Haploids can be induced in knockout mutants of OsPLA1,but not OsDMP3 or OsDMP6, in rice
Zongki Liu, Yu Zhong, Xiolong Qi, Ti An, Shuwei Guo, Dong Wng, Yuwen Wng, Bin Feng,Zuofeng Zhu, Shojing Chen,*, Chenxu Liu,*
a National Maize Improvement Center, Engineering Research Center of Ministry of Education, China Agricultural University, Beijing 100193, China
b National Center for Evaluation of Agricultural Wild Plants (Rice), Department of Plant Genetics and Breeding, China Agricultural University, Beijing 100193, China
Keywords:Doubled haploid breeding Haploid induction Mutation anlaysis Rice
ABSTRACT Doubled haploid(DH)technology is an important tool in crop breeding because it can significantly accelerate the breeding process.ZmPLA1/MATL/NLD and ZmDMP are two key genes controlling haploid induction (HI)in maize, exhibiting a synergistic effect.However, it is unknown whether knock out of ZmDMP orthologs can stimulate HI in rice.In this study, a ZmPLA1 ortholog (OsPLA1) and two ZmDMP orthologs(OsDMP3 and OsDMP6) were identified in rice.All three genes encode plasma membrane-localized proteins and were highly expressed in mature anthers.Knockout of OsPLA1 in both Minghui 63 and Nipponbare resulted in reduced seed setting rate(SSR)and caused HI.The osdmp3,osdmp6 and the double mutant failed to trigger HI independently,nor increased the haploid induction rate(HIR)when combined with ospla1.Repeated pollinations operations of QX654A with the ospla1 mutant significantly improve SSR, while reducing HIR.RNA-seq profiling of mature ospla1 mutant anthers indicated that a large number of differentially expressed genes (DEGs) were enriched in redox homeostasis and lipid metabolic GO terms, plant hormone signal transduction, and MAPK signaling pathways.These findings provide important insights towards construction of an efficient DH breeding technology and study of the molecular mechanism of HI in rice.
1.Introduction
A crucial aspect of crop breeding is the production of homozygous lines.The development of homozygous lines requires repeated rounds of self-fertilization or backcrossing, typically no less than eight generations, and thus is an expensive and timeconsuming process for most crops[1].Doubled haploid(DH)technology involves the induction of haploid embryos, followed by chromosome doubling to generate homozygous DH lines in two generations [2].The initial and crucial step in DH technology is haploid induction (HI) by various means, such as anther or ovary culture, pollen irradiation, interspecific crosses, and hybridization with a haploid inducer line [2].
HI mediated by haploid inducers is successful and efficient in maize [1–3].The first reported maize haploid inducer line was Stock6, which induced haploids at a frequency of 2.3%–3.2% [3].Haploid inducer lines with increased haploid induction rate (HIR)of 8.0%–15.0% were developed from Stock6 [4–7].HI in maize was predominantly influenced by quantitative trait loci qhir1(also known as ggi1)and qhir8 that accounted for about 66%and 20%of the genetic variance,respectively[8–11].The causal allele of qhir1 had a 4 bp insertion in the 4th exon of ZmPLA1/MATL/NLD, which encodes a pollen-specific phospholipase [12–14].The causal allele for HI in qhir8 was a substitution of thymine by cytosine at 131 bp of the pollen-expressed gene ZmDMP,the protein product of which localizes to the plasma membrane[15].Knockout of ZmDMP gave a 5-to 6-fold increase in HIR when combined with a zmpla1/matl/nld mutation [15].Another study showed that knock-out of ZmPLD3 produced haploids at a frequency of 0.85%–0.96%, and the frequency was increased 3-fold when ZmPLD3 was knocked out in a zmpla1/matl/nld mutant background [16].Increased HIR also led to a reduced seed setting rate (SSR) [15,16].ZmPLA1/MATL/NLD is a highly conserved gene in cereal species.Knockout of ZmPLA1/MATL/NLD orthologs in rice, wheat, foxtail millet and barley were also shown to induce maternal haploids [17–21].In contrast,ZmDMP is conserved in both monocots and dicots [15,22].Knockout of ZmDMP orthologs in Arabidopsis thaliana, Medicago truncatula, Solanum lycopersicum, Brassica napus and Nicotiana tabacum induce maternal haploids [22–26].
