Efficacy of MyoRing implantation in the treatment of keratoconus
Masoud Khorrami-Nejad, Rawshan Jumaah, Farshid Karimi2, Khosrow Jadidi3, Mobina Farahani4, Reza Yousefi
Abstract
•AIM: To compare the anterior and posterior corneal astigmatism and total refractive astigmatism before and after MyoRing implantation in keratoconus (KCN) patients.
•METHODS: In this historical cohort study, the preoperative and postoperative total refractive, anterior and posterior corneal astigmatism of KCN patients implanted with a 360-degree full-ring implant (MyoRing) were compared before and after four consecutive follow-up sessions at 3, 6, 9, and 12 mo after surgery.
•RESULTS: The study encompassed 79 KCN patients (85 eyes), comprising 43 males and 36 females. The mean age of the patients was 29±7.41 years, ranging from 17 to 48 years. Throughout the follow-up sessions, a gradual decrease was observed in the trend of changes for total refractive astigmatism, anterior corneal astigmatism, and posterior corneal astigmatism. Postoperatively, total refractive astigmatism measurements exhibited a significant decrease of 2.09 D at 12 mo after MyoRing implantation (4.27±3.15 vs 2.18±1.63 D, P<0.001). Additionally, post-operative measurements revealed an enhancement of approximately 3.20 D and 0.59 D for anterior and posterior corneal astigmatism, respectively [6.40±1.90 vs 3.20±1.75 D for anterior corneal astigmatism (P<0.001) and 1.30±0.55 vs 0.71±0.35 D for posterior corneal astigmatism (P<0.001)].
•CONCLUSION: MyoRing implantation demonstrates significant improvements in astigmatism parameters, encompassing total refractive astigmatism as well as anterior and posterior corneal astigmatism.
•KEYWORDS:keratoconus; intrastromal corneal ring implants; MyoRing; corneal topography; total astigmatism; corneal astigmatism
INTRODUCTION
Keratoconus (KCN) is a prevalent type of corneal ectasia characterized by non-inflammatory, progressive, bilateral, and asymmetric corneal thinning[1-4]. The aberrant corneal changes associated with KCN can potentially contribute to myopia progression, reduced visual acuity, and the development of irregular astigmatism[4-5]. Corneal thinning in KCN typically occurs in the central or para-central areas of the cornea, although it is commonly observed in the inferior and temporal regions[6]. The severity of KCN directly influences its visual and structural complications. As the severity of KCN advances, the impact on visual functions and corneal structure becomes more significant[5]. The prevalence of KCN is typically reported as approximately 1 in every 2 000 individuals, while the incidence rate is estimated to be around 1 in every 400-600 individuals. Notably, the highest incidence and prevalence rates are commonly observed within the 20- to 30-year-old population[4, 7-9].
Improving the visual quality of patients with KCN can be practically achieved through two management strategies based on the severity of the condition: surgical and non-surgical methods[10-11]. Mild to moderate cases of KCN can be effectively managed by various types of spectacles and contact lenses, including customized soft designs, GP lenses, mini-scleral and scleral designs, hybrid contact lenses, and piggyback systems[12]. In cases where other treatment strategies are no longer effective for advanced KCN, the surgical intervention of choice is penetrating keratoplasty (PKP). This procedure involves removing the affected cornea’s full thickness and replacing it with a donor cornea[13-15]. Lamellar procedures have been introduced as alternatives to full-thickness PKP. These procedures are mainly categorized into two methods: deep anterior lamellar keratoplasty (DALK) and anterior limiting lamina transplantation[13,16]. Corneal transplant procedures can give rise to severe complications, including graft rejection, significant irregular or regular astigmatism, and adverse effects associated with the prolonged administration of steroid medications in the postoperative period[17]. As a result, corneal graft surgery is considered the last management option for KCN patients. In recent years, various medical and surgical treatment modalities have been developed to slow down or halt the progression rate of KCN. However, selecting the most suitable management plan remains a topic of controversy. The sole available surgical technique that has shown potential in limiting KCN progression is called corneal crosslinking (CXL)[18]. CXL is a surgical intervention that aims to enhance the rigidity and biomechanical properties of the corneal tissue. By reinforcing the collagen bindings within the cornea, CXL effectively halts the progression of the disease[19-20].
