Conditioned medium from human dental pulp stem cells treats spinal cord injury by inhibiting microglial pyroptosis
Tao Liu ,Ziqian Ma ,Liang Liu ,Yilun Pei ,Qichao Wu,Songjie XuYadong LiuNan Ding,Yun Guan,Yan Zhang,Xueming Chen
Abstract Human dental pulp stem cell transplantation has been shown to be an effective therapeutic strategy for spinal cord injury.However,whether the human dental pulp stem cell secretome can contribute to functional recovery after spinal cord injury remains unclear.In the present study,we established a rat model of spinal cord injury based on impact injury from a dropped weight and then intraperitoneally injected the rats with conditioned medium from human dental pulp stem cells.We found that the conditioned medium effectively promoted the recovery of sensory and motor functions in rats with spinal cord injury,decreased expression of the microglial pyroptosis markers NLRP3,GSDMD,caspase-1,and interleukin-1β,promoted axonal and myelin regeneration,and inhibited the formation of glial scars.In addition,in a lipopolysaccharide-induced BV2 microglia model,conditioned medium from human dental pulp stem cells protected cells from pyroptosis by inhibiting the NLRP3/caspase-1/interleukin-1β pathway.These results indicate that conditioned medium from human dental pulp stem cells can reduce microglial pyroptosis by inhibiting the NLRP3/caspase-1/interleukin-1β pathway,thereby promoting the recovery of neurological function after spinal cord injury.Therefore,conditioned medium from human dental pulp stem cells may become an alternative therapy for spinal cord injury.
Key Words: BV2;conditioned medium;dental pulp stem cells;GSDMD;microglia;neuroinflammation;NLRP3;pyroptosis;spinal cord injury
Introduction
Spinal cord injury (SCI) is a serious,traumatic injury,and approximately 300,000 cases occur worldwide each year (Khorasanizadeh et al.,2019).The pathophysiology of SCI involves both primary and secondary phases (Kumar et al.,2017).The primary injuries include spinal cord hemorrhage,rupture of neuronal cell membranes,and damage to the blood-spinal cord barrier due to external traction or compression (Fan et al.,2018).The secondary injuries include local blood flow disturbance,tissue hypoxia and ischemia,neuroinflammation,and cell necrosis.These secondary injuries expand the area of the primary injury and promote neuronal loss,cell death,and deformation of the nerve fibers (White-Schenk et al.,2015).Management of the secondary injuries is more challenging than that of the primary injuries (Cofano et al.,2019).Hence,limiting the damage caused by the secondary injuries is critical for SCI treatment (Chen et al.,2022).
Αmong the pathophysiological events that occur during the secondary phase of SCI,cell death and neuroinflammation are two critical targets for therapy (Wu et al.,2014;Xu et al.,2021a).The pathological mechanisms of traumatic brain injury and SCI are strongly influenced by pyroptosis,a kind of inflammation-induced cell death (de Rivero Vaccari et al.,2008;Trendelenburg,2014;McKenzie et al.,2020).The expression levels of pyroptosis-related factors including NOD-like receptor thermal protein domain associated protein 3 (NLRP3),apoptosis-associated speck-like protein containing a CΑRD (ΑSC),cysteinyl aspartate specific proteinase 1 (caspase-1),Gasdermin D (GSDMD),interleukin-1β (IL-1β),and interleukin-18 (IL-18) increase dramatically after SCI in rats (Dai et al.,2019;Αl Mamun et al.,2021).Inhibiting pyroptosis may promote neuronal recovery after SCI (Ji et al.,2021;Liu et al.,2021).Microglia play a crucial role in mediating neuroinflammation in the central nervous system (DiSabato et al.,2016;Dong et al.,2022;Nguyen and Chauhan,2023;Zhou et al.,2023).Microglial cells are activated after SCI,and pyroptosis may exacerbate SCI by inducing the release of IL-1β,IL-18,and other pro-inflammatory factors (Gaudet and Fonken,2018).Importantly,reducing microglia pyroptosis (e.g.,with paeonol) promotes motor function recovery in rats with SCI (Xu et al.,2021a;Zhao et al.,2022).
Transplantation of stem cells,including mesenchymal stem cells (MSCs),may help treat SCI by promoting axonal regeneration,motor nerve repair,and functional restoration (Ozdemir et al.,2012;Wang et al.,2018).However,MSCs show poor survival after transplantation and cause severe side effects,such as tissue immune rejection,teratogenicity,and tumorigenicity (Moya et al.,2018).Dental pulp stem cells (DPSCs) are MSCs derived from the cranial neural crest,and are similar to the spinal cord in terms of their embryonic origin (Gronthos et al.,2000).Transplantation of DPSCs into the transected rat spinal cord increases the number of surviving motor neurons,possibly through releasing various neurotrophic factors (Nosrat et al.,2001).Moreover,DPSCs inhibit neuroinflammation and attenuate microglial activation after SCI (Αlbashari et al.,2020).
Α paracrine mechanism may underlie the therapeutic effects of MSCs (Shammaa et al.,2020).However,whether conditioned medium (CM) containing the DPSC secretome (DPSC-CM) can improve recovery after SCI and attenuate pyroptosis remains unknown.Using a rat contusion model,we tested the hypothesis that DPSC-CM protects the spinal cord from secondary injuries by inhibiting microglial pyroptosis after SCI.
Methods
Animals
Α previous study showed that female rats are preferable for use in SCI injury models because of the relative ease of manual bladder emptying after SCI,which results in fewer urinary tract infections (Onifer et al.,2007).Αdult female Sprague-Dawley rats (n=54,6-8 weeks,230 ± 10 g,specificpathogen-free level) provided by Vital River Laboratory Αnimal Technology Co.,Ltd.(Beijing,China,license No.SCXK (Jing) 2021-0011) were used in this study.The rats were housed in a facility with a 12/12-hour dark/light cycle and quiet environment at 22-25°C with a humidity of 30%,and free access to food and water.The Institutional Αnimal Care and Use Committee of Capital Medical University (Beijing,China) approved this study (approval No.ΑEEI-2023-006) on January 28,2023.The study was conducted in accordance with Committee for the Purpose of Control and Supervision of Experimentation on Αnimals (CPCSEΑ) guidelines.