Traditionally,haploid rice plants can be produced by anther culture [27,28].Although the method is efficient for Oryza sativa ssp.japonica geneotypes, it has limited success for ssp.indica due to early necrosis, poor callus proliferation, and high numbers of regenerated albino plantlets [27,29].Therefore, it is urgent to establish an efficient HI method that can be used for both subspecies.
In this study, we systematically generated mutant alleles of ZmPLA1 and ZmDMP orthologs in rice and studied their effects in triggering HI in both indica and japonica backgrounds.Our results showed that HI was achieved by knockout of OsPLA1 in both indica and japonica,but osdmp3 or osdmp6,or both mutants did not trigger HI,or increase the HIR when combined with the ospla1 mutation.
2.Materials and methods
2.1.Plant materials and growth conditions
The OsPLA1, OsDMP3 and OsDMP6 genes were knocked out in indica cultivar (cv.) Minghui 63 (MH63) and japonica cv.Nipponbare(NIP)using CRISPR/Cas9.Plasmid construction and rice transformation were performed by Biorun Bioscience Co., Ltd.(Wuhan,Hubei, China).Transgenic plants were self-pollinated to obtain the T3generation, in which the single ospla1, osdmp3 and osdmp6 mutants and various combinations without the Cas9 element were selected following sequencing.
Two cytoplasmic male sterile (CMS) indica lines, QX654A and QX059A,were used as female testers to evaluate SSR and HIR upon outcrossing.Rice plants were grown in the field or in a greenhouse at 28 °C and a 16 h light/8 h dark photoperiod.
2.2.Orthologous gene identification and phylogenetic analysis
The full-length amino acid sequences of ZmPLA1 and ZmDMP(https://blast.ncbi.nlm.nih.gov/Blast.cgi) were used in BLASTP against the sequence databases of Oryza sativa.Genes with amino acid sequence identity > 30% were chosen for phylogenetic analysis.Full-length candidate DMP sequences from rice along with ZmDMP, AtDMP8, AtDMP9 and SlDMP were aligned using the maximum-likelihood method by MUSCLE embedded in MEGA7[30].The ZmPLA1-like and ZmDMP-like proteins were used to construct the phylogenetic tree.
2.3.RNA extraction and qRT-PCR analysis
Fresh rice tissues, including roots, stems, leaves, panicles and mature anthers from MH63 plants were sampled and immediately frozen in liquid nitrogen.Total RNA was extracted using a RNAprep pure plant kit(TIANGEN,https://en.tiangen.com)and reverse transcribed into cDNA using a PrimeScript RT reagent Kit (TaKaRa,https://www.takarabio.com).Quantitative Reverse Transcription Polymerase Chain Reactions (qRT-PCR) were performed for the OsPLA1, OsDMP3, and OsDMP6 genes using a TB Green Premix Ex Taq kit (TakaRa) and a CFX96 Real-Time PCR detection system(Bio-Rad,https://www.bio-rad.com).OsUBQ was used as the reference gene.Three biological replicates, each of which comprised three technical replicates,of each sample were analyzed,and relative expression was calculated with the 2-ΔΔCTmethod.Primers used in qRT-PCR are listed in Table S7.