An intrastromal corneal ring segment (ICRS) is a small implant, typically in the shape of an arc or a ring, made of polymethyl methacrylate (PMMA) material[21]. Initially introduced to improve mild myopia, ICRS implants have also been utilized to correct refractive errors in patients with mild-to-moderateKCN[22]. Safety, stability, and reversibility are the main advantages of these corneal implants[23]. ICRS implants act on the corneal tissue by reducing its sagittal depth, resulting in corneal flatteningand also results in a thicker and more regular epithelium in the central corneal[24-25]. From a clinical standpoint, ICRSs are positioned between the corneal collagen fibers. Due to their compressive effect, they can reduce corneal curvature. In the case of corneal astigmatism, these implants can function as corrective lenses by exerting traction forces on steep areas of the cornea. Previous studies have shown that the effectiveness of these implanted rings is directly influenced by the thickness of the ICRS and inversely related to the inner diameter of the implant[26-27]. ICRS implants are classified into two categories based on their optical design: complete 360-degree and incomplete arc-shaped (up to 355-degree arc length) intrastromal rings[28-31]. The first group, 360-degree ICRSs, includes the MyoRing (Dioptex GmbH, Austria). The second group, consisting of incomplete ring segments, includes INTACS (Addition Technology, Sunnyvale, CA, USA), Ferrara (Mediphacos, Belo Horizonte, Brazil), and KeraRing (Mediphacos, Belo Horizonte, Brazil), among others[26,32]. The MyoRing implant is circular, with a continuous 360-degree configuration. It has a triangular cross-section and comes in various inner diameters ranging from 5 to 8 mm, with 1 mm increments. The height of the MyoRing ranges from 150 to 350 μm, with a tolerance of ±50 μm. The front surface of the MyoRing is convex, while the back surface is concave, with a radius of curvature of 8 millimeters. During the implantation process, ICRS implants are inserted into the stromal layer by creating an intrastromal pocket. Initially, the pocket creation procedure was performed using mechanical instruments[33]. This procedure imposed the risk of corneal endothelial defect, perforation, and ring asymmetry[34-35]. An innovative and highly precise method of pocket formation involves using a femtosecond laser (FSL-assisted), which creates a uniform pocket with high precision[36-38]. In prior studies, it has been explored that different types of ICRSs can have varying effects on various topographic parameters of the anterior segment, visual acuity, and refractive errors of the cornea affected by KCN. The main objective of this thesis was to compare the changes in anterior and posterior corneal astigmatism, as well as total refractive astigmatism, before and after the implantation of MyoRing in patients with KCN.
SUBJECTS AND METHODS
The present study is a historical cohort study on 85 eyes of 79 KCN patients. The protocol of the study was approved by the Ethics Committee of Tehran University of Medical Sciences (IRB No.2015-10-117) before data collection in order to review patient records and use the data, and adheres to the tenets of the Declaration of Helsinki.
The present study examined medical records from KCN patients who underwent MyoRing implantation at the ophthalmology hospital of Bina, Tehran, Iran. Data collection spanned from 2010 to July 2022, during which these patients received continuous follow-up by the ophthalmologist for 12 mo. A total of 427 files of KCN patients treated with MyoRing were reviewed, and among them, 79 files met the necessary inclusion criteria for the study. The pre-operative and post-operative data of KCN patients who were followed for at least one month after MyoRing surgery were evaluated.
The study included KCN patients who met the following characteristics: KCN stage 2 and 3 (according to Amsler-Krumich staging method)[39], progressive KCN during the last two years manifested by tomographical changes (over 1.00 D increase in steep-K, over 1.00 D increase in cylindrical manifest refraction, and more than 0.50 D change in spherical equivalent manifest refraction), intolerance to GP lenses, reduced corrected distance visual acuity (CDVA) using spectacles and GP lenses, average keratometry reading no more than 55 D, thinnest point value over 400 microns, and completion of postoperative examinations. Patients with a central corneal scar, herpetic keratitis, previous ophthalmic surgery, and any connective tissue disorders were excluded from the study. The following data were recorded in the study: demographic characteristics of the patients, uncorrected distance visual acuity (UDVA) and CDVA measured using a standard Snellen chart at a distance of 6 meters, subjective refractions for spherical and cylindrical corrections obtained using an auto-refractometer (TOPCON RM-8000), and tomography parameters measured using Pentacam HR (Oculus, Weltzar, Germany).
