Preparation of α-High Strength Gypsum from Flue Gas Desulfurization Gypsum Pretreated by Hydrothermal-aging Method
SU Yixin, GAO Lili,2, CHEN Xueqing*, LI Zhishui, LI Yun*, CAO Jilin
(1.Engineering Research Center of Seawater Utilization Technology of Ministry of Education, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China; 2.Heze Center for Disease Control and Prevention, Heze 274010, China; 3.Tianjin Bohua Yongli Chemical Industry Co., Ltd., Tianjin 300452, China)
Abstract: The synthesis of α-calcium sulfate hemihydrate (α-CSH) from flue gas desulfurization (FGD)gypsum is a good way to realize the comprehensive utilization of FGD gypsum.To obtain α-CSH with the satisfactory performances, a facile hydrothermal-aging pretreatment process for FGD gypsum raw materials was proposed, where FGD gypsum was firstly hydrothermally converted to α-CSH whiskers, and α-CSH whiskers were further hydrated to synthesize CaSO4·2H2O (CSD) by aging under the regulation of N,N'-methylenebisacrylamide (MBA).The effects of aging time, MBA addition, aging temperature, and pH on the morphology of the synthesized CSD were investigated.The synthesized CSD crystals exhibit highly uniform prismatic morphology with the length of ca 100 μm and the whiteness of 91.56%.The regulation mechanism of MBA was also illustrated.The synthesized CSD crystals with prismatic morphology were further used as raw materials to synthesize the short columnar α-CSH.The absolute dry compressive strength of paste prepared from the short columnar α-CSH is 40.85 MPa, which reaches α40 strength grade.
Key words: flue gas desulphurization gypsum; α-calcium sulfate hemihydrate; hydrothermal-aging method; N,N'-methylenebisacrylamide; prismatic CSD
1 Introduction
As an industrial by-product of coal-fired power generation, flue gas desulfurization (FGD) gypsum has an annual output of up to 70 million tons in China[1].High output and low comprehensive utilization rate have led to the bulk accumulation of FGD gypsum,which not only causes a waste of resources, but also seriously affects the environment.α-calcium sulfate hemihydrate (α-CSH), also known as α-high strength gypsum, is a high value-added cementitious material with robust mechanical properties, good compatibility and excellent stabilities, which is widely used in biomedicine[2], functional fillers[3]and building materials[4],etc.Therefore, using FGD gypsum to synthesizea-CSH is a good way to solve the low utilization rate of FGD gypsum and realize its comprehensive utilization.
The main component of FGD gypsum is CaSO4·2H2O (CSD), which could be easily converted to α-CSH by pressurized hydrothermal method[5],atmospheric salt solution method[6], and atmospheric alcohol solution method[7],etc.The morphology of α-CSH directly determines its application fields and performances.In particular, the short columnar α-CSH has significantly enhanced compressive and flexural strength, and has shown great application potential in high-grade building materials.However, α-CSH crystals possess the fastest growth rate alongcaxis thanaandbaxes without regulation, resulting in the needle-like morphology of α-CSH with high aspect ratio.Currently, various crystals modifiers have been proposed to reduce the aspect ratio of α-CSH[8-13].For example, Guanet alused inorganic salt solutions to prepareα-CSH crystals with an aspect ratio of about 3, and the highest absolute dry compressive strength reached 25.3 MPa.And after 8 cycles of mother liquor recycling, high-strengthα-CSH crystals could still be prepared[12].Jiaet alreported that trace NaCl and Na2EDTA were used to regulate the morphology ofa-CSH from acicular to lamellar, where Na2EDTA was adsorbed on the top facet to inhibit the growth rate alongcaxis, and trace NaCl promoted the nucleation and crystal growth process[13].Although the aspect ratio ofα-CSH could be dramatically decreased, the dry compressive strength of the paste prepared fromα-CSH is not satisfactory for the actual applications.This phenomenon could be attributed to the poor grain gradation and impurities of FGD gypsum.The regulation effect of the crystal modifiers on the direct transformation from FGD gypsum toα-CSH exerts a prominent change on the morphology ofα-CSH,whereas has no significant alteration on the grain gradation and impurities removal.In addition, the whiteness ofα-CSH directly prepared from FGD gypsum is low, which has certain restrictions on commercial applications.
