Camellia sinensis CsMYB4a participates in regulation of stamen growth by interaction with auxin signaling transduction repressor CsAUX/IAA4
Guoling M, Mingzhuo Li, Yingling Wu, Chngjun Jing, Yifn Chen, Dwei Xing, Yue Zho,Yjun Liu, Xioln Jing, To Xi,*, Liping Go,*
a School of Life Science, Anhui Agricultural University, Hefei 230036, Anhui, China
b Plant for Human Health Institute, Department of Plant and Microbial Biology, North Carolina State University, Kannapolis, NC 28081, USA
c State Key Laboratory of Tea Plant Biology and Utilization/Key Laboratory of Tea Biology and Tea Processing of Ministry of Agriculture/Anhui Provincial Laboratory of Tea Plant Biology and Utilization, Anhui Agricultural University, Hefei 230036, Anhui, China
Keywords:AUX/IAA4 Auxin signaling CsMYB4a Subgroup 4 R2R3-MYB
ABSTRACT Subgroup 4 (Sg4) members of the R2R3-MYB are generally known as negative regulators of the phenylpropanoid pathway in plants.Our previous research showed that a R2R3-MYB Sg4 member from Camellia sinensis(CsMYB4a)inhibits expression of some genes in the phenylpropanoid pathway,but its physiological function in the tea plant remained unknown.Here, CsMYB4a was found to be highly expressed in anther and filaments,and participated in regulating filament growth.Transcriptome analysis and exogenous auxin treatment showed that the target of CsMYB4a might be the auxin signal pathway.Auxin/indole-3-acetic acid 4 (AUX/IAA4), a repressor in auxin signal transduction, was detected from a yeast two-hybrid screen using CsMYB4a as bait.Gene silencing assays showed that both CsIAA4 and CsMYB4a regulate filament growth.Tobacco plants overexpressing CsIAA4 were insensitive to exogenous α-NAA, consistent with overexpression of CsMYB4a.Protein-protein interaction experiments revealed that CsMYB4a interacts with N-terminal of CsIAA4 to prevent CsIAA4 degradation.Knock out of the endogenous NtIAA4 gene, a CsIAA4 homolog, in tobacco alleviated filament growth inhibition and α-NAA insensitivity in plants overexpressing CsMYB4a.All results strongly suggest that CsMYB4a works synergistically with CsIAA4 and participates in regulation of the auxin pathway in stamen.
1.Introduction
MYB transcription factors form one of the largest families of transcription factors in plants.A total of 221 MYB members were identified in tea, Camellia sinensis [1].The Sg4 members of the R2R3-MYB transcription factor (TF) family typically function as negative modulators of the phenylpropanoid pathway required for lignin biosynthesis by repressing the expression of specific genes in the pathway [2,3].Overexpression of Sg4 genes always results in growth-inhibited phenotypes,such as smaller plant size,reduced leaf size, and stunted root growth [4–6], that had long been thought to result from downregulation of lignin synthesis.The growth-inhibited phenotype due to reduced lignin biosynthesis is also termed lignin modification-induced dwarfism(LMID)[7].In a previous study, using the same technical route, a R2R3-MYB Sg4 member from C.sinensis (CsMYB4a) was found to repress the expression of lignin-related genes in transgenic plants [5].
The physiological functions of R2R3-MYB Sg4 members in plants are diverse.For example, down-regulation of AtMYB4 expression in Arabidopsis thaliana is a strategy for plants to resist UV damage [4,8].AtSAD2 is an importin beta-like protein, which in responding to UV-B irradiation,transports MYB4 from the cytosol to the nucleus[9].In addition to effects on the lignin pathway,many studies have found that Sg4 member are involved in regulation of hormone signaling pathways.AtMYB7 and AtMYB3, also Sg4 members, negatively regulate ABA-induced inhibition of seed germination by suppressing expression of the gene encoding the bZIP TF, ABI5 [10].
The filament is an important part of the stamen, providing physical support for the anther and participating in the production of the pollen[11].Throughout the course of floral evolution,a myriad of morphological features has emerged.In self-incompatible species such as tea, an adaptive strategy to ensure outcrossing is approach herkogamy (spatial separation between the pistils and stamens)[12].Flowers with approach herkogamy have pistils that extend beyond the stamens [13,14].Approach herkogamy is regulated by several quantitative trait loci, DELLA and JAZ repress the transcription activity of AtMYB21 and AtMYB24 to repress filament elongation in A.thaliana[15].Filament elongation also affected by stress; AtHKT1 (a sodium ion transporter) prevents reduced filament elongation caused by salt stress to avoid male sterility.Lignin and auxin regulate flower development.In A.thaliana,auxin negatively regulates lignification and jasmonic acid synthesis in endothelial cells regulated by MYB26,thereby controlling the timing of anther dehiscence [16].Endothelial cell lignification and anther dehiscence in afb1-3 and tir1 afb2 afb3 mutants occurred earlier than in wild-type plants.As a Sg4 member,AtMYB32 is necessary for the normal pollen development in A.thaliana [17].
The AUX/IAA family includes transcription factors that function as negative regulators of the auxin signal transduction pathway[18–20].In A.thaliana, AtIAA3 interacts with AtARF7 and AtARF19 to inhibit transcription of PIN-FORMED(PIN)genes,thereby affecting auxin distribution in the root system and inhibiting the formation of lateral root primordia [21–23].atiaa16 mutant plants are resistant to abscisic acid(ABA)during seed germination and insensitive to auxin [24].Rice plants overexpressing OsIAA4 display stunting and greater tillering angle[25],whereas the osiaa6 knockdown mutants have abnormal tiller outgrowths [26].Expression levels of OsPIN1b and OsPIN10a are reduced in osiaa11 mutants and lateral root development is inhibited [27].
In the current study,we first found that the CsMYB4a and CsIAA4 genes are co-expressed in flowers and roots in tea plants,and gene silencing assays showed that down-regulated expression of both genes promoted the elongation of flower filaments.In vivo and in vitro protein interaction assays were used to investigate whether CsMYB4a directly regulates the function of CsIAA4.Our results revealed a new mechanism whereby CsMYB4a regulates auxin signal transduction by direct binding to CsIAA4 to prevent proteolytic degradation of CsIAA4 by the CsTIR1-ubiquitin complex, thereby inhibiting filament growth.This suggests that, in addition to a repressive role in the phenylpropanoid and lignin pathways,R2R3-MYB Sg4 members are directly involved in the auxin signaling pathway thereby affecting plant growth and development.
2.Materials and methods
2.1.Materials and growth conditions
All tea (Camellia sinensis var.sinensis cv.‘‘Shuchazao”) and tobacco (Nicotiana tabacum cv.‘‘G28” and Nicotiana benthamiana)plants used in this study were grown in soil or on Murashige and Skoog (MS) culture medium plates at 25 °C.CsMYB4a and CsIAA4 were cloned into a pCB2004 vector, which was then transferred into tobacco plants using the Agrobacterium-mediated leaf disk transformation method.All primers used for vector construction are listed in Table S1.Seeds of transgenic plants were stored at 4 °C until further use.
2.2.α-NAA treatment and statistical assay
T2generation seeds of transgenic tobacco (CsMYB4a, CsIAA4,ntiaa4, and CsMYB4a-ntiaa4) and control were placed on MS culture medium (Sigma, USA) and held in a greenhouse (25 °C, 60%humidity,light/darkness 16 h/8 h)for germination for determination of germination rates.The number of seeds was not less than 100 and there were three replications.