SurgicalProcedureAll surgeries were performed under anesthesia by an experienced surgeon using the same procedure (Kh J). The surgical procedure was as follows: Before the surgery, the eye was anesthetized with three drops of topical tetracaine 0.5% eye drops. The first phase of the surgery involved creating a 10 mm intrastromal pocket at a depth of 300 microns around the corneal center, utilizing a femtosecond laser machine (Femtec TECHNOLAS Perfect Vision GmbH, Bausch + Lomb, USA) with a power of 5 mJ. At this stage, the MyoRing was implanted into the stromal pocket. A bandage contact lens was applied to the corneal surface to conclude the operation[30].
All patients were instructed not to wear any contact lenses before examinations, with a minimum of 2 wk for soft lenses and 4 wk for gas-permeable lenses. While this study is not an interventional study, the criteria for the surgical procedure and MyoRing implantation were based on the following protocol. All eligible participants underwent MyoRing implantation. Several inclusion criteria were considered when recruiting the optimal candidates for MyoRing implantation:UCVA less than 0.3 LogMAR; Minimum corneal thickness over 360 μm; average central keratometry (ACK) [i.e., half of flat keratometry (Kf) + steep keratometry (Ks)] greater than 44 D; no central corneal scarring; no history of prior corneal surgery; patient’s age under 50 years old.
After meeting the criteria mentioned above, the diameter and thickness of the MyoRing implants were chosen according to the standard nomogram (Table 1)[30].
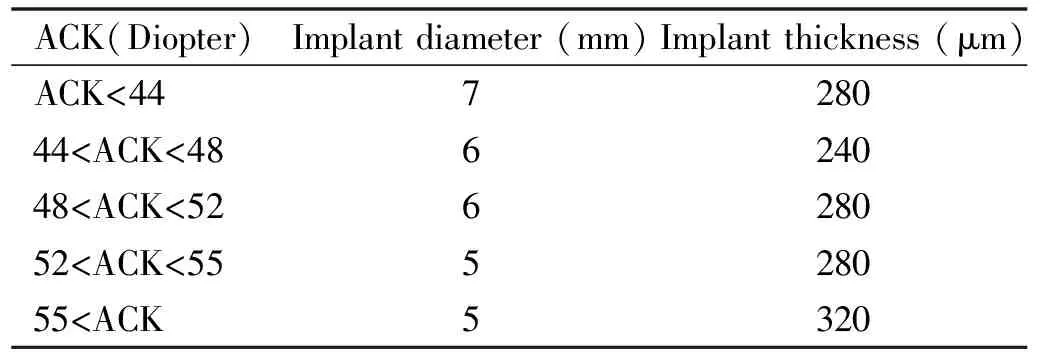
Table 1 MyoRing implantation nomogram
The power vector analysis, as outlined by Thibos and Horner[40], was employed to compare the refractive error components in the two groups under study. This approach involved converting the conventional spherocylindrical refraction into power vector coordinates, which were then represented by three dioptric powers: M, J0, and J45. In this context, M represents the spherical equivalent (SE) of the refractive error, while J0 and J45 correspond to the two Jackson cross cylinder equivalents for the conventional cylinder. The utilization of this method facilitated a comprehensive assessment of the refractive error characteristics in the study groups.
StatisticalAnalysisStatistical analyses were conducted using SPSS 24 (IBM Inc., Chicago, USA). The mean±SD was reported for each parameter during preoperative and postoperative follow-up sessions. The normal distribution of all data was assessed using the Shapiro-Wilk test. The repeated measurement analysis of variance was used for parametric analysis to compare the preoperative and postoperative measurements or consecutive postoperative examinations. In cases where parametric analysis was not appropriate, the Friedman test was employed to compare the preoperative and postoperative measurements. AP-value of less than 0.05 was considered statistically significant.
RESULTS
This study included a total of 85 eyes from 79 KCN patients, consisting of 43 males and 36 females. The mean age of the patients was 29±7.41 years (17-48 years), and a follow-up period of 3, 6, 9, and 12 mo was conducted for all participants. The results of the repeated measures analysis of variance and Friedman reveal meaningful changes in these variables over time.