Our group reported the synthesis of the uniformly small and quasi-sphericalα-CSH particles via the twostep hydrothermal method, demonstrating that the morphology and size of gypsum raw materials have an important impact on the feature of products[14].Hence, we expect thatα-CSH with the satisfactory performances could be obtained by optimizing the feature of the gypsum raw material.N,N’-methylenebisacrylamide (MBA) is a surfactant containing two double bonds, imino groups, and carbonyl groups, which shows an enhanced adsorption effect on Ca2+site ofα-CSH[14-16], thereby regulating the morphology of inorganic crystals[17].In this work, a hydrothermal-aging pretreatment process is proposed for FGD gypsum raw materials.FGD gypsum is firstly hydrothermally converted toα-CSH whiskers.Based on the property ofα-CSH that could be converted to CSD by hydration, the synthesizedα-CSH whiskers further undergoes aging process in water to be hydrated to CSD, where the surfactant MBA is added to regulate the morphology of the obtained CSD.The effects of aging time, aging temperature, MBA addition and pH on the morphology of the synthesized CSD after hydration are discussed.The variation of the whiteness of the synthesized CSD is investigated.Furthermore,the synthesized CSD is converted to short columnarα-CSH under the regulation of succinic acid.The dry compressive strength of paste prepared with short columnara-CSH is measured.
2 Experimental
2.1 Materials
FGD gypsum was produced for FGD system at a plant in Tianjin, China.Sodium chloride (NaCl),succinic acid, and MBA were obtained from Shanghai Macklin Biochemical Technology Co., Ltd., China.The reagents used in the experiment were all analysis grade.
2.2 Pretreatment of FGD gypsum
The pretreatment process of FGD gypsum is shown in Fig.1.FGD gypsum with the whiteness of 39.22% was firstly physically purified by water as reported elsewhere[14].The washed gypsum of 9 g was mixed with 50 mL of distilled water, and reacted at 120℃ for 2.5 h to obtainα-CSH whiskers slurry.After cooling, the slurry was transferred to a three-necked flask in water bath at 80 ℃.MBA of 3 g was added,and pH value of the slurry was adjusted with NaOH and HCl solutions.After aging for 60 min, the product was rinsed with distilled water, and dried at 45 ℃ to a constant weight to obtain CSD with high whiteness.
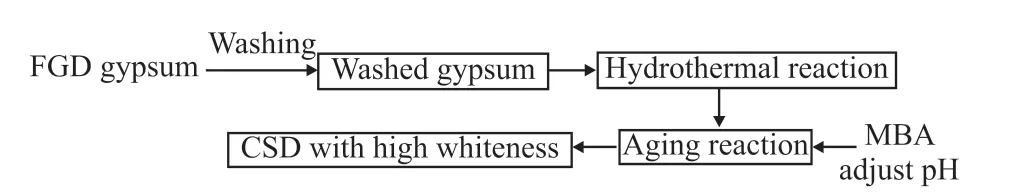
Fig.1 Pretreatment process of FGD gypsum
2.3 Synthesis of short columnar α-CSH
9 g of the synthesized CSD underwent a hydrothermal reaction in a solution containing 50 mL of distilled water, 1.5 g of NaCl, 0.01 g of succinic acid at 120 ℃ for 2.5 h.The products were rinsed and dried using the aforementioned method.
