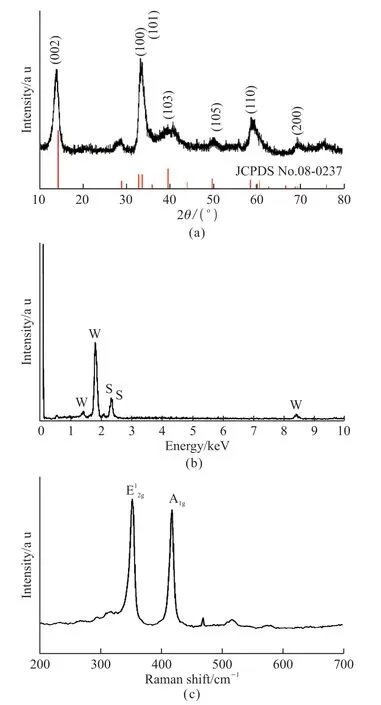
Fig.2 (a) XRD pattern, (b) EDS and (c) Raman spectrum of the asprepared WS2 samples
Fig.3 shows the SEM images and elemental maps of the WS2samples.Fig.3(a), the low magnification SEM image, demonstrates that the samples are made up of a number of hierarchical flowerlike microspheres.Fig.3(b) presents an incomplete microsphere.It can be seen more clearly that the WS2sample displays a hierarchical structure.The flowerlike WS2are composed of a number of curled nanosheets.These WS2nanosheets are assembled to form 3D WS2microspheres with diameter of about 3-5 μm.Moreover, the center of the microsphere exhibits a pore structure.Fig.3(c) is the EDS elemental maps of the WS2sample, which reveals the uniform distribution of S, and W elements throughout the flower-like WS2microspheres.
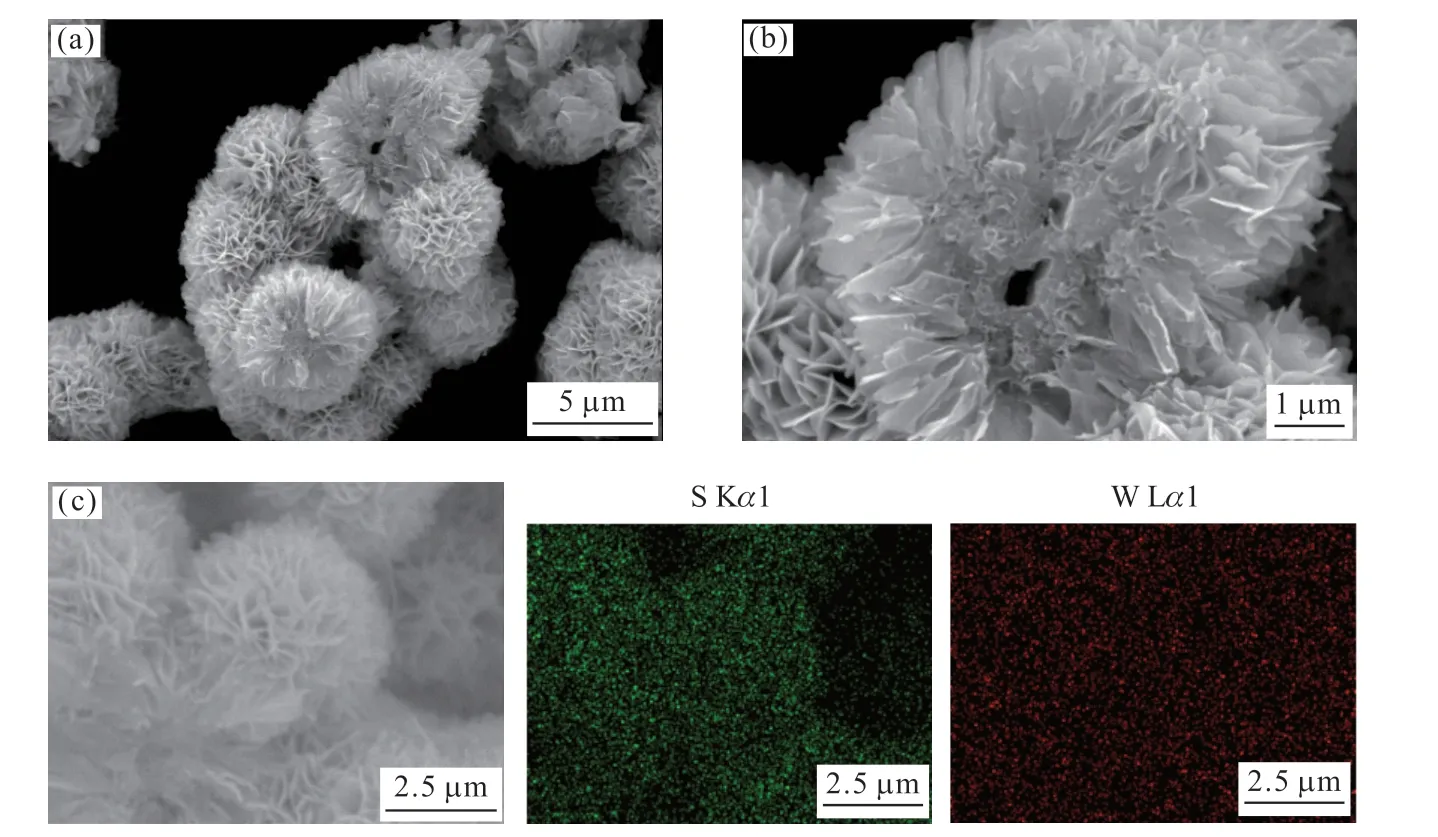
Fig.3 SEM (a,b) and elemental maps (c) images of the flower-like WS2 microspheres
In the whole reaction process, oxalic acid is used as a reducing agent, while adjusting the pH value of the reaction system, and thiourea is used as a sulfurization reagent.According to relevant literature reports, the reaction is divided into three steps[32].First, thiourea is hydrolyzed to generate hydrogen sulfide, then W+6in sodium tungstate is reduced to WO2by oxalic acid, and finally WO2reacts with hydrogen sulfide to generate WS2.
3.2 Electrochemical performance
The electrochemical reaction process of WS2have been proposed as follows[3,24,33]:
The electrochemical performance of the prepared WS2samples as a LIB anode material were firstly evaluated by cyclic voltammogram (CV).The CV curves of the anode for the first three cycles are shown in Fig.4(a).In the first cycle, two reduction peaks were observed at 0.9 and 1.6 V, which may be attributed to lithium insertion into the interlayer space of WS2to form LixWS2(Reaction (1))[18,31].The second reduction peak appearing at about 0.5 V corresponds to the reaction of WS2transforming to Li2S and metallic W (Reaction (2)), and accompanying with the formation of solid electrolyte interface (SEI) layer on the electrode[34].During the anode scan, two oxidation peaks around 1.8 and 2.3 V were corresponded to the oxidation of Li2S to S (Reaction (3))[35].In the subsequent cycle, the reduction peak at 0.4 V disappears, while the reduction peaks at 0.9 and 1.6 V move to 1.3 and 2.0 V, respectively,which means that the reversibility of the cycle is improved.The CV curves of the second and third cycles completely overlap, indicating that the WS2electrode has excellent reversibility.