Table 2 presents the mean values of UDVA and CDVA, refractive error, refractive astigmatism, and keratometry in the pre-operative phase and different post-operative follow-ups.The results of the analysis of variance with repeated measures indicate significant changes in the variables over time. The results demonstrate that following MyoRing implantation, significant improvement was observed in all visual, refractive, and keratometry parameters as shown in this table, the trend of changes in refractive astigmatism decreased gradually in each follow-up.
Figure 1 illustrates the evolution of anterior and posterior corneal astigmatism throughout the pre-operative phase and various post-operative follow-ups. As depicted in the figure,the anterior corneal astigmatism consistently decreased in each subsequent follow-up, highlighting a discernible trend in the alterations over time.
Table 2 The result of repeated measures ANOVA test or Friedman test for visual acuity, refraction, and astigmatism changes following MyoRing implantation: pre-operative and post-operative
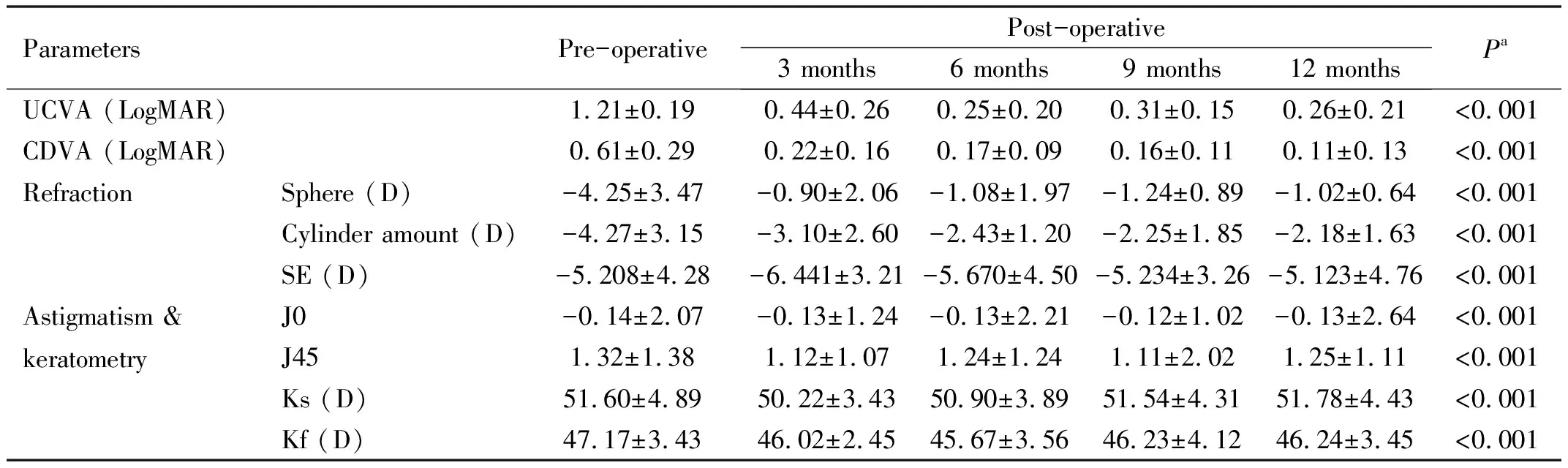