2.4 Mechanical test
This method was mainly based on JC/T 2038-2010 (China national standard forα-CSH).The synthesized short columnarα-CSH was mixed with distilled water to adjust to a standard consistency,followed by being placed in a mold of 40 mm×40 mm×160 mm.After 1 h, the sample was demolded,and put at 25 ℃ for 24 h.The synthesized paste was placed in an oven at 40 ℃ until constant weight, and was transferred in a compression testing machine for a strength test.
2.5 Characterization
Scanning electron microscopy (SEM) images of products were recorded on Nano SEM 450 with test voltage of 10 kV, and the magnification was 400-5 000.X-ray diffraction (XRD) was employed to determine the crystalline phase of products on D8-Focus ranging from 5° to 90°, using Cu Kα radiation (λ= 0.1541 8 nm)with a scanning rate of 12°·min-1.The composition of FGD gypsum andα-CSH was tested according to GB/T 5484-2000.The whiteness of the products was measured by SC-80 automatic colorimeter.The content of CSD was measured according to JC/T 2074-2011 and GB/T 5484-2000 (China national standard for gypsum).
3 Results and discussion
3.1 Pretreatment of FGD gypsum
The hydrothermal-aging method was employed to pretreat the washed FGD gypsum.As shown in Fig.2(a), the washed FGD gypsum was hydrothermally treated to preferentially form whiskers with the length of ca 170 μm without regulation, and the corresponding XRD pattern only display the diffraction peaks attributed toα-CSH (Fig.2(f)).The synthesizedα-CSH whiskers further underwent an aging process under the regulation of MBA.The effects of aging time, aging temperature, MBA addition and pH on the morphology of the synthesized CSD after hydration are investigated.

Fig.2 SEM images of products synthesized for 0 min (a), 30 min (b), 40 min (c), 50 min (d); and 60 min (e), with their corresponding XRD patterns (f)
3.1.1 The effect of aging time on the synthesized crystals
Figs.2(b-e) show SEM images of the synthesized crystals for different aging time under 3 g of MBA,4 of pH, and 80 ℃ of aging temperature.With the aging time, the diffraction peaks assigned to CSD crystals appear, and the peak intensities ofa-CSH crystals gradually diminish.The aspect ratios of the synthesized crystals are gradually decreased.When the aging time is 50 min, most of the synthesized cyrstals present a plate-like morphology (Fig.2(d)).At this time, the synthesized crystals mainly exhibit CSD crystalline phase, but a small number of diffraction peaks corresponding toα-CSH crystals could still be observed.With the aging time of 60 min, the synthesized crystals are completely transformed to uniformly prismatic crystals with the length of ca 100 μm from whiskers, and only show the diffraction peaks of CSD crystals (Fig.2(e)).The above results suggest the hydration process ofα-CSH to form CSD.Therefore, the most suitable aging time is 60 min.
3.1.2 The effect of MBA dosage on the synthesized crystals
Fig.3 shows the effect of MBA dosage on the synthesized crystals under 80 ℃ of aging temperature,4 of pH, and 60 min of aging time.When MBA is not added, the synthesized crystals exhibit various morphologies, including fan-shaped, needle-like,and a small amount of the prismatic crystals, which is not favorable for the formation of products with high strength (Fig.3(a)).As the dosage of MBA increases, the aspect ratio of the synthesized crystals shows a reduced tendency, and the morphology of the synthesized crystals is gradually changed to the flaky shape.With MBA of 3 g, the synthesized crystals appear as a highly uniform prismatic crystal with the length of ca 100 μm (Fig.3(c)).All the synthesized crystals only show the diffraction peaks corresponding to CSD, and the intensities of the diffraction peaks are gradually increased with the addition of MBA(Fig.3(e)).When continuing to increase the amount of MBA, the defects appear on the surface of the synthesized crystals, and some small grains could be observed, which is not conducive to obtainα-CSH with the superior performance (Fig.3(d)).It should be noted that the diffraction peaks assigned toα-CSH could still be observed, indicating that the synthesized crystals containa-CSH at this time (Fig.3(e)).