2.4.Subcellular localization
Protein coding sequences of OsPLA1,OsDMP3 and OsDMP6 without stop codons were cloned into the XbaI-BamHI site of PCAMBIA1305-GFP.Endoplasmic reticulum-localized mCherry was used as a plasma membrane marker.Recombinant vectors 35S::OsPLA1-GFP,35S::OsDMP3-GFP and 35S::OsDMP6-GFP,were transformed into Agrobacterium tumefaciens strain GV3101, and transiently expressed in tobacco leaf epidermal cells.A parallel control experiment was performed with an unmodified vector 35S::GFP.After culturing at 25 ℃for 48 h,green-fluorescent signals were captured by laser scanning confocal microscope (Zeiss 810,https://www.zeiss.com).Primers used in subcellular localization are listed in Table S7.
2.5.Characterization of HI-related phenotypes
Knockout lines with the pla1, dmp3, dmp6, pla1dmp3, pla1dmp6 and pla1dmp3dmp6 alleles and allelic combinations were selfpollinated to determine effects on HI.Putative haploids were identified by their short and narrow leaves compared to diploid sibs and the wild type (WT).Flow cytometry analysis to confirm the ploidy of the putative haploids was carried out following Zhong et al.[22].
The knockout lines were used as male parents in crosses with CMS lines QX654A and QX059A.MH63 was used as the control male parent.All parents were planted in an isolated plot at a ratio of 2:6 for male and female parents.During the flowering period,artificial supplementary pollination was performed twice per day for ten days.Seeds were harvested at approximately 30 days after pollination (DAP).SSR was calculated by the formula: SSR(%) = (number of filled grains/(number of filled grains + number of empty spikelets)) × 100%.Putative haploids were identified using polymorphic markers between MH63 and CMS testers;with putative haploids having only an amplified product corresponding to the female parents.Primers used for haploid identification are listed in Table S7.
In repeated pollination experiments,the pla1-2 mutant line was used as the male parent and QX654A as the female parent.At flowering stage,the upper and lower spikelets in the inflorescence were removed, and the top 1/3 part of each candidate floret glume was cut.The inflorescences were enclosed in a kraft paper bag and were pollinated with the pla1-2 mutant by shaking an anthesing spike above the top opened bags.Three pollination strategies were carried out, single pollination (SP), double pollination (DP) and triple pollination(TP).SP refers to single pollination;DP refer to pollination twice in two days;and TP refer to triple pollination over three days.Seeds were harvested at 20 DAP,SSR and HIR were calculated as described above.
2.6.Origin of haploids
To confirm the origin of haploids, genotyping work of MH63,QX654A, F1and 13 haploids were performed with GenoBaits Rice 40K Panel (https://www.molbreeding.com).DNA libraries were constructed and sequenced on the DNBSEQ-T7 platform by the MolBreeding Biotech Ltd.(Shijiazhuang, Hebei, China).Raw reads were filtered using fastp [31].Clean reads were aligned to the rice Nipponbare reference genome (downloaded from ftp://ftp.ensemblgenomes.org/pub/) using BWA [32].SNP calling was carried out with GATK [33], a total of 12,795 uniformly distributed, highquality SNPs were identified across 12 chromosomes.
2.7.RNA-seq analysis

Fig.1.Identification of candidate genes for HI.(A) Phylogenetic tree of ZmPLA1-like proteins from Oryza sativa and Zea mays; OsPLA1 is marked with a red dot.(B)Phylogenetic tree of ZmDMP-like proteins from Oryza sativa,Zea mays, Arabidopsis thaliana and Solanum lycopersicum with OsDMP3 and OsDMP6 are marked with red dots.(C) Expression patterns of OsPLA1, OsDMP3 and OsDMP6 in different tissues from MH63 analyzed by qRT-PCR.Values are the means ± SD.(D) Subcellular localization of OsPLA1, OsDMP3 and OsDMP6 proteins in tobacco epidermal cells.Confocal scanning analysis showing green fluorescent protein (GFP; outside left), ER signal (inside left),bright field (BF; inside right) and merged micrographs (outside right).Scale bars, 50 μm.