ParametersPre-operativePost-operative3 months6 months9 months12 monthsPaUCVA (LogMAR)1.21±0.190.44±0.260.25±0.200.31±0.150.26±0.21<0.001CDVA (LogMAR)0.61±0.290.22±0.160.17±0.090.16±0.110.11±0.13<0.001RefractionSphere (D)-4.25±3.47-0.90±2.06-1.08±1.97-1.24±0.89-1.02±0.64<0.001Cylinder amount (D)-4.27±3.15-3.10±2.60-2.43±1.20-2.25±1.85-2.18±1.63<0.001SE (D)-5.208±4.28-6.441±3.21-5.670±4.50-5.234±3.26-5.123±4.76<0.001Astigmatism & keratometryJ0-0.14±2.07-0.13±1.24-0.13±2.21-0.12±1.02-0.13±2.64<0.001J451.32±1.381.12±1.071.24±1.241.11±2.021.25±1.11<0.001Ks (D)51.60±4.8950.22±3.4350.90±3.8951.54±4.3151.78±4.43<0.001Kf (D)47.17±3.4346.02±2.4545.67±3.5646.23±4.1246.24±3.45<0.001
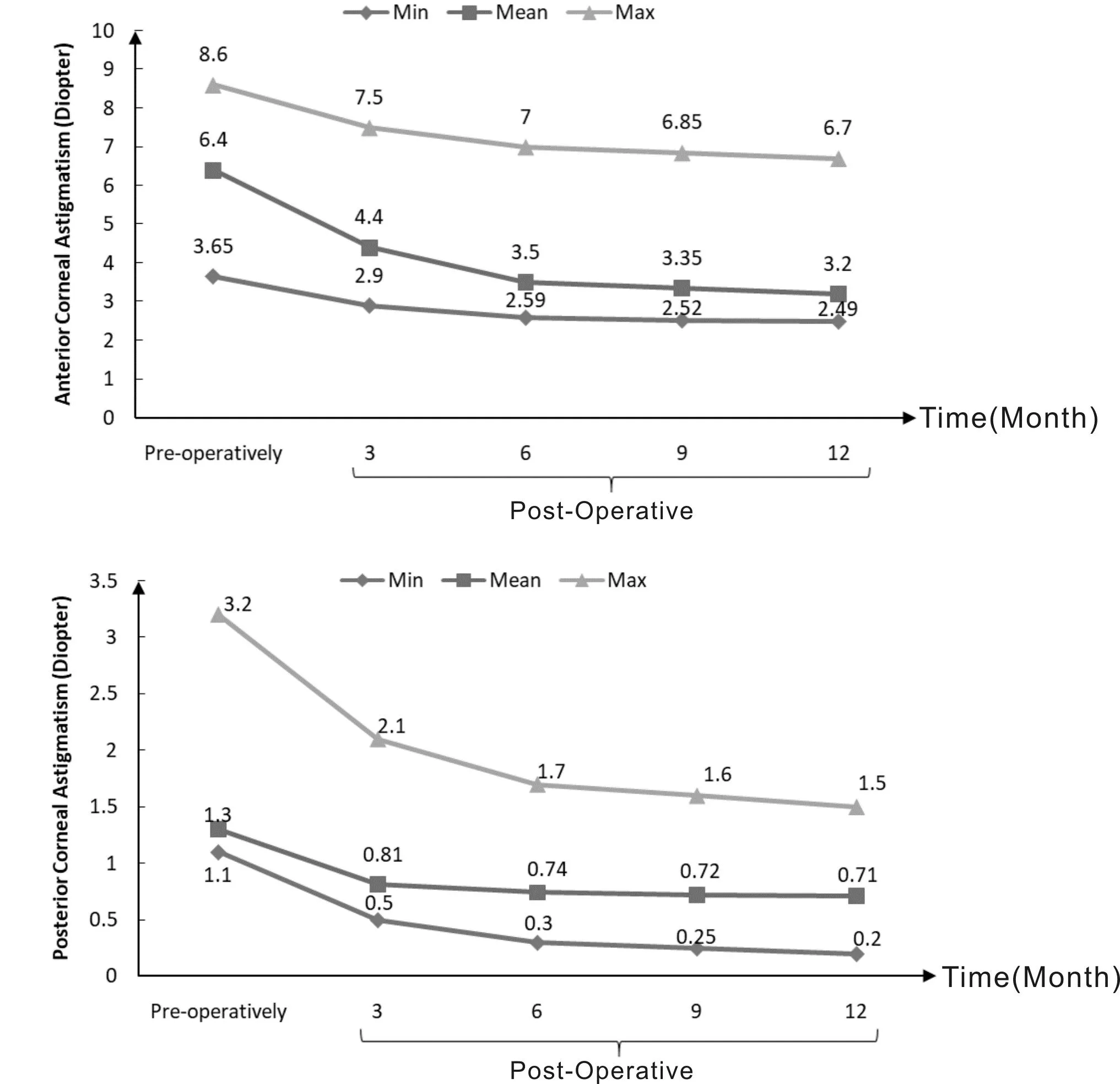
Figure 1 Changes in anterior and posterior corneal astigmatism in the pre-operative phase and different post-operative follow-ups. Max: Maximum; Min: Minimum
Figure 2 illustrates the temporal evolution of astigmatism changes following MyoRing implantation. The procedure resulted in a significant decrease in J45 (P<0.001), accompanied by a notable increase in J0 (P<0.001). Comparative analysis of postoperative values between 3 and 12 mo revealed statistically significant alterations in J45 (P<0.001), while J0 exhibited no significant changes (P<0.001). Notably, J0 values at 3 and 6 mo (aP<0.001) were smaller than those at 9 and 12 mo postoperatively. Similarly, J45 values at 3 and 9 mo (aP<0.001) were smaller compared to those at 6 and 12 mo postoperatively.