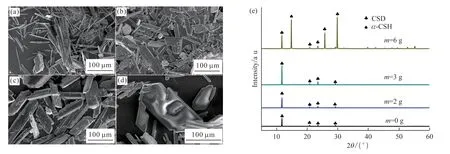
Fig.3 SEM and XRD images of products synthesized at 0 g (a), 2 g (b), 3 g (c), and 6 g (d), with their corresponding XRD patterns (e)
Table 1 shows EDS result of the prismatic CSD synthesized under 80 ℃ of aging temperature (4 of pH, and 60 min of aging time, and 3 g of MBA).The detection of C and N atoms except for the Ca, S and O atoms from CaSO4demonstrates the existence of MBA on the prismatic CSD.To further illustrate the regulation mechanism of MBA, CSD contents in the synthesized crystals with different dosages of MBA as a function of aging time are investigated.As shown in Fig.4, CSD contents in the synthesized crystals are decreased with the increase of MBA addition, demonstrating the retard effect of MBA for the hydration possess.The hydration ofα-CSH to synthesize CSD is a dissolution-recrystallization process, including the dissolution ofα-CSH and the recrystallization of CSD[18].When MBA is added to the reaction system, carbonyl groups in MBA molecules would be adsorbed on the surface ofα-CSH to form a hydrophobic and thin layer by the coordination effect between Ca2+ions and carbonyl groups, which would slow down the dissolution rate ofα-CSH crystals.As a result, low degree of supersaturation in the reaction system would provide sufficient time and space for the growth of CSD nuclei.At the same time, the preferential adsorption of MBA on (111)plane with high Ca2+ion density of CSD crystal would inhibit its growth alongcaxis.The above regulation effects of MBA promote the formation of large and prismatic CSD crystals.As the addition of MBA further increases, the adsorption layer of MBA on the surface ofα-CSH crystals would generate a serious inhibition effect for the dissolution process ofa-CSH crystals,resulting in the remarkable reduction in the hydration rate and non-uniform CSD crystals.
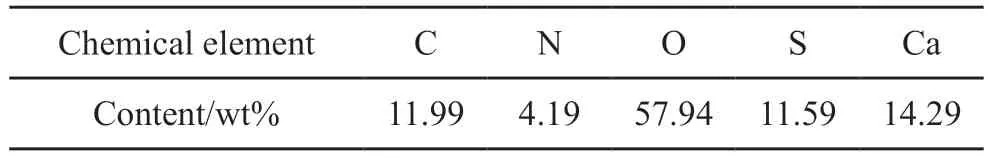
Table 1 EDS results of the prismatic CSD
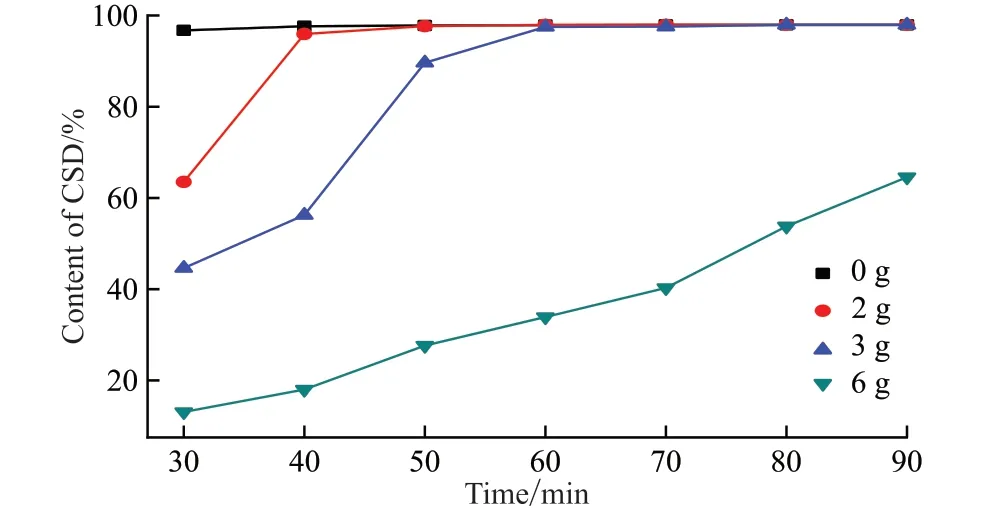
Fig.4 Variation of CSD contents in the synthesized crystals under different dosages of MBA with aging time
3.1.3 The effect of aging temperature on the synthesized crystals