Total RNA was extracted from mature anthers of MH63 and pla1-2 mutant.RNA-seq libraries were constructed and sequenced using an Illumina HiSeq 2500 by Berry Genomics Corporation(https://www.berrygenomics.com).The reads were aligned to the rice Nipponbare reference genome using HISAT2 [34].Differentially expressed genes were identified with an adjusted q value < 0.05 and fold change > 2.0 using the R software package in R[35].GO enrichment was performed using the agriGO singular enrichment analysis tool (https://systemsbiology.cau.edu.cn/agri-GOv2/),and KEGG enrichment was performed using the OmicShare tools (https://omicshare.com/tools/).
3.Results
3.1.Identification of candidate genes responsible for HI in rice
Fourteen ZmPLA1-like proteins and twelve ZmDMP-like proteins in the Oryza sativa genome were identified with sequence identity >30% (Tables S1, S2).OsPLA1 (LOC_Os03g27610, previously named OsMATL) shared amino acid identity of 78.99% with ZmPLA1, whereas the sequence identities of other ZmPLA1-like proteins were less than 70% (Table S1).OsDMP3(LOC_Os08g01530) and OsDMP6 (LOC_Os05g48840) were highly similar to ZmDMP, with a sequence identities of 46.78% and 44.83%, respectively (Table S2).Phylogenetic analysis shows that OsPLA1 assigned to a common subclade with ZmPLA1 (Fig.1A),whereas OsDMP3 and OsDMP6 were closely related to DMP orthologs with verified HI ability in Arabidopsis, tomato and maize(Fig.1B).Therefore, OsPLA1, OsDMP3 and OsDMP6 were selected as candidate genes.
3.2.Expression patterns of OsPLA1, OsDMP3 and OsDMP6
qRT-PCR experiments indicated that the expression levels of OsPLA1, OsDMP3 and OsDMP6 were high in mature anthers, but were very low in other tissues (Fig.1C).Subcellular localization experiments in tobacco epidemal system demonstrated that OsPLA1, OsDMP3 and OsDMP6 localized in the plasma membrane and merged well with the endoplasmic reticulum marker(Fig.1D).These results show that both expression patterns and subcellular localization of OsPLA1,OsDMP3 and OsDMP6 were similar to those of ZmPLA1/MATL/NLD and ZmDMP[12–15].Therefore,these findings imply that OsPLA1, OsDMP3 and OsDMP6 may have conserved functions similar to those in other species.
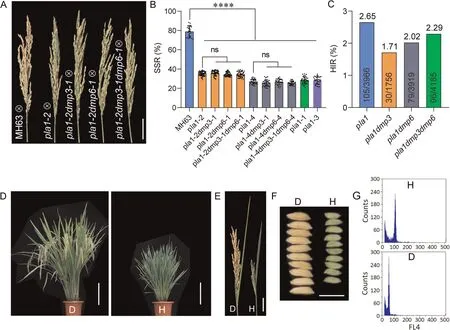
Fig.2.Mutation of OsPLA1 induced haploid progeny and synergistic effects of OsPLA1 and OsDMP mutations on HI in MH63 derivatives upon self-pollination.(A)Panicles of MH63 and various knockout combinations(pla1,pla1dmp3, pla1dmp6 and pla1dmp3dmp6).Scale bar, 5 cm.(B)Selfing SSR of MH63,and various mutant derivatives.Values are means±SD.****,P<0.0001;ns,not significant(two-tailed Student’s t-tests).(C)The selfing HIR in pla1,pla1dmp3,pla1dmp6,and pla1dmp3dmp6 mutant lines.(D)Gross morphologies of diploid(D)and a haploid(H).Scale bar,20 cm.(E)Panicles of diploid(D)and haploid(H)plants.Scale bar,5 cm.(F)Grain shape of diploid(D)and haploid(H)plants.Scale bar, 1 cm.(G) Ploidy level determination with flow cytometry.D, diploid; H, haploid.