Control and transgenic tobacco plants were treated with α-NAA after eight days of growth.Seedlings with similar size were transferred to MS culture medium containing different concentrations of α-NAA (mock; 0.01, 0.1, 1, and 5 μmol L-1), and morphological observations and measures of leaf area and root length after 20 d.were made on 20 plants of control, CsIAA4, CsMYB4a, ntiaa4 mutant,and CsMYB4a-ntiaa4 mutant seedlings.The data were analyzed using one-way ANOVA (OriginPro, Version 2022; OriginLab Corporation, Northampton, MA, USA).Tukey’s tests were used to determine the significance difference between transgenic plants and the control.
2.3.Total plant RNA extract and qRT-PCR assays
A sample of tea plants and an equal volume of polyvinylpyrrolidone(PVPP)were ground to powder in liquid nitrogen,and 100 mg of powder was transferred to a 2-mL RNase-free tube; 1 mL of Fruit-mate(Takara,catalog no.:9192)was added before incubation at 37°C for 5 min,and centrifugation at 12,000 r min-1for 5 min at 4°C.Subsequently,400 μL of supernatant was transferred to a new tube, and 400 μL of RNAiso plus (Takara, catalog no: 9108) was added for RNA isolation and centrifuged at 12,000 r min-1for 5 min at 4 °C, followed by addition of 160 μL of CHCl3and centrifuging at 12,000 r min-1for 15 min at 4 °C.Thereafter, 400 μL of the supernatant was transferred to a 1.5-mL tube, and 400 μL of isopropanol was added and incubated on ice for 5 min,followed by centrifuging at 12,000 r min-1for 5 min at 4 °C.The RNA was washed twice used with 1 mL of ethanol.A rotary steamer was used to remove ethanol,and 30 μL of RNase-free water was added to dissolve the total RNA.
The qRT-PCR primers used in the experiment are listed in Table S1.PCR programs were performed as described previously[28].There were three replications of materials, and each PCR was carried out with three technical replicates.Relative expression data was analyzed by using one-way ANOVA (OriginPro, Version 2022.OriginLab Corporation, Northampton, MA, USA), Tukey’s tests were carried out used to determine the significance of differences between transgenic plants and controls.
2.4.Gene silencing assays
Gene silencing was followed that reported by Zhao et al.[29].Candidate antisense oligonucleotides (AsODN) were designed using SOLIGO software [30] with CsMYB4a or CsIAA4 as the input sequence(Table S1).The flower stalk was soaked in 1 mL 30 μmol L-1CsMYB4a-AsODN or CsIAA4-AsODN solution to silence each respective gene in the flower.Flowers soaked with the sense oligonucleotides (sODN) solution were used as control.
2.5.CRISPR/Cas9
The vector used for CRISPR/Cas9 was pCBSG, with CRISPR-GE used to design the guide-RNA [31].Guide-RNAs were cloned into pCBSG by T4 DNA ligase.The recombination vectors were transferred into Agrobacterium (GV3101, cover pSoup-p19, Weidi Bio,Beijing,China),and transferred into tobacco plants through the leaf disc method.DNA for sequencing was extracted by DNA extract kit(Tsingke Biotechnology Co.,Ltd.,Beijing,China).Sanger sequencing results were analyzed by a TBtools (version 1.0986988) plugin named Sanger Sequencing Result Check [32].Superimposed sequencing chromatograms were decoded using DSDecode(http://skl.scau.edu.cn/dsdecode/) [33].
2.6.Purification of recombinant protein and in vitro pull-down assays
CsIAA4 was cloned into the pRSFDuet vector,and CsMYB4a was cloned into the pMAL-c2X vector (Fig.S7A).The recombined vectors were transferred into E.coli strain BL21 and used for protein expression.HIS-CsIAA4 and MBP-CsMYB4a were purified following the instructions from Novagen and NEB (Biolabs, Beijing, China).HIS-CsIAA4 and MBP-CsMYB4a contents were measured by a total protein quantitative assay kit (Jincheng Bioengineer, Nanjing,Jiangsu, China).Twenty μg of HIS and His-CsIAA4 proteins served as bait and were added to affinity chromatography columns containing 2 mL of His-Tag nickel filler.The columns were incubated at 4 °C for 3 h and washed three times with PBS buffer (0.2 mol L-1NaH2PO4, 0.2 mol L-1Na2HPO4, and 0.3 mol L-1NaCl,pH = 5.7).Twenty μg of MBP-CsMYB4 protein serving as prey was added to HIS and His-CsIAA4, respectively, and incubated overnight.The fillers were washed three times with PBS buffer;the protein was eluted with 10 mL PBS elution buffer (0.2 mol L-1NaH2PO4, 0.2 mol L-1Na2HPO4, 0.3 mol L-1NaCl, and 1 mmol L-1imidazole, pH = 5.7), and the solution was added to a 10-kD protein ultrafiltration concentrator and centrifuged at 5500 r min-1.The prey protein was analyzed by western blotting with anti-HIS and anti-MBP (TransGen Biotech, Beijing, China).
2.7.Subcellular localization assays
CsIAA4, CsMYB4a and CsMYB4aDC4were cloned in the pCambia 1305-GFP vector and transferred into Agrobacterium (GV3101,cover pSoup-p19,Weidi Bio)(Fig.S9c).These Agrobacterium were cultured at 28 °C until the OD600reached 0.6–0.8 and centrifuged at 5500 r min-1for 6 min in 50-mL tubes.The pellets were resuspended by twice vortexing using 10 mL of fresh infiltrate buffer(10 mmol L-1MgCl2,10 mmol L-1MES,and 0.1 mmol L-1acetosyringone, pH = 5.6).The OD600of the suspension was adjusted to 0.8–1.0, and the Agrobacterium was infiltrated into Nicotiana benthamiana leaves.GFP signals were detected by confocal microscopy(Leica, DM2000) after 3 d.
2.8.Yeast two-hybrid screening library
CsMYB4a was transformed into the pGBKT7 vector and transferred to AH109 chemically competent cells (Coolaber, Beijing,China) for yeast two-hybrid assays.Self-activation ability of the bait protein was detected in SD/-His-Leu-Trp-Ade (Coolaber) culture medium with 3AT (3-amino-1,2,4-triazole).The method of constructing pGADT7 yeast library vectors followed the ‘‘Mate &Plate” Library System (Clontech, Takara Bio, USA).The library vectors were transformed into yeast AH109 containing the CsMYB4a-BD vector using the Yeastmaker Yeast Transformation System 2 User Manual (Clontech).The yeasts were spread on SD/-His-Leu-Trp-Ade culture medium with 3AT and X-alpha-Gal to screen for positive candidate clones.Blue colonies were used for plasmid extraction and identification.Vectors of the positive candidate clones were extracted using a plasmid DNA mini-extraction kit(Tsingke Biotechnology Co., Ltd., Beijing, China).The vector was transferred into DH5α cells (Weidi Bio) for enrichment and extracted for sequencing.