The effect of the aging temperature on the synthesized crystals was investigated under 3 g of MBA, 4 of pH, and 60 min of aging time.As shown in Fig.5(a), when the aging temperature was 40 ℃, the synthesized crystals possess needle-shaped and fanshaped morphologies.XRD patterns demonstrate that the composition of the synthesized crystals is mainly CSD, accompanied by a small amount of incompletely dissolvedα-CSH (Fig.5(f)).As the temperature rose to 60 ℃, the whiskers dramatically diminish, and most of the synthesized crystals exhibit the flake morphology(Fig.5(b)).At this time, all the diffraction peaks in the corresponding XRD pattern could be attributed to CSD crystalline phase.With the increase of temperature, the morphology of the synthesized crystals is gradually converted to prismatic crystals.When the temperature was 80 ℃, the synthesized crystals appear as a prism with a complete structure and a smooth surface, and the length of crystals is about 100 μm (Fig.5(d)).As the temperature continued to rise to 90 ℃, the crystal morphology would be transformed to the irregularshaped flakes and a large number of small rod-shaped crystals (Fig.5(e)).
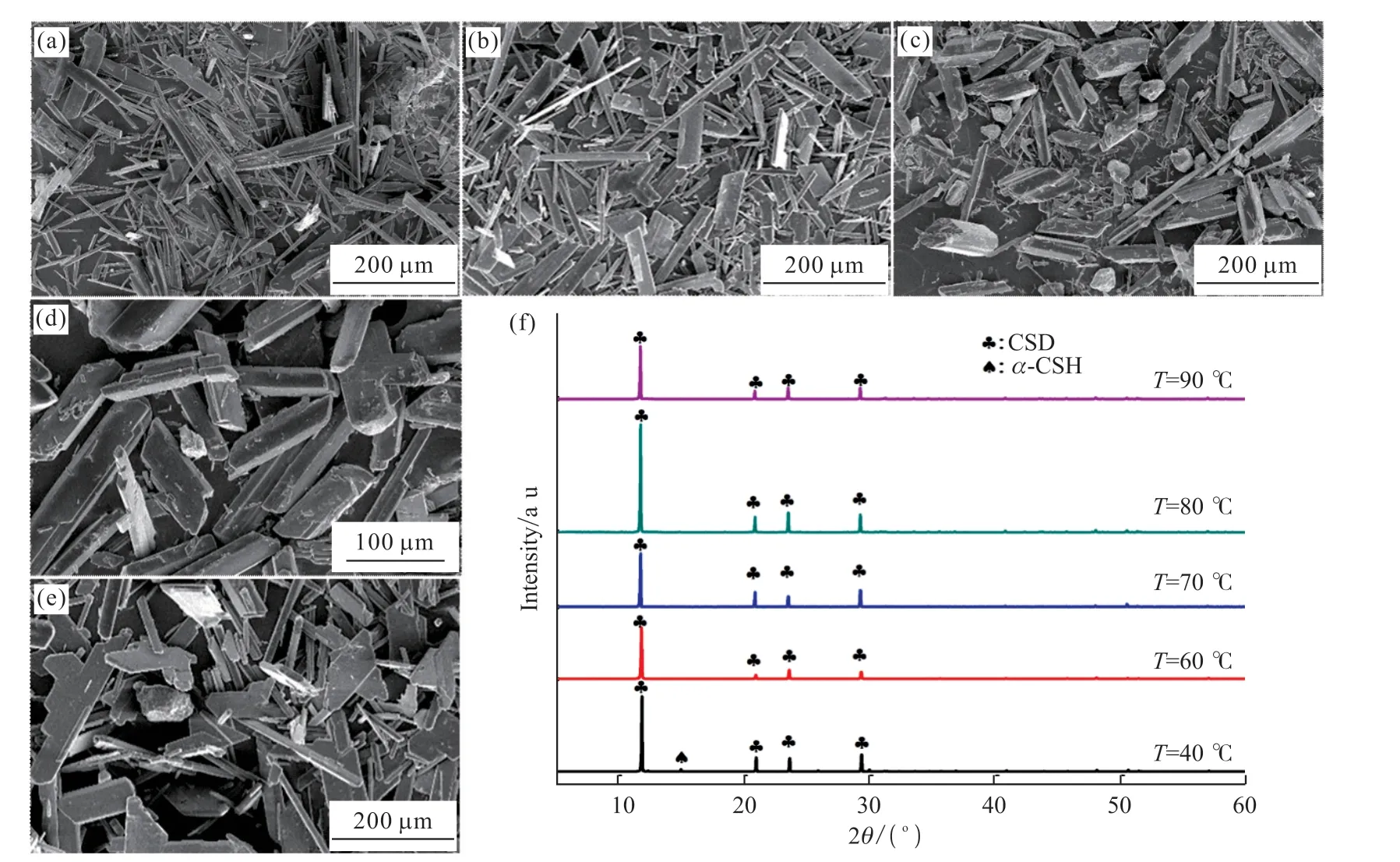
Fig.5 SEM and XRD images of products synthesized at 40 ℃ (a), 60 ℃ (b), 70 ℃ (c), 80 ℃ (d), and 90 ℃ (e), with their corresponding XRD patterns (f)
The change of temperature has great influences on the hydration process ofα-CSH and solubility of MBA.When the aging temperature was 40 ℃, low concentration MBA in the reaction system generates a limited manipulation effect on the dissolution of MBA.Although low temperature is not conducive to the dissolution ofα-CSH, the enhanced solubilities ofα-CSH at a decreased temperature would lead to the high supersaturation in the reaction system[19].As a result, massive crystal nuclei promote the formation of needle-shaped and fan-shaped CSD crystals.With increasing aging temperature, the augmented concentration of MBA in the reaction system slows down the accelerated dissolution rate ofa-CSH caused by the elevated aging temperature, while the solubilities ofa-CSH and CSD are decreased.The combined effect of the above factors is in favor of the growth of prismatic CSD crystals.Furthermore, if the temperature is too high, the increased dissolution rate ofa-CSH would lead to the prolonged supersaturation state, resulting in the generation of small CSD grains.Therefore, the optimal aging temperature is 80 °C.