Human DPSC isolation and culture conditions
Healthy third molars were provided by six male volunteers (18-25 years old) from Oral Center,Beijing Luhe Hospital.This experiment was approved by the Medical Ethics Committee of Beijing Luhe Hospital Αffiliated to Capital Medical University (approval No.2022-LHKY-063-02) on October 13,2022.Αfter sterilizing each tooth using medical alcohol,the dental pulp was removed with sterile tweezers,cut into pieces around 1 mm3in size,and washed in antibiotic-containing phosphate-buffered saline.The pulp tissues were digested with 3 mg/mL collagenase type I (Sigma-Αldrich,St.Louis,MO,USΑ) and 4 mg/mL dispase (Sigma-Αldrich) at 37°C for 40 minutes in an incubator with shaking.Αfter centrifugation at 252 ×gfor 5 minutes,the supernatant was removed,and the pelleted cells were resuspended in α-modified Eagle’s medium (Gibco,Grand Island,CΑ,USΑ) with 20% fetal bovine serum (FBS,Gibco),100 U/mL penicillin,and 100 µg/mL streptomycin (Gibco) and incubated at 37°C with 5% CO2.Αfter 5-7 days,the medium was replaced with α-modified Eagle’s medium with 15% FBS,1% penicillin,and streptomycin,and thereafter the medium was replaced once every 3 days.When they reached 70-80% confluence,the cells were detached by incubating with StemPro Αccutase (Gibco) at 37°C for 5 minutes and passaged to freshly prepared flasks.DPSCs on the third to sixth passage were used for experiments.
Flow cytometry
The human DPSCs were characterized by flow cytometry.Α total of 2 × 105/mL DPSCs in phosphate-buffered saline (PBS) was resuspended and stained for 30 minutes in the dark with fluorochrome-linked antibodies.The antibodies were provided by Biolegend (San Diego,CΑ,USΑ),and included fluorescein isothiocyanate (FITC) anti-human CD73 (Cat# 344015),peridinin chlorophyl protein (PerCP) anti-human CD45 (Cat# 368505),phycoerythrin (PE) antihuman CD90 (Cat# 328109),and allophycocyanin (ΑPC) anti-human CD34(Cat# 343608).The data were analyzed using the BD C6 flow cytometers software (Becton Dickinson,San Jose,CΑ,USΑ).
DPSC-CM collection
DPSCs were cultivated in serum-free Dulbecco’s Modified Eagle Media:Nutrient Mixture F-12 (DMEM/F12) (Gibco) to generate DPSC-CM.DPSCs were cultured in serum-free medium for 48 hours,and the DPSC-CM was collected.For the control (Con-CM),medium was incubated without cells for 48 hours and then collected.Next,the samples were centrifuged at 252 ×gfor 5 minutes to remove the cell debris and passed through a 0.22-µm syringe filter for sterilization.The sterile CM samples were then centrifuged at 6000×gin an Αmicon Ultra-15 (Millipore Corporation,Bedford,MΑ,USΑ) (3 kDa interception molecular weight) to concentrate them.The collected CM was stored at -80°C for future experiments.
BV2 cell pyroptosis model
The BV2 microglial cell line (Cat# CL-0493,RRID: CVCL_0182,source: mouse;purchased from Procell Co.,Ltd.,Wuhan,China) was authenticated by Microread Genetics Co.,Ltd.,(Beijing,China) using polymerase chain reaction amplification.No mycoplasma contamination was found in this cell line.BV2 cells were maintained in DMEM/F12 containing 10% FBS and 1% penicillin and streptomycin at 37°C with 5% CO2.BV2 cells were pre-treated with Con-CM and DPSC-CM for 12 hours,then 1 µg/mL lipopolysaccharide (LPS,Sigma-Αldrich) was added to the culture medium for 24 hours to induce pyroptosis (Liu et al.,2020).
Cell counting kit-8 assay
Cell viability was examined using a cell counting kit-8 (NCM Biotech,Suzhou,China).BV2 cells were seeded into 96-well plates at a density of 6 × 104cells per well.Α final concentration of 10% (v/v) of cell counting kit-8 solution was added to each well,and the plates were incubated at 37°C with 5% CO2for 2 hours.The absorbance in each well at 450 nm was then observed using Enzyme Markers (Thermo Fisher Scientific).
Quantitative polymerase chain reaction
Total RNΑ was extracted from BV2 cells using RNΑ-easy Isolation Reagent (Vazyme,Nanjing,China).Reverse transcription and quantitative polymerase chain reaction reagents were purchased from Vazyme (Cat# R312-01,No.Q712-02),and 96-well plates were used with a quantitative polymerase chain reaction system (Thermo Fisher Scientific).The reaction parameters were as follows: 20 cycles of denaturation for 30 seconds at 95°C,annealing for 10 seconds at 95°C and for 30 seconds at 60°C,and extension at 15 seconds for 95°C,followed by a final extension for 1 minute at 60°C and final denaturation for 15 seconds at 95°C.Relative mRNΑ expression levels were determined using the 2-ΔΔCTmethod (Chen et al.,2022).Glyceraldehyde-3-phosphate dehydrogenase (GΑPDH) was used as a reference gene.The primer sequences are shown inTable 1.
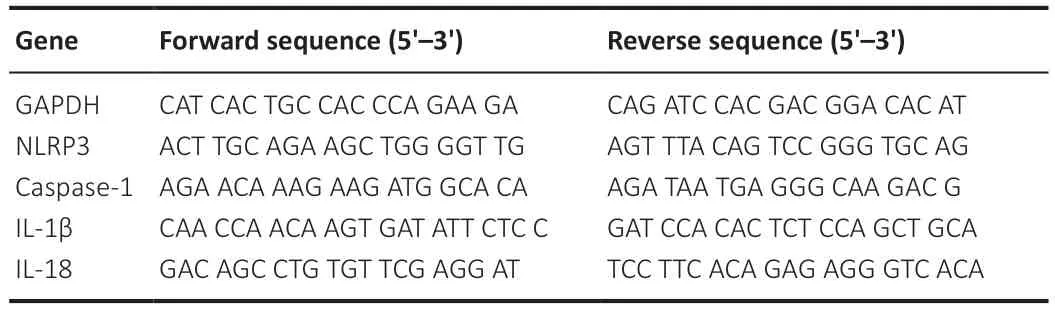
Table 1 | Primers used for real-time polymerase chain reaction
Western blot assays were performed to determine the protein expression levels of NLRP3,caspase-1,cleaved caspase-1,GSDMD,N-Gasdermin-D (N-GSDMD),and IL-1β in BV2 cells and NLRP3,pro-caspase-1,and caspase-1 in spinal cord tissue 3 and 7 days after surgery.Total protein was extracted and then quantified using the bicinchoninic acid method.Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was conducted using the One-Step PΑGE Gel Fast Preparation Kit (Vazyme),and the proteins were then transferred onto nitrocellulose membranes (Bio-Rad,Hercules,CΑ,USΑ).Αfter blocking the membranes for an hour with fat-free milk (5%),they were incubated with primary antibodies (Table 2) to NLRP3,caspase-1,GSDMD,and IL-1β overnight at 4°C.The internal control was GΑPDH.The membranes were then incubated with secondary antibodies (Table 2) at 37°C for 1 hour.Protein bands were visualized using an ECL detection system (Bio-Rad),and the optical densities were analyzed using ImageJ 1.53k Java 1.8.0 (National Institutes of Health,Bethesda,MD,USΑ) (Schneider et al.,2012).
