The Negative Thermal Expansion Property of NdMnO3 Based on Pores Effect and Phase Transition
LI Yucheng, ZHANG Yang, ZHANG Muqun, DUAN Rong, LIU Xiteng
(Department of Avionics Engineering, Aviation Maintenance NCO Academy of Air Force Engineering University, Xinyang 464000, China)
Abstract: A novel negative thermal expansion (NTE) material NdMnO3 was synthesized by solid-state method at 1 523 K.The crystal structure, phase transition, pores effect and negative expansion properties of NdMnO3 were investigated by variable temperature X-ray diffraction (XRD), scanning electron microscope(SEM) and variable temperature Raman spectra.The compound exhibits NTE properties in the orderly O' phase crystal structure.When the temperature is from 293 to 759 K, the ceramic NdMnO3 shows negative thermal expansion of -4.7×10-6 /K.As temperature increases, the ceramic NdMnO3 presents NTE property range from 759 to 1 007 K.The average linear expansion coefficient is -18.88×10-6 /K.The physical mechanism of NTE is discussed and clarified through experiments.
Key words: negative thermal expansion; NdMnO3; pores effect; phase transition
1 Introduction
As an urgent requirement of modern industrial technology, thermal expansion control is making remarkable progress.In linear distortion, even a small change of 1 mm can fatally degrade the performance of high-precision equipment and instruments.In addition, for devices composed of multiple materials,mismatched thermal expansion between components can lead to serious damage, such as interface shedding and fracture.Thermal expansion control has in recent years been strongly requested for advanced electronic equipment, such as power semiconductor devices,thermoelectric conversion systems, and fuel cells.The difficulties of thermal expansion control are common and difficult to solve.The core technology of thermal expansion control is the study of negative thermal expansion materials.Therefore, it greatly promotes the research on the characteristics of negative expansion materials, in addition, phase variant NTE materials also made significant progress.For example, ferroelectric and magnetic charge transfer and metal - insulator phase transition negative expansion material, undergo phase transformation with volume shrinkage when heated[1-16].
A large number of LaMnO3compounds have attracted extensive attention due to their very interesting physical properties, such as ferroelectric[17-20],temperature compensation[21-24]and other physical properties.Some members of this group of compounds have great potential applications, including magnetothermal effects at room temperature[25-28]and thermal expansion properties[29,30].They are attractive because their phase change diagrams are complex.
The isotropic negative thermal expansion (NTE)of ZrW2O8in a wide temperature range (0.3-1 050 K) shows the way to adjust the expansion coefficient of engineering materials.Many NTE materials have been explored, including A2M3O12, cyanide, fluoride,ABO3and anti-perovskite materials.In recent years,some ABO3materials with NTE characteristics have been studied.For example, PbTiO3, La1-xSrxMnO3,Er0.7Sr0.3NiO3-δ, Gd1-xSrxMnO3,etc.In this paper, by studying the crystal structure and microstructure of the perovskite material NdMnO3, the phenomenon and mechanism of its abnormal thermal expansion are revealed.
2 Experimental
The sample was prepared according to the conventional solid-state method.Analytic grade Nd2O3(purity 99.5%), and Mn2O3powder were used as raw materials.The Mn2O3powder was prepared at 923 K in the furnace for 10 hours by MnO2, Nd2O3and Mn2O3powders were mixed according to the mole ratio of Nd: Mn = 1:1.The mixtures were ground using an agate mortar for 1 h and then ground with ethanol for 2 h.The obtained mixtures were then dried for 1 h at 353 K in a baking oven.Afterward, the mixtures were pressed into cylindrical-shape pellets (φ10×5 mm)by using a powder pellet machine (769YP-15A,200 MPa).The pellets were initially sintered in a pipe furnace (AY-BF-555-180) at 1 273 K for 10 h in air and subsequently sintered at 1 523 K for 10 h.The sample was allowed to cool in the furnace naturally.
The linear thermal expansion coefficient was measured using a dilatometer Linseis L76 (heating and cooling rates of 5 K/min).The XRD measurement was carried out by Bruker D8 Advance with CuKα radiation.The surface morphology of the sample was observed using the FEI Quanta 250 scanning electron microscopy (SEM) and the EDS energy spectrum was obtained with Appllo XP.A Renishaw MR-2000 Raman spectrometer with 532 nm laser wavelength excitation was used for the Raman spectroscopic studies.