3.1.4 The effect of pH on the synthesized crystals
The effect of pH on the synthesized crystals was studied under 80 ℃ of aging temperature, 3 g of MBA addition, and 60 min of aging time.As shown in Fig.6(e), all samples only show the diffraction peaks corresponding to CSD crystals.When pH of the solution was 1, there are massive small grains in addition to a tiny amount of needle-like and prismatic crystals (Fig.6(a)).With pH of 4, the morphology of the synthesized crystals is mainly large and uniform prism (Fig.6(b)).When the pH was 7, most of the synthesized crystals exhibit the increased size,whereas formation of small grains could be observed(Fig.6(c)).When the reaction system is changed to an alkali solution, the synthesized crystals display a reduced size and aspect ratio (Fig.6(d)).The above phenomena could be interpreted by the variation of solubility ofα-CSH under different pH values of the solution.When pH value of the reaction solution was 1, MBA would be hydrolyzed to obtain acrylic acid.This monocarboxylic acid shows an inconspicuous restriction effect on the dissolution ofα-CSH[20].At the same time,α-CSH possesses the augmented solubility with the enhancing of the acidity.The combined effect of two above factors results in the high supersaturation in the reaction solution, generating a large number of CSD nuclei.As a result, the synthesized crystals are the tiny grains under pH value of 1.With the increase of pH, both of the solubility and dissolution rate ofa-CSH are decreased.When pH value of the solution was increased to 4, the synthesized crystals show the prism morphology with the uniform size.When the solution is alkaline, due to the reaction between OH-and Ca2+,the dissolution ofα-CSH crystals would be promoted,while the recrystallization of CSD crystals would be inhibited.At the same time, the existence of many SO42-is beneficial to the crystal growth along (110) and(200) planes[21].Therefore, the synthesized crystals in the alkaline solution display a reduced size and aspect ratio, which is not the best choice for the synthesis ofα-CSH with high strength[13].