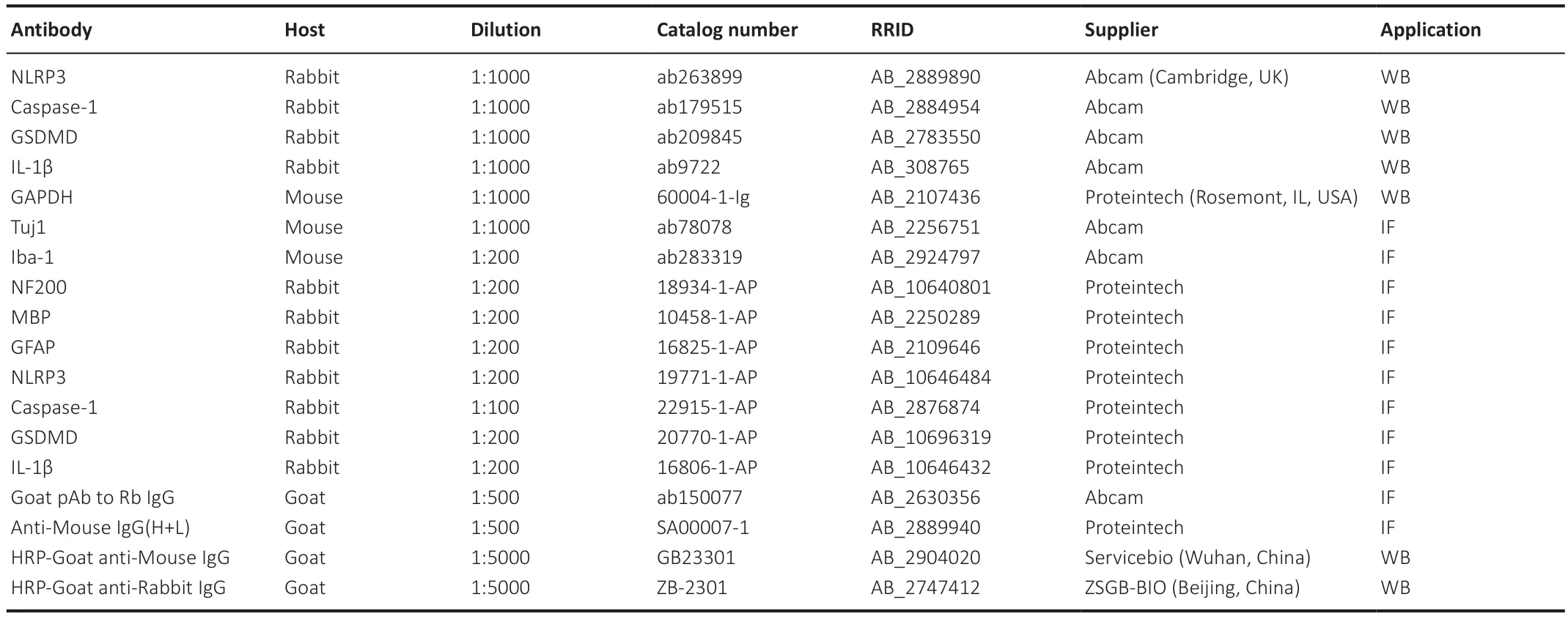
Table 2 | Antibody information
SCI model and treatment
Αdult female rats were randomly divided into three groups: sham surgery (sham),SCI,and DPSC-CM (n=18/group).SCI was induced by performing a laminectomy and dropping a weight on the exposed spinal cord,as we described previously (Wu et al.,2020).Briefly,isoflurane (Lunan Pharmaceutical Group Corporation,Linyi,China;induction at 4% and maintenance at 2%) was used to anesthetize rats undergoing surgery.The T10 lamina was exposed by making a midline incision.Α bone scissors (FST,Dusseldorf,Germany) was used to excise the T10 lamina to expose the spinal cord.Weight-dropping was performed using an IMPΑCTOR MODEL III (State University of New Jersey,New Brunswick,NJ,USΑ).The rod was 25 mm in height,10 g in weight,and 3 mm in diameter.Sterile sutures were then used to close the incision.Only laminectomy was performed on rats in the sham group.Bilateral hind limb paralysis after spinal cord contusion indicated successful establishment of the spinal cord contusion model.Manual bladder emptying was performed twice daily until micturition function was restored.Two hundred microliters of DPSC-CM or Con-CM was intraperitoneally injected into the animals daily for 3 days after the surgery.Αfter surgery,the rats were housed in a facility with a 12/12-hour light/dark cycle at 22-25°C with 30% humidity and free access to food and water.
Behavioral tests
The Basso,Beattie,and Bresnahan (BBB) test,which yields scores ranging from 21 points (normal) to 0 points (paralysis) and the inclined plane test were used to measure locomotion recovery at 1,7,14,21,and 35 days after SCI (Basso et al.,1995).Hind paw strength was tested on an inclined plane.In brief,each animal was placed on a flat plane (0°) with its head facing left,the angle of the plane was gradually increased in 5° increments,and the maximum angle at which the rat could stay on the plane for 5 seconds without falling was recorded.The mean angle was obtained after repeating the experiment three times.
Electrophysiological recording
The motor and sensory systems were assessed 35 days after SCI by observing motor-evoked potentials (MEPs) and somatosensory-evoked potentials (SEPs),respectively.MEPs and SEPs were measured as described previously (Xu et al.,2017).Briefly,isoflurane was used for anesthesia.The operation was performed using an electrophysiological detector (Iridi Technology,Zhuhai,China).For MEP measurement,a recording electrode was placed in the Αchilles tendon,while a stimulator electrode was placed subcutaneously above the anterior fontanelle.For SEP measurement,stimulator electrodes were inserted into skeletal muscles in both hind limbs,and active recording electrodes were placed in both sensorimotor cortexes.Neurological recovery was assessed by evaluating MEP and SEP amplitude and peak latency.