3.3.HI effects of OsPLA1, OsDMP3 and OsDMP6 knockout mutants
Considering the dramatic difference between ssp.indica and japonica, knockout lines were generated in MH63 and NIP, which belong to indica and japonica cultivars,respectively.Single,double and triple mutants of OsPLA1,OsDMP3 and OsDMP6 harboring base insertions or deletions and without the transgenic elements of CRISPR/Cas9 were identified in T3generations of both cultivars(Fig.S1; Table S3, S4).No obvious morphological difference was observed between any one mutant and the corresponding wild type (Fig.S2).We examined the SSR and HIR in selfed progenies of the pla1, pla1dmp3, pla1dmp6 and pla1dmp3dmp6 genotypes.The average SSR of MH63 was 78.89%, average SSR for the four pla1 mutants ranged from 26.96% to 35.11% (Fig.2A, B).There was no significant difference in SSR between the pla1 mutants and the corresponding double or triple mutants, indicating that mutations in OsDMP3 or OsDMP6 did not reduce SSR.Further analysis showed that all the mutants had the ability to induce haploids upon self-pollination.In total, we identified 105/3966, 30/1756,79/3919 and 96/4185 haploids from selfed progenies of the pla1,pla1dmp3, pla1dmp6 and pla1dmp3dmp6 mutants, respectively(Fig.2C; Table S2).The haploid plants had typical haploid characteristics, including reduced size and male sterility (Fig.2D–F).The ploidy level of putative haploids was also confirmed by flow cytometry (Fig.2G).The pla1,pla1dmp3 and pla1dmp3dmp6 mutations in NIP showed no significant differences in overall morphology, but had significantly decreased SSR when compared to NIP(Fig.S3A–C).The SSR of selfed progenies of the NIP pla1,pla1dmp3 and pla1dmp3dmp6 mutants were 7.11%, 6.71% and 6.85%, respectively, much lower than that of the corresponding mutants in MH63 background.The HIR for pla1,pla1dmp3 and pla1dmp3dmp6 mutants were 1.26%, 1.83% and 1.21%, respectively.Given no significant differences among these frequencies (Fig.S3D; Table S3),it was evident that the osdmp3 or osdmp6 mutations or their combination did not contribute to HIR in the presence of ospla1 in either rice subspecies.
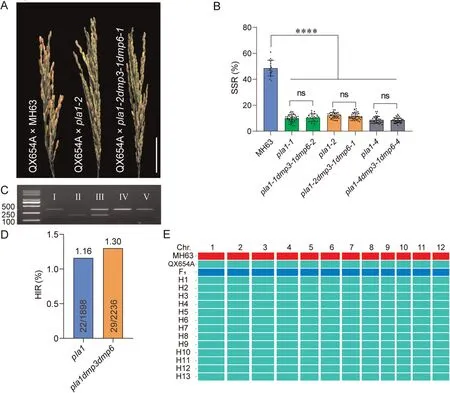
Fig.3.Effect of ospla1 in HI and synergistic effects of ospla1 and osdmps on HI in MH63 background upon crossing.(A)Panicles of QX654A cross-pollinated with MH63(left),pla1(middle)and pla1dmp3dmp6(right)mutants.Scale bar,5 cm.(B)SSR of QX654A panicles pollinated by MH63,pla1 and pla1dmp3dmp6 mutants.Values are means±SD.****, P < 0.0001; ns, not significant (two-tailed Student’s t-test).(C) Genotyping of putative haploid seedlings with molecular marker L3-7.Left lane, DNA marker; I-V, PCR bands of QX654A,MH63,F1 hybrid from QX654A×MH63,and two QX654A haploids,respectively.(D)HIR of pla1 and pla1dmp3dmp6 mutants.(E)SNP analysis of haploids and their corresponding parents.H1-13,haploids derived from QX654A×pla1-2 mutant.Vertical blocks represent 12 rice chromosomes.Genotypes of MH63,QX654A and F1 are marked in red, green, and blue respectively.