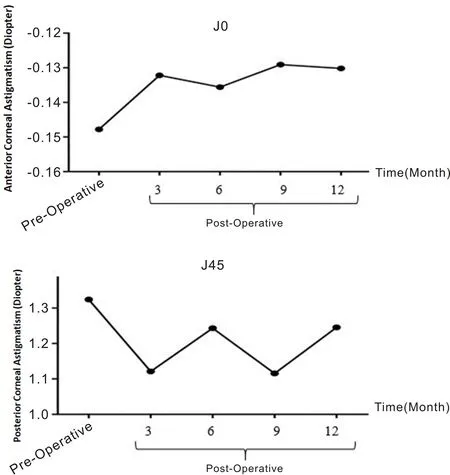
Figure 2 Time course of changes in astigmatism. MyoRing implantation significantly decreased J45 (P<0.001), but significantly increased J0 (P<0.001). When postoperative values from 3 to 12 mo were compared, statistically significant changes were found in J45 (P<0.001) but not in J0 (P<0.001). The J0 at 3 and 6 mo (aP<0.001) were smaller than the J0 at 9 and 12 mo postoperatively. The J45 at 3 and 9 mo (aP<0.001) were smaller than the J45 at 6 and 12 mo postoperatively.
Figure 3 illustrates a vector plot that visually represents variations in both magnitude and direction for each individual during the pre-operative phase and various post-operative follow-ups.
DISCUSSION
ICRS implants function as corrective lenses by exerting traction forces on steepened areas of the cornea, thereby altering both corneal and refractive astigmatism. Previous studies have asserted that various types of ICRS can significantly improve postoperative refractive errors and potentially delay the necessity for corneal graft surgery in affected individuals[41-42]. In this study, we investigated 85 eyes of 79 patients with KCN (43 males and 36 females). The average patient age was 29±7.41 years, ranging from 17 to 48 years. Follow-ups at 3, 6, 9, and 12 mo were conducted for all participants. Our findings revealed a gradual decrease in the trend of changes in total refractive astigmatism, as well as anterior and posterior corneal astigmatism during each follow-up session.
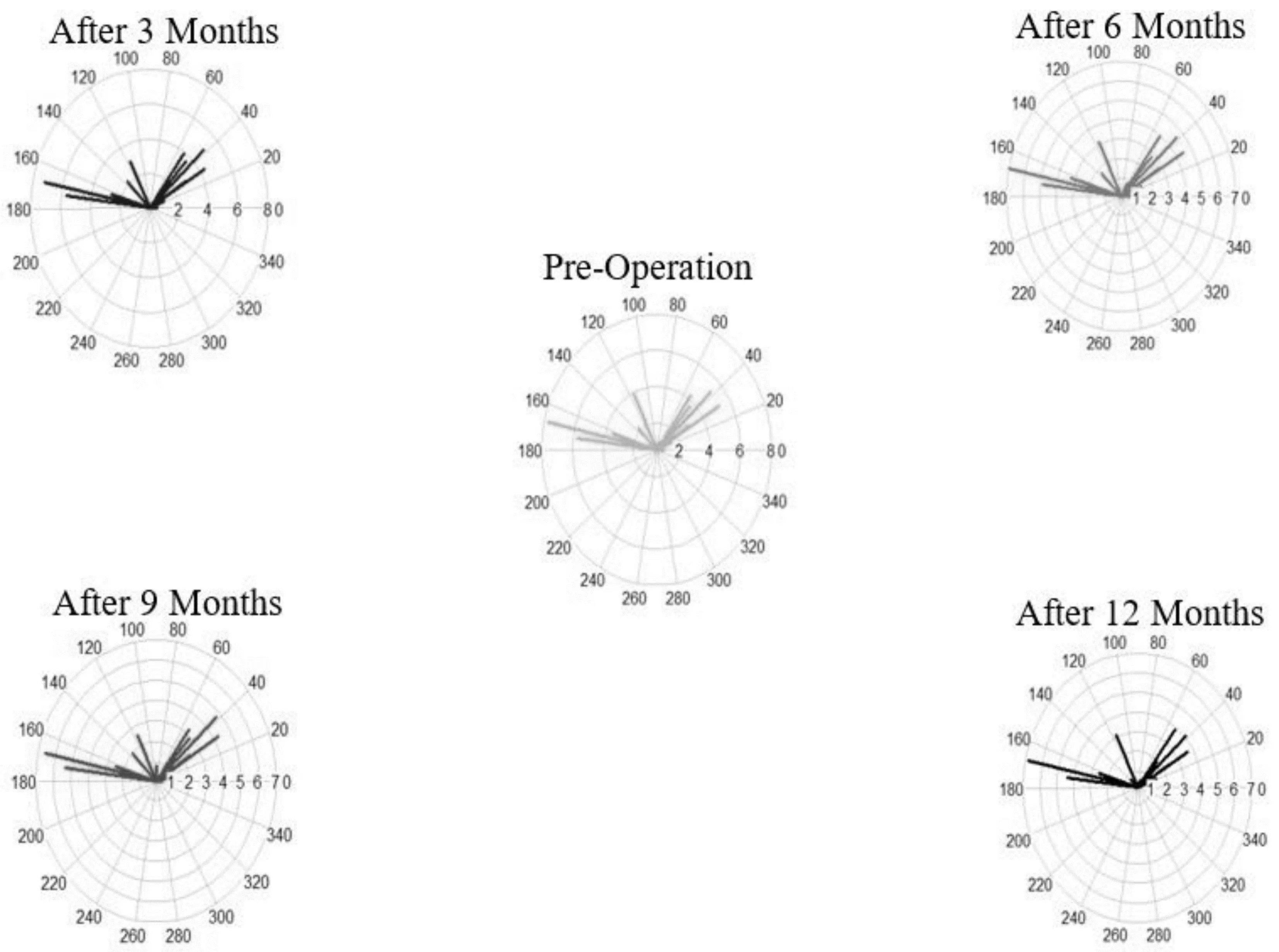
Figure 3 The vector plot depicting changes in magnitude and direction for each individual in the pre-operative phase and different post-operative follow-ups.