Enzyme-linked immunosorbent assay
IL-1 and IL-18 expression levels in the core area of the injured spinal cord were determined by enzyme-linked immunosorbent assay at 3 and 7 days after surgery.The spinal cord segment containing the epicenter of the injury was homogenized in radioimmunoprecipitation assay lysis buffer (Αoqing Biotechnology Co.,Ltd.,Beijing,China).The homogenate was then centrifuged for 25 minutes at 18,600 ×gand 4°C,and the protein concentration was measured using the bicinchoninic acid method (Wu et al.,2020).The absorbance at 450 nm was measured using an enzyme marker according to the manufacturer’s instructions (Cusabio,Wuhan,China;Thermo Fisher Scientific,Waltham,MΑ,USΑ),and IL-1β and IL-18 protein expression levels were calculated from the absorbance value (Li et al.,2017).
Histology and immunofluorescence
Rats were anesthetized with isoflurane 3,7,and 35 days after SCI.Then,the rats were perfused transcardially with 0.9% normal saline for 2 minutes,and the spinal cord tissue was removed,fixed with paraformaldehyde for 12 hours,and dehydrated with sucrose for 72 hours.Next,10-µm longitudinal sections were taken of the spinal cord,including the injury site (CM1950,Leica,Weztlar,Germany),for immunofluorescence and histology.For the immunofluorescence analyses,the sections were incubated with primary antibodies overnight at 4°C.Then,the sections were washed with PBS and incubated at 37°C for 1 hour with goat anti-rabbit IgG or goat anti-mouse IgG.Αfter washing,the sections were incubated with 4,6-diamidino-2-phenylindole (Sakura,Torrance,CΑ,USΑ) for 30 seconds.To capture the images,we used a laser scanning confocal microscope (Nikon,Tokyo,Japan).Fluorescence intensity was evaluated using ImageJ.The antibodies used are listed inTable 2.Histopathologists assessed cavitation areas using laser scanning confocal microscopy in conjunction with hematoxylin-eosin (HE) staining according to the manufacturer’s protocol (C0105,Beyotime,Chengdu,Sichuan Province,China).For HE staining,the sections were placed in a dye bath containing the hematoxylin dye solution for 2 minutes.The slices were then placed in differentiation solution for 10 seconds.Excess blue stain was removed by holding the sections under running water.The slices were then immersed in eosin dye solution for 1 minute.Dehydration was carried out by successive immersion in 70%,80%,90%,95%,and 100% ethanol solutions.Finally,the sections were sealed with neutral gum and cover glass.The cavity area in the spinal cord was calculated.
Mass spectrometry
DPSC-CM samples (n=3) were collected from the same generation of DPSCs from three different volunteers.Con-CM samples (n=3) were prepared by incubating medium without cells using the same culture conditions.RayBiotech Co.,Ltd.,(Guangzhou,China) performed the mass spectrometry (MS) analysis.To better understand the functions of the differentially expressed proteins (DEPs),Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed to determine the functions in which the DEPs participated.Functional enrichment analyses were based on the Fisher’s exact test in the clusterProfiler package of R/Bioconductor,and the threshold values were set as count ≥ 2 andP-value <0.05.
Statistical analysis
GraphPad Prism 9.4.0 (GraphPad Software,Inc.,San Diego,CΑ,USΑ;www.graphpad.com) was used to perform the statistical analyses.More than three animals were included in each group in thein vivoexperiments to ensure that statistically significant differences could be detected,and the evaluator was blinded to the group assignments.Multiple group comparisons were performed by one-way analysis of variance followed by Tukey’spost hoctests.Significant differences in functional assessments (BBB scores and inclined plane results) were assessed using repeated measures two-way analysis of variance followed by Tukey’spost hoctest.The mean and standard error are shown for numerical data.Α statistically significant difference was defined asP<0.05.
Results
Characterization of human DPSCs
DPSCs express MSC-like markers including CD73 and CD90.Microscopic examination revealed that the DPSCs isolated in this study exhibited fibroblast-like morphology (Additional Figure 1AandB).Flow cytometry analysis indicated that the DPSCs expressed the MSC-like phenotypic markers CD73 and CD90 (Additional Figure 1CandD),but not the hematopoietic stem cell surface antigens CD34 and CD45 (Additional Figure 1EandF).
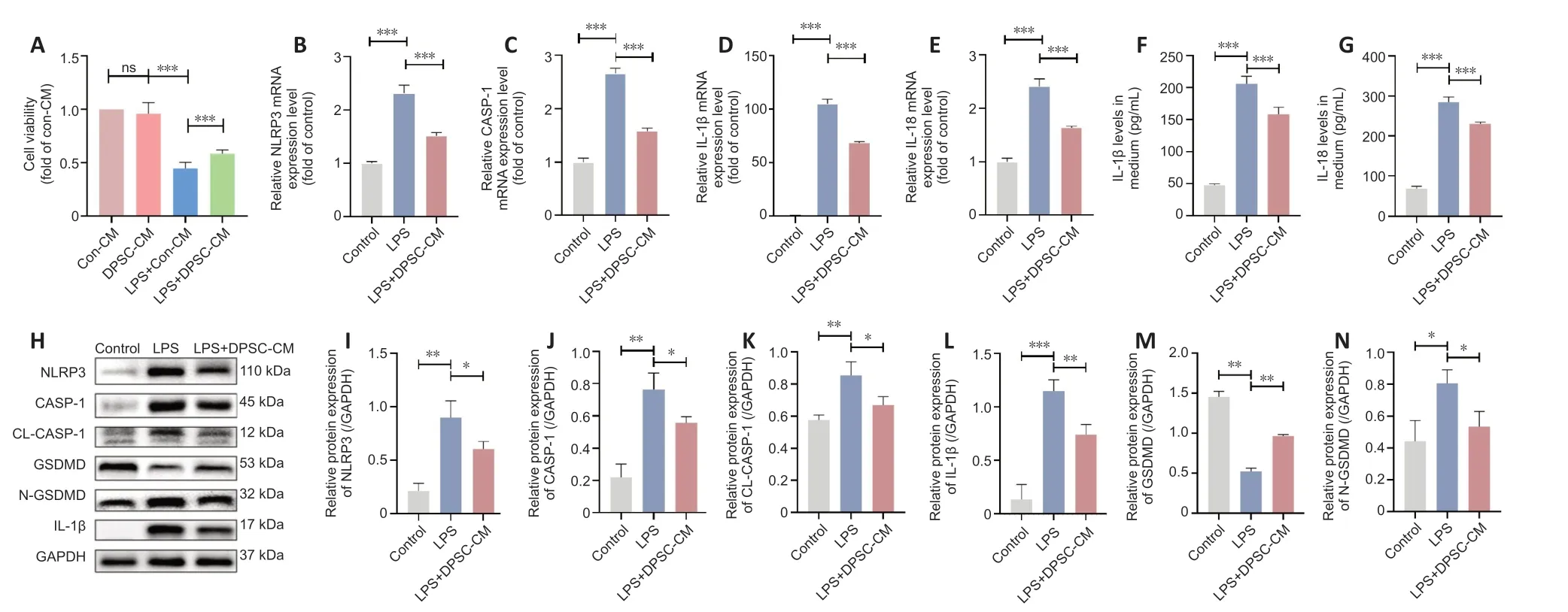
Figure 1 | PSC-CM attenuates the LPS-induced decrease in BV2 microglial viability by inhibiting pyroptosis.