3 Results and discussion
3.1 Phase analysis
Fig.1 is the XRD pattern of the sample at RT.Comparing the XRD pattern with JCPDS cards Nd0.973Mn0.95O3(01-085-2204), we find the diffraction peaks are similar to these JCPDS cards except some shifts, which suggests that the as-prepared sample has similar structure with Nd0.973Mn0.95O3.It can be confirmed that the ceramic Nd0.973Mn0.95O3crystallizes in orthorhombic structure.
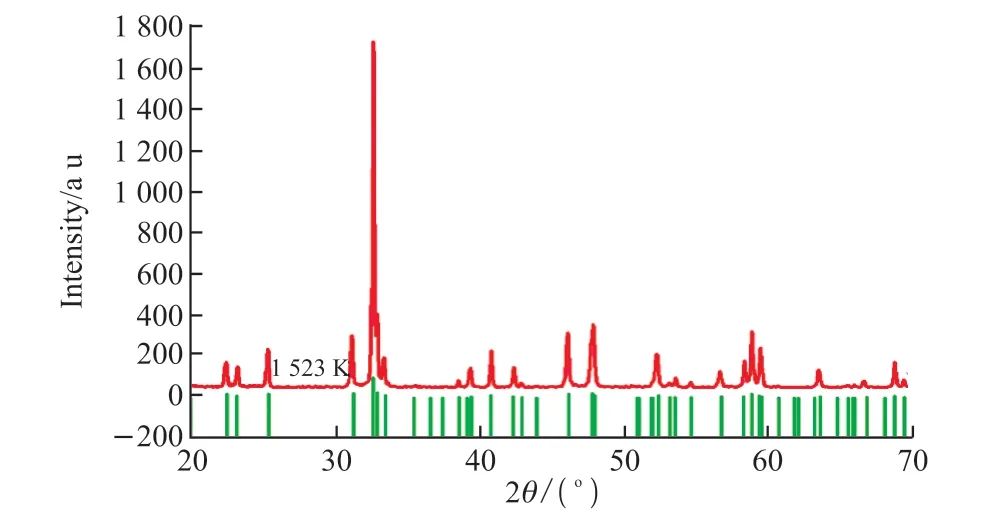
Fig.1 The XRD patterns of Nd0.973Mn0.95O3 at 1 523 K
3.2 SEM analysis
Fig.2 shows the SEM image of the sample in 1 523 K.We find that the ceramic sample is composed of blocky structure with some obvious agglomerations,and there are pores and microcracks in the sintered body.The size of the particles is uniform with average grain size about 6-7 μm.The EDS analysis of the sample presents the primary elements of Nd, Mn and O and their atomic ratio (Nd: Mn: O) is about 1:1:3 (Table 1).Combining with the XRD analysis, we identify the composition of the samples is NdMnO3.
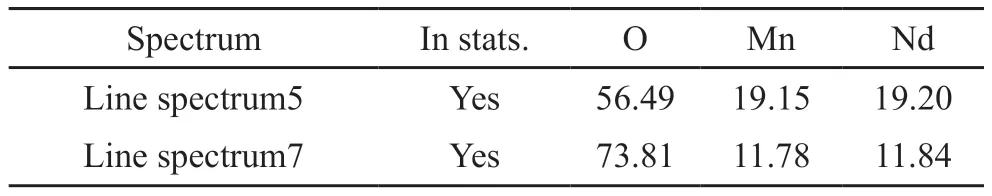
Table 1 Atomic ratio of Nd, Mn and O in NdMnO3 at 1 523 K by EDS
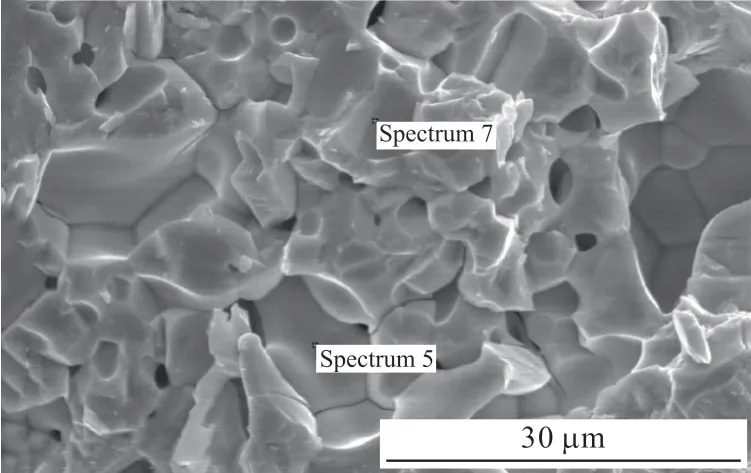
Fig.2 SEM image of the ceramic NdMnO3 at 1 523 K
3.3.Thermal expansion property
Fig.3 shows the relative length (dL/L) as temperature increasing of NdMnO3in 1 523 K.When temperature is from 233 to 269 K, the ceramic NdMnO3(Fig.3(a))shows negative thermal expansion of -0.848 7×10-6/K.As temperature increases, the ceramic NdMnO3(Fig.3(a)) presents NTE property range from 280 to 673 K.The average linear expansion coefficient is -6.3×10-6/K; When the temperature is from 293 to 759 K, the ceramic NdMnO3(Fig.3(b)) shows negative thermal expansion of -4.7×10-6/K.As temperature increases, the ceramic NdMnO3(Fig.3(b)) presents NTE property range from 759 to 1 007 K, and the average linear expansion coefficient is -18.88×10-6/K.