2.9.Simulation and analysis of the protein heterodimer model
The setup and running of Alpha Fold 2 followed the method described on GitHub (https://github.com/deepmind/alphafold)[34,35].Beikunyun cloud server (Beikun Cloud Computing Co.,Ltd., Shenzhen, Guangdong, China) was accessed as the server.A fasta file containing the amino acid sequence of CsIAA4 or CsMYB4a as input, and the model ‘‘ranked_0” that showed a prediction with the highest confidence was analyzed by PyMOL(version 2.5),and the interaction site was analyzed using the InterfaceResidues script (https://pymolwiki.org/index.php/InterfaceResidues).
2.10.Split-LUC complementation assays
The vectors used for LUC assays were pC1300-cLuc and pC1300-nLuc, which contained fragments encoding the C- and N-terminal halves of Luc (cLuc and nLuc, respectively).CsIAA4, CsIAA4D2,CsIAA7, NtIAA4, CsMYB4a, and CsMYB4aDC4were cloned into the pC1300-cLuc and pC1300-nLuc vectors.Recombination vectors were then transferred into Agrobacterium (GV3101, cover pSoupp19, Widi Bio).The Agrobacterium infiltration method was the same as that used for the subcellular localization assays.pC1300 recombination vectors mixed with empty pC1300 vectors served as negative controls.IAA recombination vectors were mixed with CsMYB4a as the test group.The two negative controls and the experimental group were infiltrated into leaves of tobacco plants which were incubated in darkness for 48 h and then under white light for 16 h.The tobacco leaves were excised and sprayed with D-luciferin and potassium salt, and Luc fluorescence was detected using an in vivo imaging system.
2.11.BIFC assays
The vectors used for BIFC assays were pBI221-cYFP and pBI221-nYFP, which contain fragments encoding the C- and N-terminal halves of YFP (cYFP and nYFP, respectively).The recombination vectors were transferred into Agrobacterium (GV3101, cover pSoup-p19, Weidi Bio).nYFP-CsIAA4 was mixed with pBI221-cYFP as negative control and with CsMYB4a-cYFP as the test group.CsIAA4-cYFP was mixed with pBI221-nYFP as negative control and with nYFP-CsMYB4a as the test group.Protoplast extraction in A.thaliana and transfection followed the protocol described by Mituła et al.[36].YFP signals were detected through confocal microscopy(Leica, DM2000).
2.12.In vitro and in vivo protein degradation assays
The vector used for transient protein expression was p1300-MYC (Fig.S9A).CsIAA4-MYC was transiently expressed in tobacco plants and after three days the same weights of leaf tissue and of PVPP were ground in liquid nitrogen.Ten mL of phosphatebuffered saline (PBS) (0.2 mol L-1NaH2PO4, 0.2 mol L-1Na2HPO4,0.3 mol L-1NaCl,and 1 mmol L-1PMSF,pH=5.7)was added to the powder, with additional grinding for 8 min.The PBS solution was transferred to a 50 mL centrifuge tube and centrifuged at 6500 r min-1.The supernatant was transferred to a new centrifuge tube,85% (w/v) ammonium sulfate was added, then shaken on a decolorization shaker for 2 h, and centrifuged at 6500 r min-1for 10 min.The precipitate was dissolved in 1 mL of PBS and centrifuged at 6500 r min-1for 10 min.The crude protein containing CsIAA4-MYC was transferred to a new centrifuge tube and its content was measure using a total protein quantitative assay kit(Jincheng Bioengineer, Nanjing, Jiangsu, China).
For assaying degradation rate,0.1 μmol L-1of α-NAA treatment,20 μg of IAA4-MYC crude protein were added to the buffer(0.2 mol L-1NaH2PO4,0.2 mol L-1Na2HPO4,0.3 mol L-1NaCl,10 mmol L-1ATP,2 mmol L-1DTT,pH=5.7 and 0.1 μmol L-1α-NAA or 5 μmol L-1MG132)and incubated at 25°C for 4 h.NaOH or dimethyl sulfoxide served as the control.IAA4-MYC content was determined by western blotting.
2.13.In vitro degradation assay of IAA4-MYC
MBP,MBP-CsMYB4a,and MBP-CsMYB4aDC4were purified using E.coli BL21.Subsequently, 20 μg CsIAA4-MYC crude protein was mixed with 20 μg MBP-MYB4a and 20 μg MBP-CsMYB4aDC4in buffer (0.2 mol L-1NaH2PO4, 0.2 mol L-1Na2HPO4, 0.3 mol L-1NaCl,10 mmol L-1ATP, 2 mmol L-1DTT, and 0.1 μmol L-1α-NAA pH = 5.7) and incubated at 25 °C for 4 h; 20 μg MBP served as the control and IAA4-MYC content was determined by western blotting.
2.14.Protein degradation assay by LUC in tobacco
An additional 35S promoter was inserted before the LUC cassette of pGreenII 0800, and IAA4 was then cloned into the reconstructed vector pGreenII 0800 (Fig.S9D) which was transferred into Agrobacterium (GV3101, cover pSoup-p19, Weidi Bio).The method of transient expression and LUC signal detection in tobacco was the same as that of the split-LUC assay.GFP signals were detected by confocal microscopy(Leica,DM2000)after three days.Three 0.3 cm radius circular leaf segments in the injection area were removed from three leaves with a hole punch, then pooled,and LUC and REN activities were detected using dual luciferase kits(Yeasen Biotechnology, Shanghai, China).
3.Results
3.1.Determination of CsMYB4a and CsIAA4 gene function
Although CsMYB4a had been proven to inhibit lignin synthesis through heterologous expression in transgenic plants, its actual physiological function in tea plants was unclear.We observed a unique phenomenon of 35S promoter-driven CsMYB4a overexpression (CsMYB4a-OE) whereby growth inhibition induced by highconcentrations of exogenous auxin disappeared.Transgenic plants showed insensitivity to exogenous alpha-naphthalene acetic acid(α-NAA) at concentrations of 5 and 10 μmol L-1(Fig.S1).Based on the results of RNA-sequencing (RNA-seq) and quantitative reverse transcription-polymerase chain reaction (qRT-PCR), the expression genes in the auxin signal pathway in transgenic tobacco,such as NtSAUR (SMALL AUXIN UP RNA)family members,were affected, and degree of down-regulated expression was greater than that caused by phenylpropanol pathway genes in 14-day-old transgenic lines (Fig.S2A–C).These results indicated that CsMYB4a played an indeterminate role in the auxin signaling pathway in addition to regulating the phenylpropanoid pathway,thereby affecting seed germination and plant growth.
A yeast two-hybrid library was screened for CsMYB4a’s interacting proteins by using CsMYB4a as bait(Table S2).Using CsMYB4 as the bait protein, AUX/IAA protein was identified from the tea cDNA library.An unrooted PHYLIP phylogenetic tree showed that the AUX/IAA family in tea contained 24 members, and the identified AUX/IAA protein clustered in the same branch as the AtIAA1–4 proteins(Fig.S3).Based on high amino acid identity with AtIAA4, we named it CsIAA4 (NCBI ID: XP_028087156.1).