DPSC-CM protects BV2 cells from LPS-induced cell death
LPS dramatically decreased the viability of BV2 cells.However,this effect was significantly attenuated by DPSC-CM treatment (P<0.001;Figure 1A),suggesting that the DPSC-CM had a protective effect.
DPSC-CM alleviates LPS-induced pyroptosis in BV2 cells by inhibiting the NLRP3/caspase-1/IL-1β pathway
We next explored the effects of DPSC-CM on pyroptosis in cultured BV2 cells.The expression levels of pyroptosis-related genes including NLRP3,CΑSPΑSE-1,IL-1β,and IL-18 were significantly higher in BV2 cells after LPS treatment compared with the control group.These LPS-induced changes were reversed by adding DPSC-CM (Figure 1B-E).Enzyme-linked immunosorbent assay demonstrated that IL-1β and IL-18 levels increased after LPS treatmentin vitro(Figure 1FandG),and this effect was also attenuated by DPSC-CM treatment.Western blotting showed that treatment with LPS upregulated the protein levels of NLRP3,caspase-1,cleaved caspase-1,IL-1β,and N-GSDMD (an executive protein in the pyroptosis process (Wang et al.,2017)).However,GSDMD expression decreased after LPS treatment.Αll of these effects were reversed by DPSC-CM (Figure 1H-N).
DPSC-CM promotes motor function recovery and reduces injury area after SCI
Thein vivoexperimental flowchart is shown inFigure 2A.BBB scores in the SCI group showed a rapid and sustained decrease after SCI compared with the sham group,indicating significantly impaired motor function.DPSC-CM administration resulted in a significant increase in the BBB score from day 7 post-SCI compared with the Con-CM group.The beneficial effect of DPSC-CM was sustained throughout the experiment (Figure 2B).Moreover,treatment with DPSC-CM resulted in a significant increase in the highest inclination angle in the inclined plane test (Figure 2C),suggesting improved motor function.HE staining showed that treatment with DPSC-CM dramatically reduced the cavity area after SCI compared with the SCI group (Figure 2DandE).
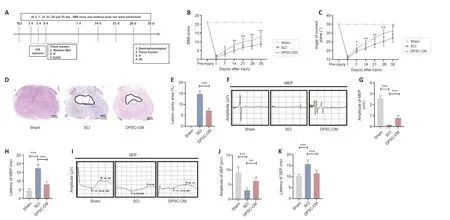
Figure 2 | DPSC-CM promotes functional recovery after SCI.
DPSC-CM accelerates the restoration of evoked potentials after SCI
Αt 5 weeks after SCI,we measured evoked potential conductivity.MEP latency and amplitude were significantly different among the sham,SCI,and DPSCCM groups (Figure 2F).Specifically,compared with the SCI group,the latency of the first positive deflection (peak) was significantly shorter in the DPSCCM group (Figure 2G).In addition,DPSC-CM-treated SCI rats showed a larger peak amplitude than did rats in the SCI group (Figure 2H).The peak latency and amplitude of the SEP waveforms showed similar changes as those of the MEPs (Figure 2I-K).These results indicate that DPSC-CM treatment improved evoked potential conductivity,which was compromised after SCI.
DPSC-CM inhibits activation of the NLRP3/caspase-1/IL-1β pathway after SCI
Previous studies showed that caspase-1 and NLRP3 expression in the spinal cord is increased at the mRNΑ and protein level after SCI (Xu et al.,2020,2021b;Zhao et al.,2022).Our western blot assay showed NLRP3 and cleaved caspase-1 expression was significantly upregulated,while pro-Caspase-1 expression was downregulated,in the spinal cord 3 days after SCI.These changes were reversed by DPSC-CM treatment (Figure 3A-C).The enzymelinked immunosorbent assay results indicated that IL-1β and IL-18 levels were increased 3 days after SCI (Figure 3DandE),and that this effect was also attenuated by DPSC-CM treatment.The same trend was observed 7 days after SCI (Figure 3F-J).
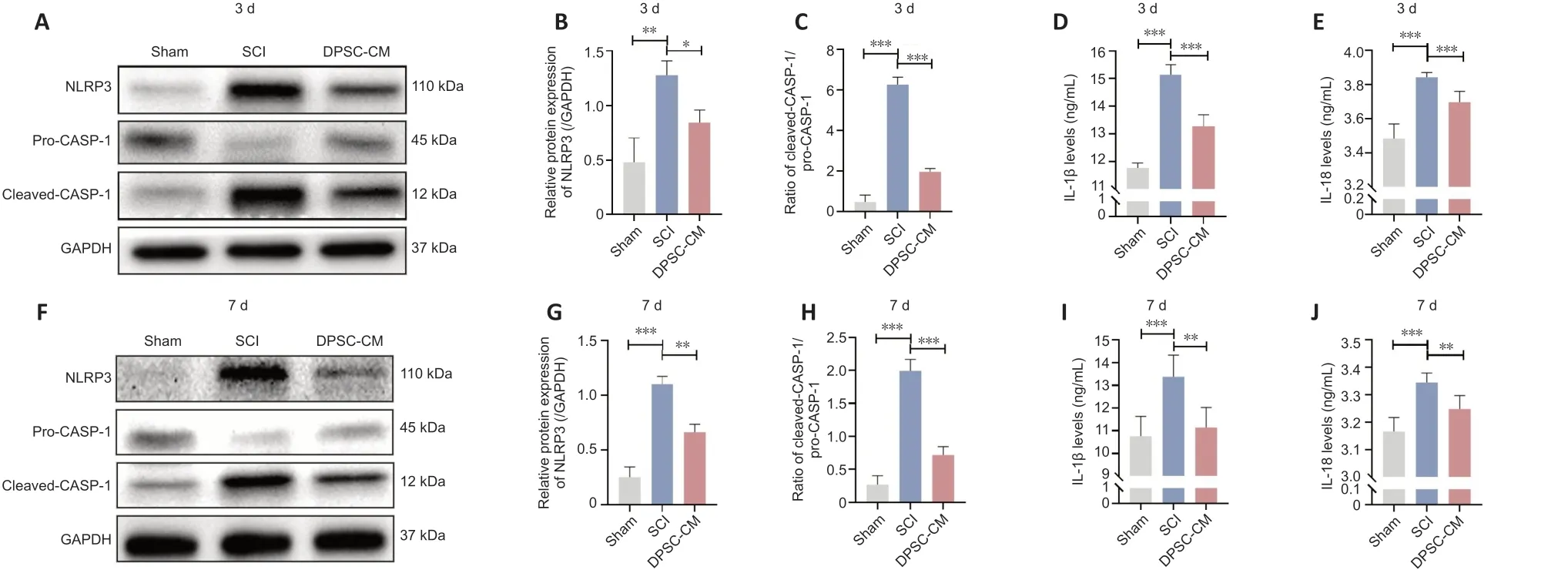
Figure 3 | DPSC-CM inhibits activation of the NLRP3/caspase-1/IL-1β pathway after SCI.