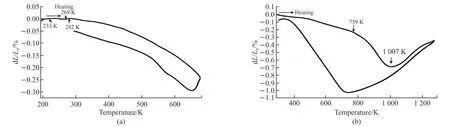
Fig.3 Relative length change (dL/L) with temperature: (a)(193-673 K) at 1523 K, (b) (RT-1 273 K) at 1 523 K
3.4 Variable temperature XRD analysis
Fig.4 is the variable temperature (RT-1 173 K) XRD patterns.As is shown in the figure, there are obvious changes in XRD patterns from room temperature to 1 023 K.For example, the shape of XRD has changed greatly at 45.9°, 51.8°, 57.4°, 67.3°and 76.9° respectively , which is considered to be a characteristic of phase transition.
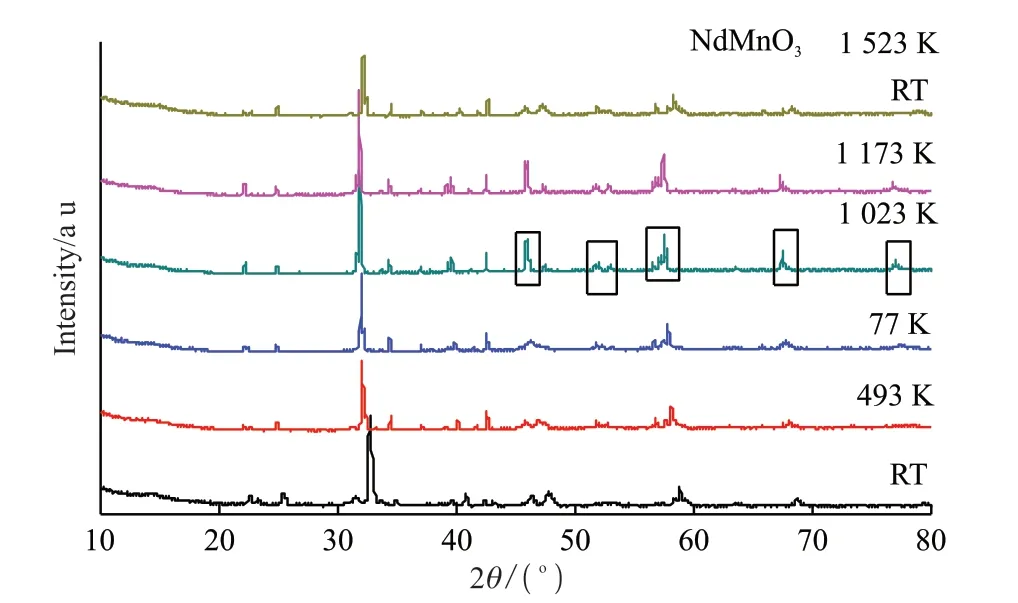
Fig.4 Variable temperature XRD of NdMnO3 at 1 523 K
Furthermore, the theta positions of some peaks are slightly offset, which means that the lattice constants have changed.The diffraction peaks gradually shift to small angle direction with increasing temperature,that is, thea-axis,b-axis andc-axis lattice constants gradually increase with increasing temperature.But the coefficient of thermal expansion has changed strangely,and then it goes down as the temperature goes up.