Cross-pollination experiments revealed that SSR of QX654A × MH63 was 48.60%, whereas average SSR for QX654A pollinated by pla1-1, pla1-2 and pla1-4 mutant plants were 10.02%, 12.21% and 8.69%, respectively, and average SSR of QX654A pollinated by their corresponding, pla1-1dmp3-1dmp6-2,pla1-2dmp3-1dmp6-1 and pla1-4dmp3-1dmp6-4 triple mutants were 10.41%,11.50%and 8.43%,respectively.QX654A panicles pollinated by pla1 and the corresponding triple mutants had significantly more aborted seeds than that pollinated by MH63(Fig.3A).However, differences in SSR between single mutant and corresponding triple mutation lines were not significant (Fig.3B).We next determined the frequencies of haploids in crosspollinated progenies with a molecular marker L3-7(Fig.3C).A total of 22/1898 and 29/2236 haploids were identified from progenies pollinated by pla1 and pla1dmp3dmp6, respectively, whereas no haploid individual was detected in 864 individuals obtaine by pollination with MH63(Fig.3D).The HIR in crosses involving pla1 and pla1dmp3dmp6 mutants were 1.16% and 1.30%, respectively(Fig.3D; Table S4).Upon crossing of pla1 and pla1dmp3dmp6 with QX059A, the SSR of QX059A × MH63 was 22.64%, whereas the average SSR of QX059A pollinated by pla1-2 and pla1-2dmp3-1dmp6-1 were 5.91% and 6.09%, respectively (Fig.S4A, B).The HIR in pla1 and pla1dmp3dmp6 mutant crosses were 1.26% and 1.10%,respectively(Fig.S4C,D).Genotyping result of 13 randomly selected haploids confirmed that ospla1 mutant induces only maternal haploids (Fig.3E).The overall HIR in crosses involving the pla1 and pla1dmp3dmp6 mutants were similar.Together,these results showed that loss-of-function of OsPLA1 in both indica and japonica could induce haploid, but osdmp3 or osdmp6 muations failed to increase the HIR in the presence of ospla1.
3.4.Independent HI ability analysis of OsDMP3 and OsDMP6
Previous studies showed that dmp mutations induced haploids in maize [15], Arabidopsis [22] and tomato [25].We therefore investigated whether osdmp3, osdmp6 or osdmp3osdmp6 mutants of rice could induce haploids upon self-pollination or crossing to QX654A as male parents.Upon selfing or serving as pollen donors in crosses with QX654A,there was no significant difference in SSR between MH63 and the mutants (Fig.4A–D), implying that mutations in OsDMP3 or OsDMP6 did not affect SSR.No haploid individual was detected among 7728 selfing progeny and 2274 crossing progeny (Tables S3, S5).These results implied that knockout of OsDMP3 or OsDMP6 did not lead to HI.
3.5.Effect of pollination methods on HIR
In order achieve efficient HI, we trialed a repeated pollination strategy and analyzed SSR and HIR.Compared to SP (mean SSR 2.15%), DP and TP trails significantly improved SSR to 4.85% and 6.96%, respectively (Fig.5A, B).However, there was no increase in the haploid production with these procedures.The HIR of SP,DP and TP were 4.97%, 2.92% and 1.88%, respectively (Fig.5C;Table S5).Thus, repeated pollination increased seed set, but did not contribute to increased haploid production.
3.6.Transcriptome analysis of the ospla1 mutant
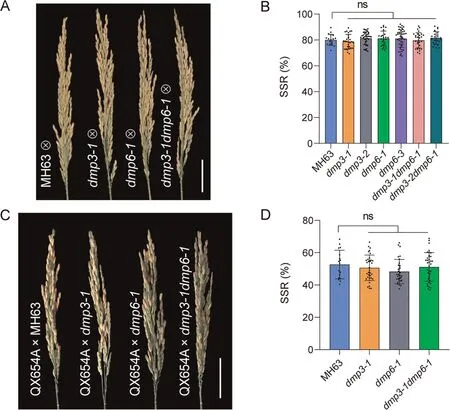
Fig.4.Effects of dmp3, dmp6 and dmp3dmp6 mutations on SSR in MH63.(A) Representative panicles from selfed MH63 and knockout mutants.Scale bar, 5 cm.(B) SSR of selfed MH63 and knockout mutants.Values are the means ± SD.ns, not significant.(C) Panicle morphologies of QX654A after cross-pollination with MH63 and knockout mutants.Scale bar, 5 cm.(D) SSR of QX654A after cross-pollination with MH63 and knockout mutants.Values are means ± SD.ns, not significant.