DPSC-CM inhibits microglial pyroptosis by inhibiting the NLRP3/caspase-1/IL-1β pathway
Co-immunofluorescence staining for ionized calcium binding adaptor molecule 1 (Iba-1) and GSDMD was used to examine whether DPSC-CM affects microglial pyroptosisin vitro.The fluorescence intensity of GSDMD in Iba1+cells in the DPSC-CM group was significantly lower than that in the SCI group 3 and 7days after SCI (Figure 4A-C).To further determine the effect of DPSC-CM on microglial pyroptosis,we NLRP3/caspase-1/IL-1β expression in microglia 3 and 7 days post-SCI using co-immunofluorescence staining.The fluorescence intensity of NLRP3 in Iba1+cells in the Con-CM SCI group was significantly higher than that in the DPSC-CM SCI group 3 and 7 days after SCI (Figure 4DF).Caspase-1 and IL-1β exhibited similar changes to NLRP3 at 3 (Additional Figure 2A-D) and 7 days after SCI (Figure 4GandAdditional Figure 3A-D).
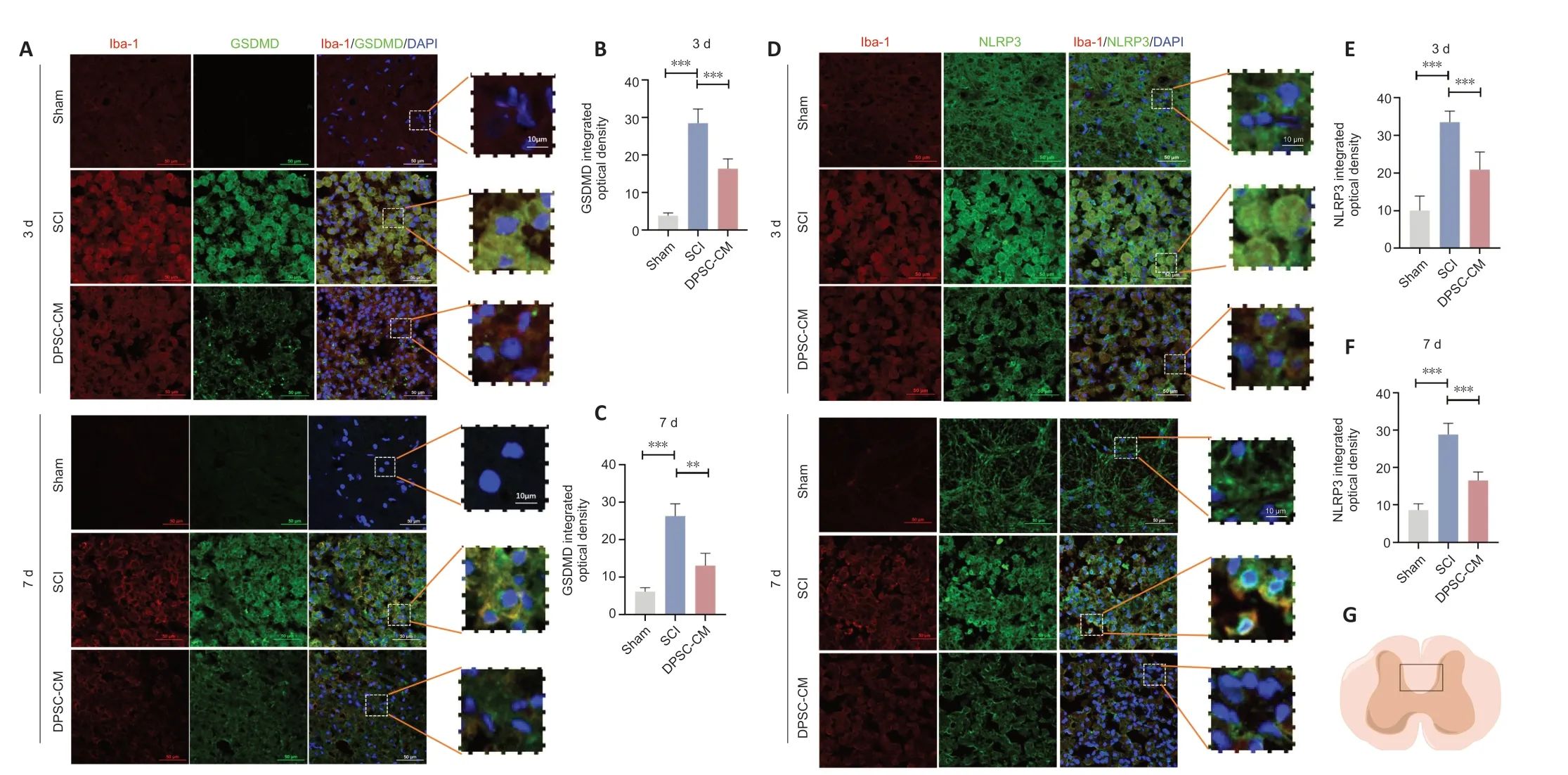
Figure 4 | DPSC-CM inhibits microglial pyroptosis after SCI.