The repeated measurement analysis of variance test demonstrated a significant difference between cylinder values in minimum, maximum, and mean modes during all follow-up sessions compared to the pre-operative phase. Notably, the 3-month follow-up exhibited a significant difference in cylinder values compared to other sessions, while the subsequent follow-ups at 6, 9, and 12 mo did not show any significant differences. Furthermore, all visual, refractive, and keratometry parameters, except steep keratometry, showed significant improvement following MyoRing implantation. The mean cylindrical refractive error pre-operatively and at 12 mo post-MyoRing implantation were -5.25±2.03 and -1.54±0.21, respectively (P<0.001).
Numerous studies have highlighted various types of ICRS implants as a safe and effective strategy for managing patients with KCN[20, 43-46]. However, only a limited number of studies have been dedicated to assessing corneal, refractive, and topographic cylindrical changes following MyoRing implantation. The MyoRing, a 360-degree corneal implant, boasts several advantages compared to segmental rings. Its comprehensive design renders it particularly beneficial in managing severe cases of KCN[47]. Moreover, it exhibits enhanced capabilities in fortifying the corneal tissue of KCN patients, leading to a flattening of corneal curvature[47-48]. Consistent with our findings, previous studies have suggested that the utilization of 360-degree ICRS can result in a reduction of sphero-cylindrical refractive error and a decrease in central corneal curvature[47]. Additionally, these implants have demonstrated the capacity to minimize high-order aberrations in corneal surface measurements[41].
The initial technique proposed for MyoRing implantation involves the continuous and flexible insertion of the ring into the corneal tissue, known as the Corneal Intrastromal Implantation System (CISIS)[49]. A comprehensive literature review supports the notion that these 360-degree ICRSs represent a reasonable alternative for managing KCN and possess the potential to delay the need for corneal graft surgery in affected patients[41-42].