Fig.4 (a) Cyclic voltammetry curves at 0.01-3 V; (b) Galvanostatic discharge/charge curves at 100 mA·g-1; (c) Cycling performances at current density of 100 mA·g-1 and (d) Rate capacities at current densities from 0.1 to 2 A·g-1 of the WS2 samples
The first three typical galvanostatic discharge/charge curves of WS2with the potential range of 0.01-3 V and the current density of 100 mA·g-1are shown in Fig.4(b).It can be clearly seen from the figure that the plateau in the voltage curve is consistent with the CV curve.In the first discharge curve, the plateau at 1.6 V is attributed to the decomposition of the electrolyte and the formation of an SEI film.The long plateau appearing at approximately 0.5 V is attributed to the reaction of WS2to form W and LiS2(Reaction (4))[23].In subsequent cycles, a higher and more inclined discharge plateau at 1.8 V can be observed, indicating that it is easier to insert Li+in the continuous cycles[4].The charge plateau at about 2.3 V represents the reverse reaction of the formation of WS2[36].The following discharge curves have a high degree of overlap.It can be seen that the layered nanostructure has excellent Li+insertion and extraction capabilities and electrochemical reversibility.
Galvanostatical charge/discharge test of WS2was carried out 120 cycles in a current density of 0.1 A·g-1and a voltage range of 0.01 to 3 V.Fig.4(c) shows the cycle performance of the WS2electrode.The first discharge/charge capacity of the WS2electrode is 870/715.6 mA·h·g-1with a coulombic efficiency of 82.3%, which is much higher than most of the previous reports of WS2materials.The irreversible capacity in the first cycle may be caused by the formation of a solid electrolyte interface (SEI) layer and electrolyte decomposition.The discharge capacity of WS2maintains at 647.8 mA·h·g-1after the 120th cycle, indicating an excellent electrochemical performance.
In order to test the rate capability of WS2, cyclic testing was performed at various current densities from 0.1 to 2 A·g-1.The test results are shown in Fig.4(d).When the current densities are 0.1, 0.2, 0.3, 0.5, 1 and 2A·g-1, the discharge capacities of the samples are 709.6, 665.1, 573.5, 516.8, 390.1 and 365.3 mA·h·g-1,respectively.When the current density returns to 100 mA·g-1, the discharge specific capacity also returns to 668.5 mAh·g-1, and it can still be maintained at 600 mA·h·g-1after 100 cycles, indicating that the material has good rate performance.
Table 1 lists the electrochemical cycling performance of WS2materials with different morphologies published in previous literature.Compared with other studies, it can be observed that the charge capacity of the as-synthesized hierarchical flower-like WS2microspheres is better than other shape materials at the same current density.The excellent electrochemical performance of WS2can be attributed to its special three-dimensional (3D) hierarchical structure.Previous reports[29,36,37]have shown that 3D hierarchical structures have better electrochemical performance than simple nanostructures.The flower-like hierarchical WS2microspheres is composed of many ultra-thin nanosheets.Firstly, these nanosheet can significantly shorten the diffusion distance of Li+ions and reduce the volume change during the lithiation and delithiation process, thereby ensuring the structural stability and charge transport capability of the material[38].Secondly,the hierarchical flower-like structure can provide large interfacial surface area to ensure high availability of active sites, promote the rapid transfer of lithium ions from all directions, and shorten the diffusion path of lithium ions during circulation.

Table 1 Comparison of WS2 anode materials between this study and related references
4 Conclusions
In summary, hierarchical flower-like WS2microspheres have been successfully synthesized by a facile one-pot hydrothermal process.The prepared WS2sample as a LIB anode material shows excellent electrochemical performance.3D hierarchical structure may be the reason for improving the electrochemical performance of materials.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Controllable Synthesis of Au NRs and Its Flexible SERS Optical Fiber Probe with High Sensitivity
- Effciient Direct Decomposition of NO over La0.8A0.2NiO3(A=K, Ba, Y) Catalysts under Microwave Irradiation
- Appreciable Enhancement of Photocatalytic Performance for N-doped SrMoO4via the Vapor-thermal Method
- Infulence of Current Density on the Photocatalytic Activity of Nd:TiO2Coatings
- The Negative Thermal Expansion Property of NdMnO3 Based on Pores Effect and Phase Transition
- Efficient Removal of Phosphate from Aqueous Solutions Using Corundum- hollow-spheres Supported Caclined Hydrotalcite Porous Thin Films