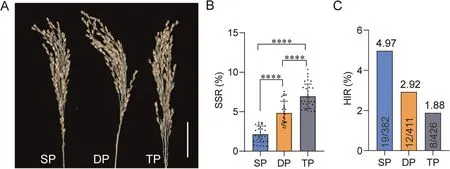
Fig.5.Effect of repeated pollination on SSR and HI by crossing pla1-2 to QX654A.(A) Panicles of QX654A pollination with the pla1-2 mutant after single pollination (SP),double pollination (DP) and triple pollination (TP), Scale bar, 5 cm.(B) SSR of QX654A panicles after SP, DP and TP with the pla1-2 mutant.Values are means ± SD, ****,P < 0.0001 (two-tailed Student’s t-tests).(C) HIR of QX654A panicles after SP, DP and TP with the pla1-2 mutant.
RNA-seq analysis was undertaken to explore the molecular mechanism of OsPLA1 in regulating HI.In comparison to MH63, a total of 2510 DEGs were identified in the pla1-2 mutant, among them 939 were up-regulated and 1571 were down-regulated(Fig.6A).Gene Ontology(GO)term enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed that these DEGs were enriched in multiple processes.According to the GO analysis, many down-regulated DEGs were involved in‘oxidation reduction’and‘lipid metabolic process’under the biological process category; both up-regulated and down-regulated DEGs were enriched in the ‘membrane’ under the cellular component category;and in the‘oxidoreductase activity’under the molecular function category(Fig.6B).KEGG pathway enrichment analysis demonstrated that ‘plant hormone signal transduction’ and ‘mitogen-activated protein kinase (MAPK) signaling pathway’ were significantly enriched, and the majority of associated DEGs were down-regulated (Fig.6C).In the hormone signal transduction pathway, DEGs were involved multiple plant hormone pathways, such as abscisic acid (ABA), indole-3-acetic acid(IAA),jasmonic acid(JA),ethylene(ETH),and brassinosteroids(BR) (Fig.6D).In the JA signal transduction pathway, seven OsJAZs were significantly downregulated in the pla1-2 mutant (Fig.6D).

Fig.6.Transcriptome analysis of an ospla1 mutant mutant.(A) Volcano plot displaying significantly upregulated and downregulated genes in ospla1 mutant compared to MH63.The Y axis is the-log10 of the q value for significance of differential expression.(B)Gene Ontology(GO)enrichment analysis of up-and down-regulated genes in MH63 and the ospla1.The size of dots indicates DEGs numbers,the color of dots indicates enrichment significance.(C)Kyoto Encyclopedia of Genes and Genomes(KEGG)pathway enrichment analysis of up-and down-regulated genes of MH63 and ospla1.(D) DEGs associated with ABA, IAA, JA, ETH, BR, CK and SA signal transduction.
4.Discussion
DH lines are valuable materials for breeding and genetic studies[36].Establishing an efficient, background-independent rice HI system is crucial for breeding.Based on sequence information of ZmPLA1/MATL/NLD,we identified ZmPLA1 orthologs in rice,OsPLA1,and demonstrated that ospla1 muants can produce maternal haploid progeny in both indica and japonica backgrounds.Our findings support previous reports,and demonstrate the feasibility of establishing an intraspecific HI system in rice based on OsPLA1 mutants in both subspecies [19,37].Moreover, by comparing HIR and SSR between MH63 and NIP, we found that OsPLA1 mutants produced significantly higher SSR in MH63 than that in NIP,whereas the HIR were similar.This phenomenon is probably not unique to MH63 and NIP, but more likely a universal difference between japonica and indica.The underlying mechanism needs to be further studied.