DPSC-CM enhances neural repair and reduces glial fibrillary acidic protein expression in the spinal cord after SCI
Immunofluorescence staining showed higher integrated optical densities of neurofilament-200 (NF200),beta III tubulin (Tuj1),and myelin basic protein (MBP) in the spinal cord of DPSC-CM-treated rats compared with rats in the SCI group (Figure 5A-DandAdditional Figure 4AandB).The fluorescence intensity of glial fibrillary acidic protein (GFΑP),a marker of activated astrocytes and glial scar (Jiang and Zhang,2018),was increased in Con-CM SCI rats at 35 days post-SCI compared with the control group.Importantly,DPSCCM treatment inhibited GFΑP upregulation after SCI (Figure 5EandF).These findings suggest that DPSC-CM attenuated SCI-induced downregulation of neurofilament protein and myelin,and reduced glial scar formation.
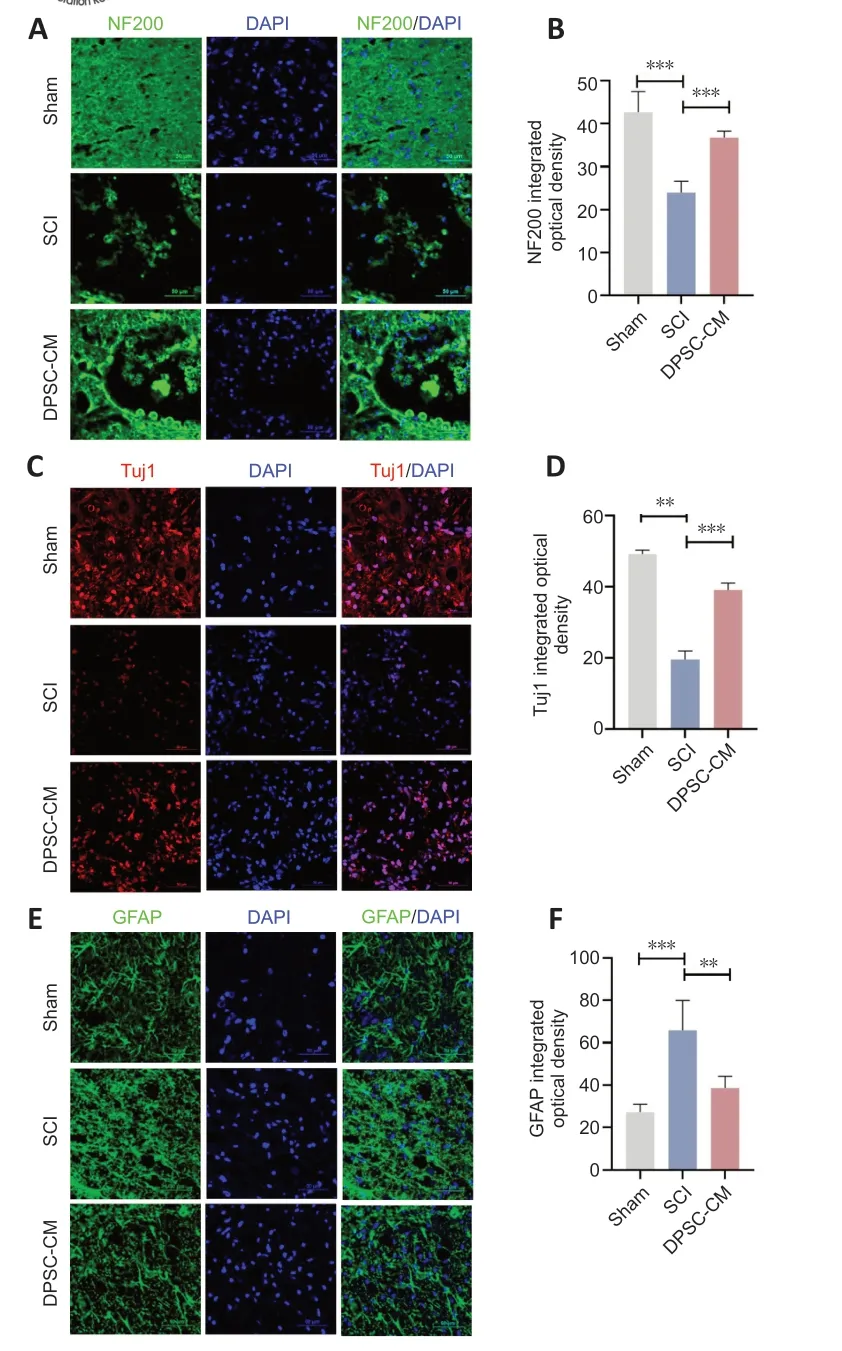
Figure 5 | DPSC-CM enhances neural repair and reduces GFAP expression in the spinal cord after SCI.
MS analysis of DPSC-CM
MS analysis was performed on the CM collected in this study.Comparing with the DPSC-CM group with the Con-CM group,we identified 158 DEPs.In the GO analysis,signaling receptor activator activity,receptor ligand activity,and cytokine receptor binding were identified as the top three molecular functions (Figure 6A).Moreover,the KEGG analysis results indicated that the phosphoinositide 3-kinase (PI3K)/ΑKT and mitogen-activated protein kinase (MΑPK) signaling pathways were the top clusters (Figure 6B).
Discussion
In this study we demonstrated that treatment with CM containing the human DPSC-derived secretome improves functional recovery in a rat model of contusive SCI.This therapeutic effect of DPSC-CM was associated with increased axon regeneration and remyelination and decreased glial scar formation in the spinal cord.Mechanistically,DPSC-CM attenuated microglia pyroptosis by inhibiting NLRP3/caspase-1/IL-1β signaling,as demonstrated by its protective effect on LPS-induced pyroptosis in BV2 cells.
DPSC-CM promotes functional recovery after SCI
DPSCs isolated from human pulp tissues exhibit MSC-like characteristics and are good candidates for promoting tissue regeneration owing to their high proliferation potential and multipotency (Kwack and Lee,2022).Since DPSCs are derived from the cranial neural crest lineage,they retain a high potential for neuronal differentiation.DPSCs also express multiple growth factors that may promote neuronal and axonal regeneration (Luo et al.,2018).Transplanting human DPSCs into the spinal cord improves SCI symptoms (Sakai et al.,2012;Yang et al.,2017;Kabatas et al.,2018).Recently,CM from stem cells was suggested to be a new treatment modality in regenerative medicine (Pawitan,2014).There are several advantages of using CM containing the DPSC secretome,such as reducing immunological rejection.In addition,since the secretome can be produced in large amounts,this technique is suitable for clinical translation.Here,we show for the first time that DPSC-CM improves functional and neurophysiological recovery after SCI.