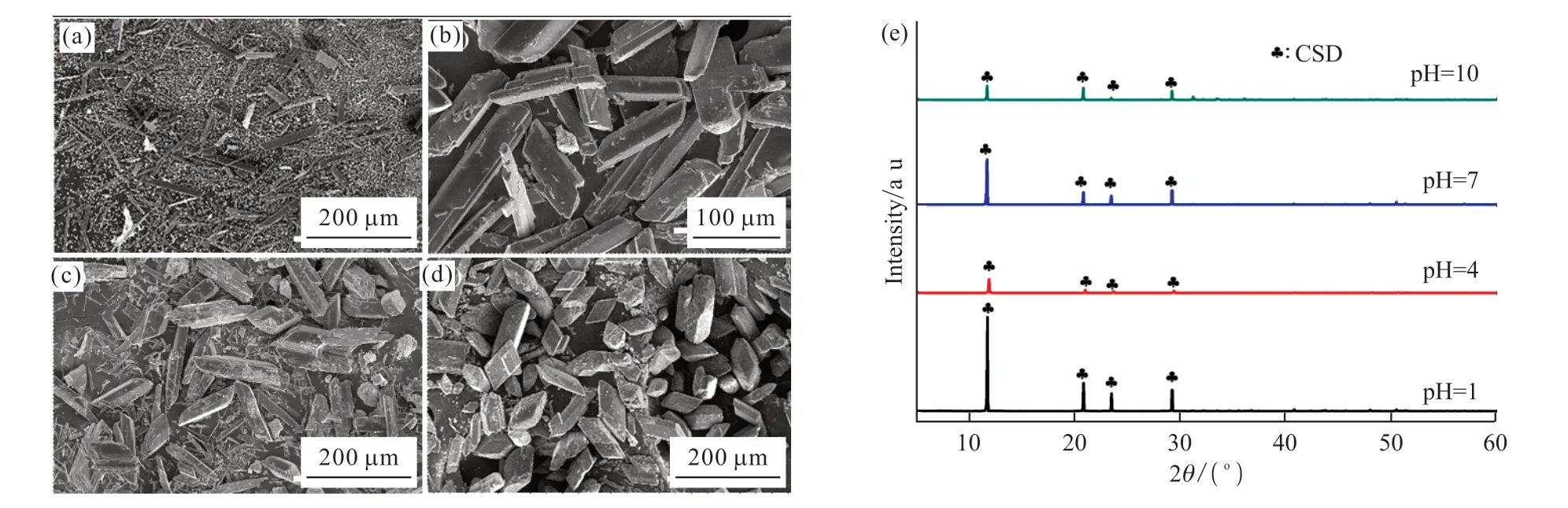
Fig.6 SEM and XRD images of products synthesized under pH of 1 (a), 4 (b), 7 (c), and 10 (d), with their corresponding XRD patterns (e)
3.1.5 Whiteness of CSD aged for different time
The change of CSD contents in the products and whiteness of products synthesized for different aging time are shown in Fig.7.It can be seen that whiteness ofα-CSH before aging is 44.19%, and CSD content in the product is 0.4%.With the extension of the aging time, the whiteness and CSD content in the product show an augmented trend.Until the aging time is 60 min, the whiteness of the product could reach 91.56%, and the content of CSD is 99.29%.During the hydration process ofa-CSH, with the dissolution ofa-CSH crystals, the impurities in the gypsum are gradually released to the solution.When dissolved Ca2+and SO42-are recrystallized to obtain CSD, the concentration of impurities in the solution is far from their supersaturated state, leading to a result that the impurities remain in the mother liquor.Therefore,the hydration process could realize the removal of impurities from the gypsum.