To explore its function, CsIAA4 was cloned into the pCB2004 vector(containing the 35S promoter)and transformed into tobacco plants by Agrobacterium (GV3101).CsIAA4 expression leveldependent growth inhibition was observed in CsIAA4 transgenic plants, including high-level expression transgenic lines 6 and 8,and low-level expression transgenic line 4 (Fig.S4A, B).Overexpression of CsIAA4 significantly inhibited germination of transgenic seeds,with decreases of 16.3%,36.8%,and 39.8%in transgenic lines 4, 6, and 8, respectively, compared with the control plants(Fig.S4C).However, the lignin content in 60-day-old transgenic lines was not significantly different from that in control plants(Fig.S4D), demonstrating that CsIAA4 has no functional effect on lignin biosynthesis in transgenic plants.qRT-PCR showed that expression levels of NtSAUR were significantly decreased in CsIAA4 transgenic lines 6 and 8 compared to line 4 and control plants(Fig.S4E).These experiments showed that CsIAA4 is a negative regulator of plant growth.
3.2.Co-expression characteristics of the CsMYB4a and CsIAA4 genes and their effects on filament elongation
qRT-PCR results revealed that CsIAA4 and CsMYB4a showed similar transcriptional features in different tea plant tissues, and both were highly expressed in fully developed flowers and primary roots (Fig.1A,B).The co-expression characteristics CsMYB4a and CsIAA4 in flower parts were also queried from a transcriptome database of flower organs detected by our research group.CsMYB4a and CsIAA4 were both highly expressed in filaments and petals,less so in calyx (Fig.S5).A subcellular localization assay in tobacco showed that both CsIAA4 and CsMYB4a were localized in the nuclei of epidermal cells (Fig.S6).These results showed that the two genes have co-expression characteristics in flowers.
We silenced CsMYB4a in S4 flowers by treatment with candidate antisense oligonucleotide (AsODN), with sense oligonucleotide(sODN) as control (Fig.1C–F).CsMYB4a-silenced flowers showed an increase in filament length and abnormal anther development(Fig.1C,D).qRT-PCR assays verified that expression of CsMYB4a in flowers treated with AsODN for four days was reduced by 60%,compared with flowers treated with sODN(Fig.1E).However,filament length was significantly increased in CsMYB4a-silenced flowers (Fig.1F).
We also silenced CsIAA4 expression in S4 flowers (Fig.1G–J).Like CsMYB4a-silenced flowers, the CsIAA4-silenced flowers also had elongated filaments but the anthers were normal (Fig.1G,H).qRT-PCR assays showed that expression of CsIAA4 was reduced by 59%, and CsIAA4-silenced flowers showed increased growth(Fig.1I), but the filament length was significantly relative to the control (Fig.1J).
The above results indicated that both CsMYB4a and CsIAA4 have the function in controlling filament length.
3.3.Tobacco plants overexpressing CsMYB4a and CsIAA4 exhibit abnormal responses to α-NAA
A low concentration of applied auxin promoted plant root elongation whereas a high concentration auxin inhibited root elongation and growth, a typical physiological response to auxin.To confirm whether transgenic tobacco had abnormal hormone response characteristics,8-day-old tobacco seedlings overexpressing CsMYB4a-and CsIAA4 and controls were cultured in MS medium with different concentrations of α-NAA (Fig.2).
Transgenic seedlings overexpressing both genes individually showed similar morphological characteristics (Fig.2A,D).In addition to CsIAA4 transgenic line4 with low level expression,seedlings overexpressing CsMYB4a and CsIAA4 had abnormal responses,confirmed by measures of root length and leaf area (Fig.2B,C,E,F).At low concentrations of α-NAA (0.01 μmol L-1), the root lengths of almost all plants represented by two transgenics and the control plants were significantly longer than those of plants without α-NAA treatment,demonstrating the promoting effect of low concentrations of α-NAA on plant root growth.However,the inhibition of root elongation by high concentrations of α-NAA (1 μmol L-1)occurred only in control plants and CsIAA4 transgenic line4(Fig.2B,E).The leaf areas of transgenic plants under high concentration α-NAA treatment were significantly higher than those treated with low concentrations of α-NAA and the control (Fig.2C,F).Taken together, seedlings overexpressing CsMYB4a and CsIAA4 transgenic exhibited altered responses to exogenous α-NAA treatment.
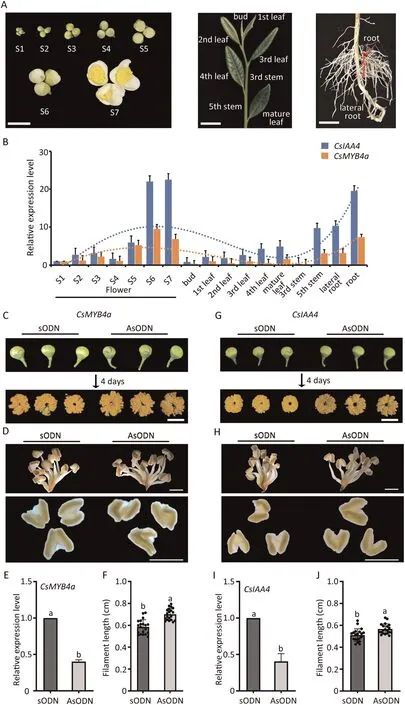
Fig.1.CsMYB4a and CsIAA4 regulate the growth of stamens in tea plants.(A)Tissues of tea plants used for qRT-PCR assays.Flowers at seven developmental stages(S1–7),arial parts including buds,leaves,stem internodal regions and primary and lateral roots.Scale bars,2 cm.(B)qRT-PCR assay showing expression levels of CsIAA4 and CsMYB4a in tissues and growth stages.Blue, relative expression of CsIAA4; orange, relative expression of CsMYB4a.Dotted lines represent the expression trends of CsIAA4 and CsMYB4a,across the tissues.(C)Phenotypes of S4 flowers following CsMYB4a-AsODN treatment for 4 d;sODN served as the control.Scale bar,1 cm.(D)Stamens after CsMYB4a-AsODN treatment for 4 d, with sODN as the control.Scale bar, 2 mm.(E) Relative expression of CsMYB4a in CsMYB4a-silenced flowers; sODN served as the control.(F) Statistics of filament length in CsMYB4a-silenced flowers; sODN served as control.Data are means ± SD (n = 20).Different letters indicate a significant difference at P < 0.05 (one-way ANOVA, Tukey’s test).(G) Phenotype of S4 flowers after CsIAA4-AsODN treatment for 4 d, with sODN serving as control.Scale bar, 1 cm.(H)Stamens after CsMYB4a-AsODN treatment for 4 d with sODN as the control.Scale bar,2 mm.(I)Relative expression of CsIAA4 in CsIAA4-silenced flowers with sODN as control.(J)Statistics of filament length of CsIAA4-silenced flowers with sODN as control.Data are means ± SD (n = 20).Different letters indicate a significant difference at P < 0.05 (one-way ANOVA, Tukey’s test).
3.4.Determination of the domain of interaction between CsIAA4 and CsMYB4a
Further tests were used to verify interaction between CsMYB4a and CsIAA4 in tea plants.Pull-down assays using maltose binding protein (MBP)-tagged CsMYB4a as prey protein and His-tagged CsIAA4 as bait protein (Fig.S7A,B) confirmed that CsIAA4 pulled down CsMYB4a in vitro(Fig.3A).Bimolecular fluorescence complementation (BIFC) assays showed that YFP signals were reconstituted in the nucleus when CsIAA4 and CsMYB4a were coexpressed (Fig.3B).A split-LUC assay showed that co-expression of CsIAA4 and CsMYB4a proteins produced LUC activity (Fig.3C).These assays showed that CsMYB4a interacts with CsIAA4 in vivo and in vitro.