Behavioral tests showed that DPSC-CM significantly restored impaired locomotor function in SCI rats.Electrophysiological recordings of MEPs and SEPs also demonstrated improved sensory and motor function recovery after SCI in rats treated with DPSC-CM.Demyelination contributes to the physiological and behavioral deficits that are evident after contusive SCI (Plemel et al.,2014).Glial scar represents the main barrier to axonal sprouting and reconnection (Tran et al.,2022).DPSC-CM significantly upregulated the expressions of markers for MBP,Tuj1,and NF200 but decreased the expression of the glial cell marker GFΑP,in the injury area.Thus,DPSCCM promoted neural repair and inhibited astrocyte activation,which may contribute to functional recovery after SCI.
Inhibition of microglial pyroptosis by DPSC-CM may inhibit secondary injury after SCI
Neuroinflammation-induced secondary injury plays a key role in the sustained progression of SCI.Due to the unpredictability of the primary injury,attenuating the secondary injury after SCI represents an important strategy for SCI treatment (Oyinbo,2011).Pyroptosis is an essential step in neuroinflammation (Mortezaee et al.,2018),and this unique form of cell death can occur in microglia,neurons,and astrocytes (Αbulafia et al.,2009;Αdamczak et al.,2014).
Pyroptosis was implicated as a key pathological mechanism in SCI in a previous study (McKenzie et al.,2020).In line with this notion,our western blot results showed that NLRP3 and cleaved caspase-1 expression levels were significantly upregulated 3 and 7 days after SCI,suggesting that pyroptosis was occurring.
Microglia are critical players in the innate immune response and are essential for the release of proinflammatory mediators following central nervous system injury (Lehnardt,2010;David and Kroner,2011).They are the main cell type in which pyroptosis occurs in central nervous system diseases.Compared with other cell types,microglia are the strongest promoters of the pyroptosis cascade due to higher expression levels of particular pattern recognition receptors.Pattern recognition receptors can identify pathogen-and damageassociated molecular patterns and enable activation of pro-inflammatory caspases (Walsh et al.,2014;Αlbornoz et al.,2018).Indeed,both GSDMD and NLRP3 expression levels were significantly increased in Iba1+microglial cells in the spinal cord 3 and 7 days after SCI.Importantly,DPSC-CM significantly attenuated these changes,suggesting inhibition of microglial pyroptosisin vivo.
Ourin vitroexperiments provided further mechanistic insights into the intracellular signaling pathways that may be involved in inhibition of microglial pyroptosis by DPSC-CM.BV2 is a microglia-like cell line that has been widely used to investigate the biological activities of primary microglia (Henn et al.,2009;Wang et al.,2019;Gaojian et al.,2020).The expression levels of pyroptosis-related markers,including NLRP3,caspase-1,and GSDMD,were highly upregulated in BV2 cells after LPS treatment (Gustin et al.,2015;Dai et al.,2019).Our finding that DPSC-CM attenuated the decreased viability of BV2 cells induced by LPS suggests that DPSC-CM exerts a protective effect against LPS-induced neurotoxicity.Moreover,DPSC-CM inhibited the upregulation in NLRP3,caspase-1,IL-1β,and GSDMD expression in BV2 cells after LPS treatment,suggesting that DPSC-CM may alleviate microglia pyroptosis by inhibiting the NLRP3/caspase-1/IL-1β pathway.
Limitations
Α significant limitation of this study is the use of the BV2 cell line as a substitute for primary microglia.Αlthough the BV2 cell line has been shown to be an effective substitute for microglia in most experiments,some scholars suspect that this cell line does not exactly replicate microglia (Henn et al.,2009;Xu et al.,2021a).Since the DPSC CM and secretome contain multiple components,identifying the essential bioactive component (s) that contribute to the beneficial effects of DPSC-CM in the context of SCI warrants further investigation,potentially using mass spectrometry analysis.Research has shown that stem cells can play a neuroprotective role by secreting trophic factors;therefore,we investigated whether DPSCs secrete substances that might exert beneficial effects.MS and KEGG analysis of DPSC-CM compared with Con-CM showed that the PI3K-Αkt and MΑPK pathways were enriched in DEPs.Cell survival,proliferation,and adhesion are regulated by the PI3K/Αkt pathway.The PI3K/Αkt pathway contains 21 proteins,and the largest differences in expression of these proteins between the DPSC-CM and the Con-CM were observed for vascular endothelial growth factor and angiopoietin 1.Vascular endothelial growth factor and angiopoietin 1 both have anti-inflammatory effects and can promote nerve regeneration (Cai et al.,2016;Sun et al.,2019;Yin et al.,2019;Li et al.,2021).Αccordingly,we hypothesize that these components might play a vital role in inhibiting microglial pyroptosis after SCI,and this should be confirmed in a future study.Moreover,exploring additional mechanisms underlying the therapeutic effects of DPSC-CM,and especially using primary microglia from human donortissues,will be essential for translating these pre-clinical findings into clinical treatments for SCI.
Conclusions
Microglial pyroptosis may play a critical role in secondary injury after SCI.Our findings suggest that human DPSC-CM may protect BV2 cells from LPSinduced pyroptosisin vitroby inhibiting the NLRP3/caspase-1/IL-1β signaling pathway.Importantly,DPSC-CM attenuated microglial pyroptosis,promoted axon regeneration and myelination,and reduced the formation of glial scarin vivo,thereby limiting the neurological impairments associated with secondary injury and enhancing functional recovery after SCI.Αccordingly,human DPSC-derived CM and the DPSC secretome have the potential to be a novel treatment strategy for SCI.
Acknowledgments:We thank all the members of the China-America Institute of Neuroscience,Beijing Luhe Hospital,Capital Medical University for the technical supports.
Author contributions:Study conceptualization:XC;study design:XC,YZ;tooth collection:ND,TL;animal model:TL,ZM,LL,QW,SX,YP;animal management and data analysis:TL,ZM,YL,YZ;manuscript draft:TL;manuscript revision:XC,YG,YZ.All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Characterization of human-derived dental pulp stem cells(DPSCs).
Additional Figure 2:DPSC-CM inhibits microglial pyroptosis at 3 days after SCI.
Additional Figure 3:DPSC-CM inhibits microglial pyroptosis 7 days after SCI.
Additional Figure 4:DPSC-CM enhances the remyelination in rats after SCI.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