The NdMnO3sample has an orthorhombic phase structure (space groupPbnm) at room temperature, and the unit cell parameters of NdMnO3sample are :a=0.542 1 nm,b= 0.579 5 nm, andc= 0.757 7 nm, which correspond to the orbital phase.As the temperature increases to 1 023 K, the NdMnO3compound undergoes a Yahn-teller phase transition from the orderly O’ phase to the disordered O phase[38], and the unit cell parameters of NdMnO3sample area= 0.548 1 nm,b= 0.556 9 nm,c= 0.775 9 nm[34].It can be seen thatb-axis contracts with the temperature rising, whilea-axis andc-axis both expand with the temperature rising.However, as a whole, the unit volume of NdMnO3shrinks with the temperature rising.
3.5 Variable temperature Raman analysis
Fig.5 is the high-temperature (RT-1 023 K)Raman patterns of sample.From Fig.5, the wave shape of NdMnO3has changed.This suggests that there is phase transition throughout the tested temperature range (RT-493 K).From the temperature of 493 K, the Raman shape is almost invariable, indicating that the chemical structure of NdMnO3can remain stable in the experimental temperature range.
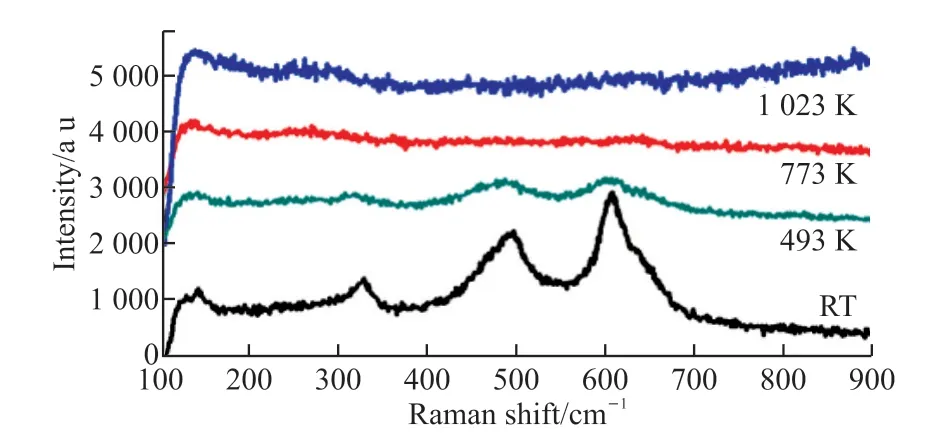
Fig.5 Variable temperature Raman of NdMnO3 at 1 523 K
3.6 Discussion
Fig.6 is schematic of NdMnO3with the perovskite orthorhombic structure.As can be seen from the figure,the structure of NdMnO3is composed of large network polyhedron, such as NdO4tetrahedron and MnO6octahedron, with obvious gaps or crystal cavities.The bond lengths in the polyhedron change anisotropy during heating or cooling, and the subsequent contraction of the crystal voids leads to a more compact structure of the lattice which results in negative expansion properties.
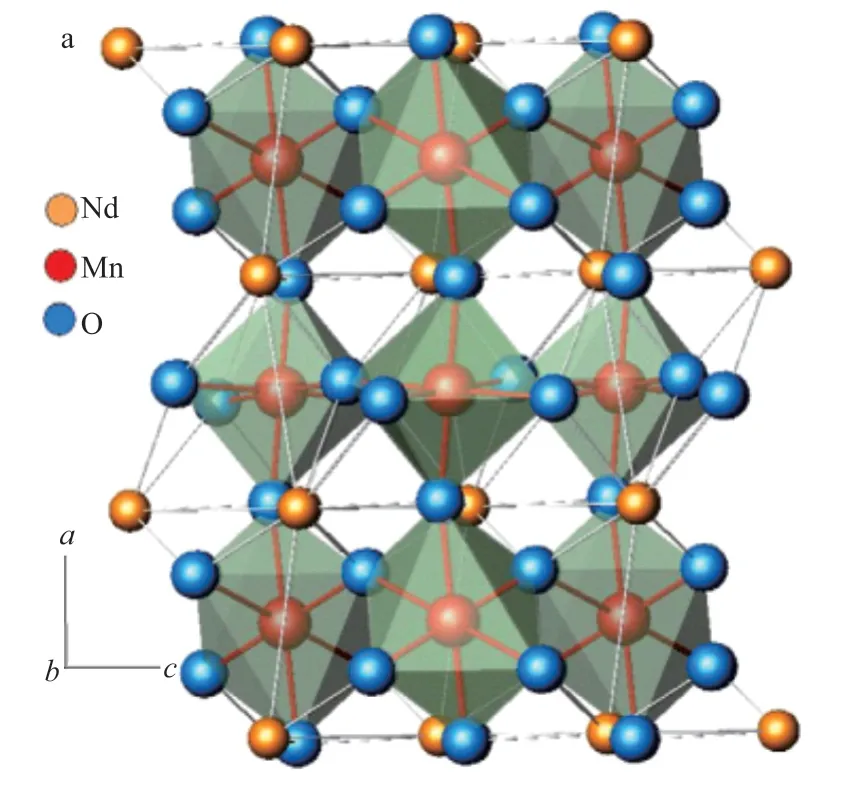
Fig.6 Schematic of NdMnO3 with the perovskite orthorhombic structure
Fig.7 is schematic of negative thermal expansion in a flexible network.We know that rigid units are formed by strong covalent bonds.For NdMnO3, Mn-O covalent bonds are strong and they do not expand due to heat.In contrast, the Nd-O-Mn bond is soft,although the distance between Mn-O and Nd-O bonds does not change.It is easy to produce lateral oxygen displacement during heating, and these displacements consume the open space in the crystal structure,resulting in negative expansion[39].