Our aim was to determine the domains of CsIAA4 and CsMYB4a mediating their interaction.We modelled the interaction using Alphafold2 (Fig.3D).The predicted potential interaction sites spanned the R2, R3, C2, ZF, C4 domains of CsMYB4a, and domains I,II,and III of CsIAA4(Fig.3E).To identify the domains that mediate the interaction, we generated deletion constructs by removing individual domains and assessing their effect on protein interaction (Fig.3F).Removal of the C4 domain of CsMYB4a (named CsMYB4aDC4) abolished its ability to interact with CsIAA4 in the split-LUC assay (Fig.3G).Domain II of IAAs is an important site for ubiquitination of AtIAA6 and AtIAA19[37].We designed a truncated CsIAA4, CsIAA4D2(with domain II deleted), and a Val-74-Ser(within domain II) mutant CsIAA4V74S(Fig.3F).Neither CsIAA4D2nor CsIAA4V74Sinteracted with CsMYB4a in the split-LUC assay(Fig.3H,I).These results suggest that domains II of CsIAA4 and C4 domain of CsMYB4a were key motifs for the interaction.
To determine whether other CsAUX/IAA paralogous proteins with domain II interact with CsMYB4a we performed a similar experiment using CsIAA7(Gene ID:TEA014506.1)as the CsMYB4a prey protein in the split-LUC assay.Amino acid alignment showed that the amino acid sequence between CsIAA4 and CsIAA7 was 30.15%, but their domain II differed by a single one amino acid(Fig.S8A).LUC activity was not reconstituted when CsIAA7 was the prey protein of CsMYB4a, suggesting that CsIAA7 does not physically interact with CsMYB4a (Fig.S8B).
3.5.CsMYB4a prevents degradation of CsIAA4
Transport inhibitor response 1(AtTIR1)in A.thaliana interacted with domain II of AtIAA6 and AtIAA19 to promote ubiquitinationmediated degradation responses [37].To test our speculation that interaction between CsMYB4a and CsIAA4 blocked ubiquitinmediated CsIAA4 degradation, we expressed CsIAA4 fused with MYC-tag in tobacco, extracted the crude protein from transgenic plants, and simultaneously expressed and purified the recombinant MBP-CsMYB4a or MBP-CsMYB4aDC4protein from E.coli strain BL21.The expression vector structures of genes fused with the antigenic epitope tags are shown in Fig.S9A,B.The extract containing CsIAA4-MYC protein was incubated alone or together with recombinant MBP-CsMYB4a or MBP-CsMYB4aDC4protein in vitro for protein degradation experiments (Fig.4A–C).Western blotting showed that in the presence of α-NAA, most of the CsIAA4-MYC protein was degraded within 4 h,whereas the degradation was significantly slower in the absence of α-NAA(Fig.4A)and completely inhibited by MG132 (a potent proteasome inhibitor) (Fig.4B).These results indicated that the α-NAA-induced CsIAA4-MYC protein degradation reaction that occurred in the in vitro experiments was caused by the residual protease in the crude enzyme extract.However, α-NAA-induced CsIAA4-MYC degradation was blocked when CsIAA4-MYC was co-incubated with MBP-MYB4a, but not with MBP or MBP-CsMYB4aDC4(Fig.4C).The results showed that CsMYB4a inhibits the degradation of CsIAA4 in vitro.
Protein degradation assay were carried out to examine whether CsMYB4a protects CsIAA4 from degradation in vivo, First, we expressed CsIAA4-LUC together with GFP (control), CsMYB4a-GFP, and CsMYB4aDC4-GFP in transient expression assays in tobacco.The expression vector structures of CsIAA4-LUC,CsMYB4a-GFP, and CsMYB4aDC4-GFP are shown in Fig.S9C,D.The efficiency of Agrobacterium infiltration was normalized using Renilla luciferase activity and expression of the GFP signal(Fig.S9E,F).
The infiltrated tobacco plants were then treated with exogenous α-NAA (0.1 μmol L-1) and MG132 (5 μmol L-1) for 4 h and their LUC activity was quantified (Fig.4D).Fluorescence signal detection showed no obvious CsIAA4-LUC fluorescence signal coexpressed with CsIAA4-LUC and GFP or CsIAA4-LUC and CsMYB4aDC4-GFP, but signals were present in tobacco coexpressing CsIAA4-LUC and CsMYB4a-GFP under the 0.1 μmol L-1α-NAA treatment (Fig.4D) indicating that CsMYB4 protects CsIAA4-LUC from degradation.The addition of MG132 prevented the α-NAA-induced degradation of CsIAA4-LUC, indicating that α-NAA-induced CsIAA4-LUC degradation probably occurs through the ubiquitin proteolytic pathway.
Furthermore,Luc/REN activity in the above plants(Fig.4E)was significantly reduced in plants co-expressing CsIAA4-LUC and GFP,or CsIAA4-LUC and CsMYB4aDC4-GFP, upon α-NAA treatment.However, the Luc/REN activity of plants co-expressing CsIAA4-LUC and CsMYB4a-GFP was not significantly different from that of the control plants.MG132 treatment restored the Luc/REN activity.
We expressed CsIAA4-MYC with GFP (control), CsMYB4a-GFP,or CsMYB4aDC4-GFP in a transient expression system in tobacco.The infiltrated tobacco plants were treated with exogenous α-NAA(0.1 μmol L-1)for 4 h,and western blotting showed that only CsMYB4 protected CsIAA4-MYC from degradation under α-NAA treatment(Fig.4F).In summary,our results showed that CsMYB4a interacts with CsIAA4 to prevent its proteolytic degradation.
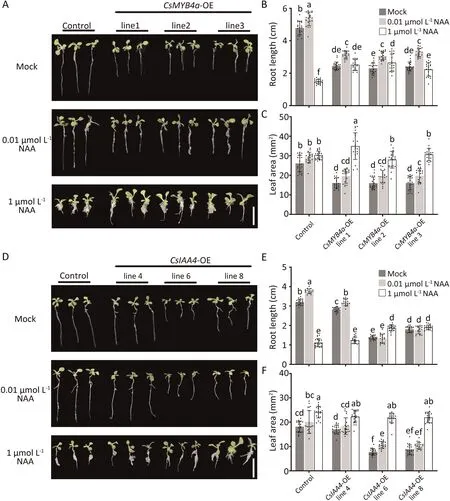
Fig.2.Responses of transgenic tobacco possessing CsMYB4a or CsIAA4 to exogenous α-NAA.(A)Phenotypes of control and CsMYB4a-OE lines after α-NAA treatments for 20 d.Scale bar,2 cm.(B)and(C)Statistical data for root elongation and leaf area of CsMYB4a-OE seedlings in response to α-NAA treatments.Data are means±standard deviation(SD) (n = 20).Different letters denote significant differences at P < 0.05 (one-way ANOVA, Tukey’s tests).(D) Phenotypes of control and CsIAA4-OE seedlings after α-NAA treatments for 20 d.Scale bar,2 cm.(E)and(F)Statistical data for root elongation and leaf area of CsIAA4-OE seedlings in response to α-NAA treatments.Data are expressed as means ± SD (n = 20).Different letters denote significant differences at P < 0.05 (one-way ANOVA, Tukey’s tests).