In a retrospective observational study, Khosravietal[50]investigated postoperative astigmatism characteristics at 6 mo following MyoRing implantation surgery, comparing the results to preoperative data. The study revealed a significant decrease in mean values of total refractive astigmatism by 2.09 D. Similarly, anterior and posterior corneal astigmatism measurements significantly decreased by 1.16 D and 0.24 D, respectively. Our study aligns with these findings, demonstrating a gradual reduction in the trend of changes in total refractive astigmatism, anterior, and posterior corneal astigmatism across each follow-up session.
Our results indicate that postoperative measurements of total refractive astigmatism improved by approximately 2.09 D after 12 mo of follow-up following MyoRing implantation (4.27±3.15vs2.18±1.63 D). Additionally, measurements of anterior and posterior corneal astigmatism improved by approximately 3.20 D and 0.59 D, respectively (6.40±1.90vs3.20±1.75 D for anterior corneal astigmatism, and 1.30±0.55vs0.71±0.35 D for posterior corneal astigmatism). Consistent with our findings, the authors of the initial study concluded that MyoRing implantation significantly decreases the magnitude of total refractive astigmatism and anterior and posterior corneal astigmatism measurements.
In a cross-sectional observational study, Khorrami-Nejadetal[28]assessed postoperative corneal and refractive changes 6 mo after MyoRing implantation in moderate and severe KCN patients. They reported a significant reduction (2.19 D) in refractive astigmatism and corneal flattening, with decreased corneal toricity (1.78 D). Our 6-month follow-up examinations similarly showed a 2.02 D improvement in refractive cylindrical measurements, aligning closely with Khorrami-Nejadetal’s results.
Naderietal[51]conducted a retrospective study examining the long-term effects of MyoRing on corneal and refractive astigmatism measurements over five years. They reported a significant decrease in the cylindrical component of refractive error from -5.25±2.03 D before surgery to -1.99±0.93 D five years after MyoRing implantation. Corneal astigmatism values also significantly reduced from 6.13±2.99 to 3.09±0.79. Our findings corroborate these results, emphasizing the sustained safety and efficacy of MyoRing implantation over an extended period.
In a 12-month study of MyoRing efficacy on KCN, Jabbarvandetal[52]reported significant improvements in UDVA, CDVA, and refractive error, with a substantial reduction in spherical and cylindrical components. Our study similarly demonstrated enhanced postoperative measurements of total refractive astigmatism by approximately 2.09 D following 12 months of MyoRing implantation. However, notable differences were observed in corneal astigmatism measurements, which could be attributed to variations in KCN severity, sample size, and surgical techniques among different studies.However, notable differences were observed in the changes of anterior and posterior corneal astigmatism compared to Jabbarvandetal’s results. This variance may be attributed to differences in KCN severity among participants, sample size, and variations in surgical techniques employed by different surgeons.
Several limitations should be acknowledged in this study. First, the limited sample size prevented the categorization of patients based on their specific stage of KCN. The absence of a comparison group, comprising patients treated with alternative strategies such as INTACS, Ferrara, or KeraRing, hinders the ability to assess the relative effectiveness of MyoRing compared to other modalities for treating KCN. Furthermore, a time-related limitation was encountered in this study. The constrained post-operative follow-up period restricted our ability to conduct evaluations over more extended durations, such as 5 years after the operation. Future studies are recommended to include a larger and more diverse population, covering a broad age range and varying degrees of KCN severity. This approach would provide a more comprehensive understanding of the impact of MyoRing implantation across different age groups and KCN severities.
In summary, our study demonstrates the significant improvement of astigmatism parameters in the eye, specifically total refractive astigmatism and anterior and posterior corneal astigmatism, through the implantation of MyoRing as a complete 360-degree ICRS. Notably, we observed a gradual reduction in the trend of changes for total refractive astigmatism, as well as anterior and posterior corneal astigmatism, across successive follow-up sessions (3, 6, 9, and 12-month follow-up sessions). As a practical recommendation, we suggest that optometrists and ophthalmologists involved in the examination of patients with KCN should place heightened emphasis on monitoring long-term changes in different ocular parameters, with a specific focus on cylindrical components. This proactive approach to extended follow-up assessments is crucial for a comprehensive understanding of the sustained positive impact and efficacy of MyoRing implantation in managing astigmatism in KCN patients.