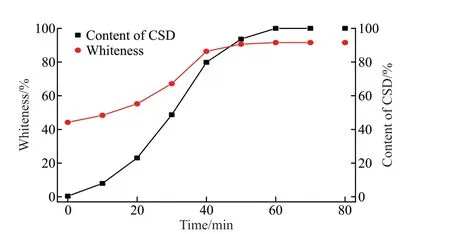
Fig.7 Variation of the content of CSD and whiteness with the aging time
3.2 Preparation of short columnar α-CSH
CSD prepared by hydrothermal-aging process under the optimal conditions was used to synthesizeα-CSH crystals.As shown in Fig.8, the synthesizedα-CSH exhibits short columnar morphology with an average length of ca 80 μm.The corresponding XRD pattern shows no other diffraction peaks assigned to impurities.Furthermore, the absolute dry compressive strength of the paste prepared by the synthesized short columnarα-CSH is 40.85 MPa, which reachesa40 strength grade according to the industrial standard ofα-high strength gypsum plaster.The above results suggest that the synthesizedα-CSH is in favor of obtaining high-quality building materials.
4 Conclusions
We proposed a facile hydrothermal-aging pretreatment process for FGD gypsum raw materials.FGD gypsum was firstly hydrothermally converted toα-CSH whiskers, andα-CSH whiskers was further hydrated to synthesize CSD under the regulation of MBA.The optimal hydration conditions were 60 min of aging time, 3 g of MBA addition, 80 ℃ of aging temperature, and 4 of pH.CSD crystals prepared from aging ofα-CSH whiskers exhibit the highly uniform prismatic morphology with the length of ca 100 μm and the whiteness of 91.56%.MBA could be adsorbed on the surface ofα-CSH to slow down the dissolution rate ofα-CSH crystals, providing the sufficient time and space for growth of CSD.At the same time, the coordination between Ca2+ion and carbonyl groups of MBA would inhibit the growth of CSD crystal alongcaxis.Furthermore, the synthesized CSD crystals with prismatic morphology were used as raw materials to synthesize the short columnara-CSH with an average length of ca 80 μm.The absolute dry compressive strength of paste prepared from short columnara-CSH is 40.85 MPa, which could reacha40 strength grade according to the industrial standard ofa-high strength gypsum plaster.This process effectively improves the performances of the synthesizeda-high strength gypsum by optimizing the morphology, size and whiteness of gypsum raw materials, paving a new way to realize the high value-added utilization of FGD gypsum.
Conflict of interest
All authors declare that there are no competing interests.
 Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
Journal of Wuhan University of Technology(Materials Science Edition)2024年1期
- Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- One-pot Synthesis of Hierarchical Flower-like WS2 Microspheres as Anode Materials for Lithium-ion Batteries
- Controllable Synthesis of Au NRs and Its Flexible SERS Optical Fiber Probe with High Sensitivity
- Effciient Direct Decomposition of NO over La0.8A0.2NiO3(A=K, Ba, Y) Catalysts under Microwave Irradiation
- Appreciable Enhancement of Photocatalytic Performance for N-doped SrMoO4via the Vapor-thermal Method
- Infulence of Current Density on the Photocatalytic Activity of Nd:TiO2Coatings
- The Negative Thermal Expansion Property of NdMnO3 Based on Pores Effect and Phase Transition