3.6.CsMYB4a interacts with NtIAA4 to suppress growth of transgenic tobacco plants
To investigate whether CsMYB4a altered auxin-dependent plant development by interfering with CsIAA4 function of orthologous genes in tobacco transgenic plants, we first tested whether CsMYB4a interacted with NtIAA4 (Gene ID:107830067), an ortholog of CsIAA4 with an amino acid sequence identity of 69.54%(Fig.S10A,B).Split-luciferase complementation (Split-LUC) imaging revealed that CsMYB4a and NtIAA4 physically interacted with each other (Fig.S10C).
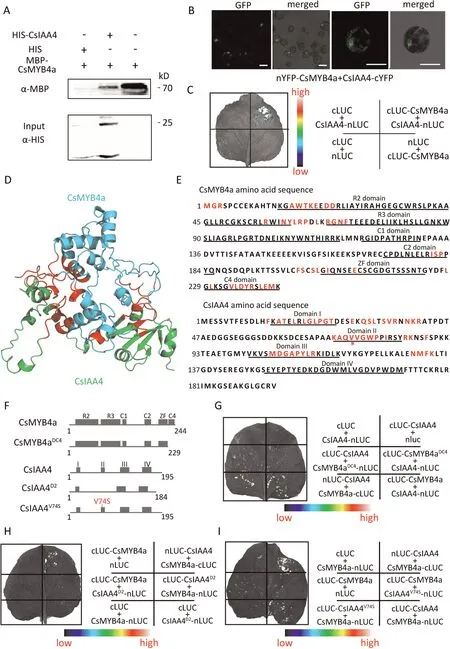
Fig.3.Confirmation the interaction sites between CsMYB4 and CsIAA4.(A)Pull-down assay showing interaction of CsIAA4 and CsMYB4a in vitro.HIS served as the control.Protein molecular weights of CsMYB4a-MBP and CslAA4 were 70 and 25 kDa,respectively.(B)Bimolecular fluorescence complementation(BIFC)assay showing interaction of CsIAA4 and CsMYB4a in Arabidopsis protoplast.Empty vectors cYFP and nYFP served as internal controls for each combination.Scale bars,50 μm.(C)Split-LUC assay showing interaction of CslAA4 and CsMYB4a in a tobacco leaf.Seale bar denotes wavelengths for the in vivo imaging system.(D) Protein model showing interaction of CslAA4 and CsMYB4a.Blue chain, CsMYB4a; green chain, CsIAA4; red regions, interacting regions of the proteins.(E) Amino acid sequences of CsMYB4a and CsIAA4; underscoring indicates domains of CsMYB4a and CsIAA4;‘‘*”under valine 74 in CsIAA4 denotes the residue that interacts with CsMYB4a.(F)Schematics of truncated amino acid sequences of CsMYB4a and CslAA4.CsMYB4aDC4;mutant protein of CsMYB4a with knocked out C4 domain;CsIAA4D2 mutant protein of CslAA4 with knocked out domain II;CslAA4V74S,protein of CsIAA4 with valine 74 mutated to serine.(G to I)Split-LUC assays showing protein interactions in tobacco leaves:(G)CsIAA4 with CsMYB4a and CsMYB4aDC4;(H)CsIAA4D2 with CsMYB4a; (I) CsIAA4 and CsIAA4V74s with CsMYB4a.Scale bars in (G) to (I) denote wavelengths of the in vivo imaging system.

Fig.4.CsMYB4a prevents CsIAA4 degradation in vitro and in vivo.(A to C) Western blots showing: (A)α-NAA-induced (0.1 μmol L-1) degradation of CslAA4-MYC in vitro.Molecular weights:CsIAA4-MYC,25 kDa:actin,42 kDa;(B)MG132 inhibition of the degradation of CslAA4-MYC under α-NAA treatment at 25°C for 4 h.Molecular weights:CsIAA4-MYC,25 kDa;actin,42 kDa;(C)CsMYB4a inhibition of degradation of CslAA4-CsMYC in vitro.CsIAA4-MYC protein was extracted from transiently expressed tobacco;MBP-CsMYB4a and MBP-CsMYB4aDC4 MBP protein was extracted by prokaryotic expression.Molecular weights: MBP, 40 kDa; MBP-CsMYB4a, 70 kDa; MBP-CsMYB4aDC4,68 kDa.(D)Protein degradation assay showing α-NAA-induced degradation of CsIAA4-LUC in vivo.CslAA4-LUC was co-expressed with GFP,CsMYB4a-GFP,and CsMYB4aDC4-GFP.Tobacco plants were treated with 0.1 μmol L-1 α-NAA and 5 μmol L-1 MG132.(E) Relative activity of LUC/REN (LUC, firefly luciferase; REN, renilla luciferase) in the protein degradation assays.Data are means ± SD (n = 3).Different letters above the bars indicate significant differences at P < 0.05 (one-way ANOVA, Tukey’s tests).(F)Western blots showing MYB4a inhibition of degradation of CslAA4-MYC in vivo.CsIAA4-MYC was co-expressed with GFP, CsMYB4a-GFP, and CsMYB4aDC4-GFP.Tobacco plants were treated with 0.1 μmol/L α-NAA after 2 d.Molecular weights GFP, 27 kDa; CsMYB4a-GFP, 55 kDa; CsMYB4aDC4-GFP, 53 kDa.
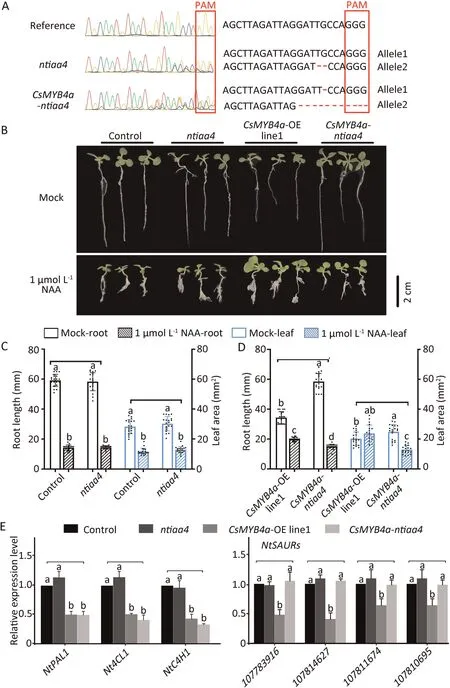
Fig.5.NtIAA4 knockout by CRISPR/Cas9-mediated genome targeting.(A) Sequence chromatograms of gene edited control, ntiaa4, and CsMYB4a-ntiaa4 first-generation transgenic plants(T0).Red box indicates the PAM site;red horizontal lines denote deletion of bases.(B)Morphologies of the control,and ntiaa4,CsMYB4a-linel and CsMYB4antiaa4 transgenic seedlings after α-NAA treatment for 20 d.Scale bar,2 cm.(C,D)Statistical data for root elongation and leaf area of control and transgenic plants in response to α-NAA treatment.Data are mean SD(n=20).Different letters denote significant differences at P< 0.05(one-way ANOVA,Tukey’s tests).(E)qRT-PCR assays showing the relative expression of genes in the phenylpropane pathway and NtSAUR family in control seedlings and ntiaa4,CsMYB4a-linel,CsMYB4a-ntiaa4 transgenic seedlings.Data are means ± SD (n = 3).Different letters indicate significant differences, at P < 0.05 (one-way ANOVA, Tukey’s test).