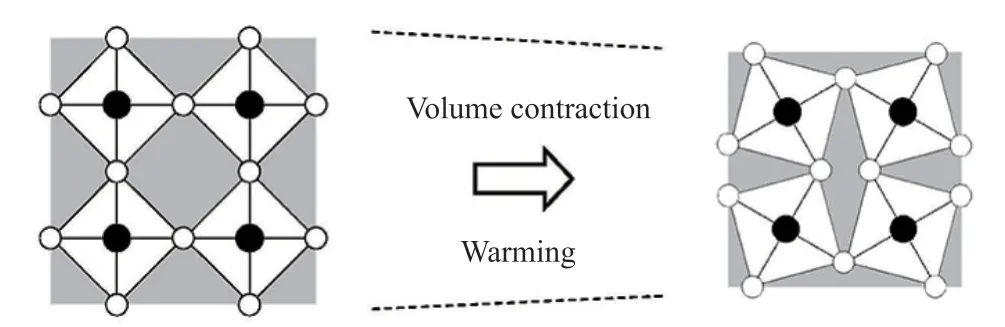
Fig.7 Schematic of negative thermal expansion in a flexible network
Fig.8 is schematic explanation ΔL/Lof microstructural effect for bulk negative thermal expansion.NdMnO3is composed of microcracks and pores with anisotropic thermal expansion.As the temperature increases, the crystal particle expands in one direction and shrinks in the vertical direction.When there is open space in the direction of crystal particle expansion, the ceramic NdMnO3will shrink[40].
4 Conclusions
The NTE properties of NdMnO3at different temperatures were studied experimentally.The experimental results show that NdMnO3has two structural phases at different temperatures: the orderly O’ phase structure at lower temperature and the disorderly O phase structure at higher temperature.As temperature increases, the ceramic NdMnO3presents NTE property range from 280 to 673 K.The average linear expansion coefficient is -6.3×10-6/K; As temperature increases, the ceramic NdMnO3presents NTE property range from 759 to 1 007 K.The average linear expansion coefficient is -18.88×10-6/K.
There are two reasons for negative expansion about NdMnO3: a) There are microcracks and pores in NdMnO3, and when the temperature rises, the crystal squeezes the open space, resulting in negative thermal expansion; b) NdMnO3is prone to jahn-teller effect,resulting in distortion and atomic movement, especially when heated, it prone to lateral oxygen displacement.These displacements consume open space in the crystal structure, resulting in volume contraction and negative expansion.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- One-pot Synthesis of Hierarchical Flower-like WS2 Microspheres as Anode Materials for Lithium-ion Batteries
- Controllable Synthesis of Au NRs and Its Flexible SERS Optical Fiber Probe with High Sensitivity
- Effciient Direct Decomposition of NO over La0.8A0.2NiO3(A=K, Ba, Y) Catalysts under Microwave Irradiation
- Appreciable Enhancement of Photocatalytic Performance for N-doped SrMoO4via the Vapor-thermal Method
- Infulence of Current Density on the Photocatalytic Activity of Nd:TiO2Coatings
- Efficient Removal of Phosphate from Aqueous Solutions Using Corundum- hollow-spheres Supported Caclined Hydrotalcite Porous Thin Films