Improving the HIR is important for DH breeding.In maize,ZmDMP mutants induced haploids at a frequency of 0.1%–0.3%and that level was increased 5- to 6-fold in the presence of zmpla1/matl/nld mutations [15].Orthologs of the ZmDMP are functionally conserved in dicots, and no functional orthologs have been identified in monocots [15,22–26,38].In the current study,the two ZmDMP orthologs in rice were knocked out in both indica and japonica rice.Nevertheless, we failed to detect a synergistic effect on HIR in osdmp3 or osdmp6 or their combination in the presence presence of ospla1, these results were different from zmdmp in improve HIR in maize.
In addition to ZmPLA1/MATL/NLD and ZmDMP, a recent study showed that mutations of ZmPLD3 exhibited synergistic effects with zmpla1/matl/nld rather than functional redundancy, resulting in a significant increase in HIR from 1.19%to 4.13%[16].ZmPOD65 mutants also induce haploid progeny in maize [39].The discovery of ZmPLD3 and ZmPOD65 provided potential targets to further improve HIR not only in maize,but also in other crop species such as rice.In addition, optimization of cross-pollination strategy is also an important way to produce more haploids.
Two main hypotheses have been proposed to explain the mechanism of HI in maize, i.e., chromosome elimination and single fertilization [2].There is compelling evidence supporting chromosome elimination, particularly the detection of chromosome segments in haploids [40–44].It was observed that the maternal and paternal gametes fail to fuse, but the central cell and sperm cell fusion remain normal when using pollen from haploid inducers that support single fertilization [45,46].Recent discoveries of ROS burst and DNA fragmentation in pollen of haploid inducers reemphasized chromosome elimination [21,39].In this study,‘oxidation reduction’and‘lipid metabolic process’were two highly enriched GO terms, which is in accordance with previous studies.Moreover, we also detected plant hormone signal transduction and mitogen-activated protein kinase signaling pathway activity.These pathways were known to affect male gametophyte development and double fertilization.These findings provide potential evidence to reveal the mechanism of HI.
CRediT authorship contribution statement
Zongkai Liu:Investigation, Data curation, Writing – review &editing.Yu Zhong:Investigation,Writing–review&editing.Xiaolong Qi:Investigation,Writing–review&editing.Tai An:Investigation.Shuwei Guo:Investigation.Dong Wang:Investigation.Yuwen Wang:Investigation.Bin Feng:Investigation.Zuofeng Zhu:Writing – review & editing.Shaojiang Chen:Conceptualization,Funding acquisition,Project administration,Writing–review& editing.Chenxu Liu:Conceptualization, Funding acquisition,Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Prof.Pengcheng Wei (Anhui Agricultural University),Mr.Xianyou Sun (China Agricultural University), and Prof.Yuping Hu (Jiangxi Academy of Agricultural Sciences), for assistance in field experiments,and Mr.Yulong Feng(Neijiang Academy of Agricultural Sciences) for providing seeds of the CMS lines.This work was supported by the National Key Research and Development Program of China (2022YFD1200800), the China Agriculture Research System (CARS-02-05), Beijing Nova Program (2023067),and Yunnan Province Science and Technology Department(202305AF150026).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.11.005.
- The Crop Journal的其它文章
- Flowering-time regulation by the circadian clock: From Arabidopsis to crops
- Global characterization of OsPIP aquaporins reveals that the H2O2 transporter OsPIP2;6 increases resistance to rice blast
- Drought-triggered repression of miR166 promotes drought tolerance in soybean
- The OsBSK1-2-MAPK module regulates blast resistance in rice
- Natural variation of an autophagy-family gene among rice subspecies affects grain size and weight
- Rice gene OsUGT75A regulates seedling emergence under deep-sowing conditions