To determine whether the plant phenotype was affected by this interaction,we performed CRISPR/Cas9 to knock out NtIAA4 in control plants and CsMYB4a-line 1,designated as ntiaa4 and CsMYB4antiaa4,respectively.DNA sequencing showed that the NtIAA4 gene was successfully deleted in these genotypes (Fig.5A), the corresponding amino acid of edited NtIAA4 were shown in (Fig.S11).Morphological examination revealed no visible differences in root length and leaf area between the ntiaa4 mutants and control(Fig.5B,C), nor any significant difference in expression of genes involved in the phenylpropanoid pathway, lignin biosynthesis, or auxin signaling pathway (Fig.5E).We believe that knocking out NtIAA4 alone did not affect plant growth in tobacco due to redundancy of AUX/IAA genes.
Root elongation and leaf growth of CsMYB4a-ntiaa4 plants were significantly promoted compared with plants overexpressing CsMYB4a(Fig.5B,D),suggesting that knock out of NtIAA4 alleviated the growth inhibition imposed by CsMYB4a.Moreover,root length and leaf area in CsMYB4a-ntiaa4 plants were significantly less than those of CsMYB4a-line 1 after α-NAA(1 μmol L-1)treatment for 20 d (Fig.5D).This suggests that the symptoms of the plant insensitive response to α-NAA caused by CsMYB4a overexpression were alleviated.
qRT-PCR assays detected no difference in expression levels phenylpropane pathway genes PAL1, 4CL1, and C4H1 between CsMYB4a-line 1and CsMYB4a-ntiaa4 transgenic plants or between the control and ntiaa4 plants (Fig.5E).However, NtSAUR gene expression in CsMYB4a-ntiaa4 transgenic plants was significantly increased to the level of the control(Fig.5E).These results suggest that overexpression of CsMYB4a affects growth of tobacco plants by interference with the functional role of NtIAA4.
3.7.CsMYB4a and CsIAA4 regulate filament growth in tobacco flowers
To determine whether CsMYB4a and CsIAA4 regulate flower filament growth in tobacco we compared the control, and ntiaa4,CsMYB4a-ntiaa4, CsMYB4a-OE and CsIAA4-OE plants at the same stage (Fig.6AB).Filament length of the ntiaa4 mutant increased but not statistically so.CsMYB4a-OE plants had significantly decreased filament length relative to the control and knock out of NtIAA4 alleviated the growth inhibition of filament length in CsMYB4a-ntiaa4.The filaments of CsIAA4-OE flowers showed repressed growth in a similar manner to CsMYB4a-OE (Fig.6C).These results suggested that CsMYB4a and CsIAA4 inhibit filament growth, and that CsMYB4a can synergistically act with NtIAA4 in flower controlling filament growth.
3.8.NtMYB4a also prevents degradation of NtIAA4 in tobacco
We choose NtMYB4a (XP_016501430.1) and NtIAA4 in tobacco to verify conserved interaction between MYB4 and IAA4.NtMYB4a,a Sg4 member of R2R3 MYB, is a nuclear localized transcription repressor [38].
To determine whether NtMYB4a regulates degradation of NtIAA4 in tobacco, we cloned NtMYB4a and tested whether NtMYB4a can interact with NtIAA4 (Fig.S12A).Split-LUC assays revealed that NtMYB4a physically interacted with NtIAA4.Western blotting analysis showed that NtMYB4, but not GFP or NtMYB4aDC4-GFP, prevented degradation of NtIAA4-MYC under α-NAA treatment (Fig.S12B).Fluorescence signal detection and LUC/REN relative activity in transient expression assays when NtIAA4-LUC was simultaneously expressed with GFP (control),NtMYB4a-GFP, and NtMYB4aDC4-GFP showed that NtMYB4a repressed the degradation of NtIAA4-MYC under α-NAA treatment(Fig.S12C,D).These results showed that NtMYB4a inhibits degradation of NtIAA4, and that interaction between MYB4 and IAA4 is conserved in tobacco.
4.Discussion
4.1.CsMYB4a is a transcription factor with multiple functions
There are many reports on the functions of MYB repressors,including those involved in regulation of secondary cell wall synthesis, vascular tissue development and pollen development, and their effects on oxidative stress [3].Their target genes are distributed in the lignin, sinapic acid ester [4], flavonoid [39] and anthocyanin biosynthesis [40] pathways.Sg4 members of R2R3-MYB repressors in many plants directly bind to AC elements and inhibit the promoter activities of PAL,C4H and COMT in the phenylpropane pathway and synthesis of lignin [41].There are many reports indicating that genes in hormone pathways are also targets for R2R3-MYB4 Sg4 members[10,42]In the current study,we confirmed the repression function of CsMYB4a in the plant growth and CsMYB4a-OE plants showed abnormal auxin response (Fig.2).
Lignin modification induced dwarfism (LMID) caused by MYB4 family members hinders the modification of Sg4 members in improving crop quality, down-regulating C3H or HCT in alfalfa resulted in a 40% reduction in biomass [43].CsWRKY13 is a negative regulator in regulation of lignin synthesis in tea plants,CsWRKY13 overexpressing in Arabidopsis cause a weaker stem phenotype [44].Although it remains a practical challenge, there are attempts to reduce lignin content but at the same time avoiding LMID.A recent trial showed that expressing PvMYB4 with a green tissue-specific promoter resulted in a reduction in lignin content without biomass reduction [45].Interestingly, mutations in SAD2(also known as GIR1 or growth inhibition relieved 1)alleviate LMID in ref8 (defective in p-coumaroyl shikimate 3′-hydroxylase, C3′H)mutants without restoring lignin content [7].Our results suggest that there is a way to eliminate interaction between CsMYB4a and CsIAA4 to reduce lignin content without affecting plant growth.
4.2.The model of MYB4a interaction with IAA4
In previous research, both an atmyb32 insertion mutant and AtMYB32 overexpression lines had distorted pollen[17].CsMYB4asilenced flowers showed exhibited aberrant pollen sacs, but CsIAA4-silenced flower did not (Fig.1D,H).Although both lignin and auxin are essential for flower development, our results suggested that CsMYB4a regulates filament elongation through CsIAA4, but not anther development, which is independent of lignin regulation.
We also found that CsIAA4 and CsMYB4a were co-expressed in lateral and main roots (Fig.1A).The inhibition of root elongation by high concentrations of α-NAA(1 μmol L-1)in control seedlings did not occur in other transgenic genotypes (Fig.2).Auxin is the main signaling system involved in inducing lateral and adventitious root development.Endogenous AtMYB32 transcription level in roots was significantly induced by exogenous auxin [17].Whether CsIAA4 and CsMYB4a are jointly involved in the development of lateral roots or adventitious roots in tea plants should be further studied.
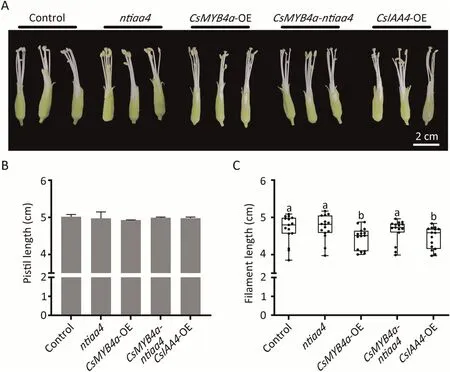
Fig.6.CsMYB4a and CsIAA4 regulate flower filament growth in tobacco.(A)Flowers of control,and ntiaa4 mutants,CsMYB4-OE,CsMYB4-ntiaa4 mutants and CslAA4-OE.Scale bar, 2 cm.(B, C) Statistical data for in genotypes shown in (B) pistil length and (C) filament length.Data are means ± SD (n = 15).Different letters above the bars indicate significant differences at P < 0.05 (one-way ANOVA, Fisher’s test).

Fig.7.A Proposed model of CsMYB4a regulating auxin signaling by interacting with CsIAA4 to prevent its degradation.(A)The flower of tea plant contains seven stages(S1–S7).Scale bar,1 cm.(B)The relative expression of CsIAA4 and CsMYB4a in flowers.scale bar denotes the relative expression level of CslAA4 or CsMYB4a.(C)IAA4 interacts with auxin response factors (ARFs) to repress the expression of the auxin response genes.(D) MYB4a interacts with the domain II of the IAA4 to compete with TIR1.The degradation of IAA4 is prevented when the expression of MYB4a is increased.(E) Transport inhibitor response 1 (TIR1) interacts with IAA4 via auxin mediation and causes IAA4 degradation by ubiquitination; thus, the transcriptional activity of ARFs is alleviated.
The SCFTIR1-Aux/IAA-ARF-dependent auxin signaling pathway regulates almost all aspects of plant growth and development and participates in plant responses to environmental signals [46].Altered AUX/IAA protein stability is a key factor in the auxin signaling pathway.AUX/IAAs interact with auxin response factors(ARFs)via their III and IV domains to suppress the transcription of ARFactivated genes [47,48].High concentrations of auxin are thought to produce a corresponding growth-inhibiting effect by stabilizing interaction between TIR1 and the AUX/IAA transcriptional repressor to promote of AUX/IAA proteins produced from SCFTIR1/AFB E3 ligase-dependent ubiquitination [49].Some proteins prevent degradation of AUX/IAAs by interacting with domain II.For example, rice plants infected with the rice dwarf virus (RDV) show dwarfing symptoms [50] caused by interaction of RDV P2 protein and domain II of OsIAA10,resulting in OsIAA10 not being ubiquitinated by OsTIR1.Similarly, in deep shade, phytochrome (PHY)-A competes with TIR1 by directly binding to the N-terminus of IAA3 and IAA17 to prevent shade-induced degradation of IAA3 and IAA17 [51].Under blue and red light, cryptochrome 1 (CRY1)and PHYB inhibit hypocotyl growth by interacting with AUX/IAA proteins [52,53].
Here, we confirmed that interaction occurred between CsIAA4 and CsMYB4a (Fig.3).We simulated a complex model of CsIAA4 and CsMYB4a using the AlphaFold2 algorithm and found that possible interaction sites mainly distributed in the N-terminal domain of CsIAA4 (Fig.3D,E).Interaction experiments carried out using point mutations to determine the interacting sites of CsIAA4 and CsMYB4a(Fig.3H,I)confirmed that CsMYB4a interacts with CsIAA4 through domain II of CsIAA4,and valine 74 of CsIAA4 was a key site for mediating this interaction.
Interaction between CsMYB4a and CsIAA4 appears to be specific, as CsMYB4 did not interact with CsIAA7 (a close homolog of CsIAA4)in split-LUC assays(Fig.S8),whereas NtIAA4 from tobacco did interact with CsMYB4a (Fig.S10).More importantly, through in vitro and in vivo protein degradation assays we verified that the interaction between CsMYB4a and CsIAA4 helped to prevent the degradation of CsIAA4 (Fig.4).We also verified the NtMYB4a interact with NtIAA4 to strengthen the stability of NtIAA4 in tobacco (Fig.S12).When NtIAA4 (a homologue of CsIAA4) was knocked out in a transgenic tobacco plant with CsMYB4a, the above abnormal phenotype was alleviated, indicating that CsMYB4a participated in regulation of NtIAA4 function (Figs.5,6).These results implied that CsMYB4a participates in the regulation of auxin signal transduction.To our knowledge,this is the first report that R2R3-MYB Sg4 members are involved in the regulation of auxin signaling.
Stigma exertion is beneficial for increasing outcrossing [54,55].Stamen filament growth was inhibited during the S4–S6 developmental stage (Fig.7A,B) when expression of CsIAA4 and CsMYB4a was gradually increasing.Finally,we proposed a model for regulation of plant growth using CsMYB4a and CsIAA4 (Fig.7C–E).TIR1 interacts with IAA4 by auxin mediation and causes IAA4 degradation by ubiquitination.This alleviates the transcriptional activity of ARFs.However,CsMYB4a can disturb this interaction by protecting CsIAA4 from CsTIR1,thereby increasing the stability of CsIAA4 and preventing its ubiquitination and degradation.CsSAURs were downregulated and flower filament development was inhibited.CsMYB4a interacts with CsIAA4 to prevent excessive filament growth, and spatial separation of stamens and pistils, one of the strategies to ensure cross pollination.
In summary, our results reveal the physiology function of CsMYB4a and CsIAA4 in filament growth of tea plant, in contrast to those of previous studies of the regulation of CsMYB4a in phenylpropanoid and lignin pathway, CsMYB4a stabilizes CsIAA4 to repress the auxin signaling pathway, result in reduced filament elongation.Together,our findings extend our understanding of the diversity of function of CsMYB4a and suggest the regulation of CsMYB4a and CsIAA4 in the morphogenesis of tea floral organs.
Accession numbers
All gene information for tea plants can be found in TPIA(https://tpia.teaplant.org/): CsMYB4a (TEA003515.1), CsIAA4(TEA006724.1), CsIAA7 (TEA014506.1).All gene information for tobacco can be found in NCBI: NtIAA4 (107830067), NtMYB4a(107819789), NtPAL1 (107786762), Nt4CL1 (107785405), NtC4H1(107826530).
CRediT authorship contribution statement
Guoliang Ma:Data curation,Writing–original draft.Mingzhuo Li:Data curation, Writing – original draft.Yingling Wu:Project administration, Writing – review & editing, Funding acquisition.Changjuan Jiang:Methodology.Yifan Chen:Methodology.Dawei Xing:Methodology.Yue Zhao:Methodology.Yajun Liu:Methodology.Xiaolan Jiang:Project administration, Writing – review &editing,Funding acquisition.Tao Xia:Project administration,Writing – review & editing, Funding acquisition.Liping Gao:Project administration, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the joint funds of National Natural Science Foundation of China (U21A20232), the Natural Science Foundation of China (32072621, 32002088,31870676), and Collegiate Collaborative Innovation Foundation of Anhui Province (GXXT-2020-081).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.11.006.
- The Crop Journal的其它文章
- Flowering-time regulation by the circadian clock: From Arabidopsis to crops
- Global characterization of OsPIP aquaporins reveals that the H2O2 transporter OsPIP2;6 increases resistance to rice blast
- Drought-triggered repression of miR166 promotes drought tolerance in soybean
- The OsBSK1-2-MAPK module regulates blast resistance in rice
- Natural variation of an autophagy-family gene among rice subspecies affects grain size and weight
- Rice gene OsUGT75A regulates seedling emergence under deep-sowing conditions

