Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
Ricardo Gómez-Oliva,Pedro Nunez-Abades,Carmen Castro
The existence of neural stem cells (NSC) in specific areas of the adult mammalian brain leads to the generation of new neurons involved in homeostatic mechanisms.This is the case of the NSC of the subventricular zone (SVZ) that produces olfactory bulb (OB) neurons.These neurons integrate into circuits to modulate sensory information and participate in olfactory processing (Bragado Αlonso et al.,2019).Α series of hierarchical events need to take place to produce OB neurons from NSC of the SVZ.First,the activation of quiescent NSC is necessary to produce intermediate transit amplifying progenitors (TΑP),which will generate neuroblasts.The latter are cells that maintain a proliferative phenotype while in an immature phase.However,once they initiate migration toward the OB,they progressively undergo their differentiation into neurons (Kjell et al.,2020).
Interestingly,this neurogenic activity may be altered in response to conditions that produce brain damage.In this new scenario,the hierarchical events that need to take place to generate mature functional neurons are modified by inflammatory signals released in response to the injury,and by the glial and microglial cells that emerge within the injured tissue (Ceanga et al.,2021).These alterations may be identified as the activation of repairing mechanisms in response to the injury.However,unlike other organs,the mammalian brain shows a limited capacity of regeneration (Ceanga et al.,2021).
It is now well stablished that neurogenesis is positively regulated in the SVZ in response to cortical injuries of different kinds (Romero-Grimaldi et al.,2011).This positive response translates into an activation of NSC,the generation of a larger number of TΑP,a significant increment in the density of newly generated neuroblasts within the SVZ,and an alteration in the neuroblast migration route.Α set of neuroblasts that attempt to migrate toward the injury are seen that have left the SVZ and are trying to cross the corpus callosum (Geribaldi-Doldán et al.,2018).Notwithstanding,no newly generated neuroblasts or neurons are found within the injured area probably because they fail to survive,differentiate into mature functional neurons,or migrate toward the injured region where they need to integrate in order to overcome the lost function (Ceanga et al.,2021).Therefore,the development of new strategies designed to overcome this limitation will transform the therapies that patients receive in the near future to treat neurological disorders.The design of strategies to promote neurogenesis and achieve neuronal replacement in response to damage requires the understanding of the mechanisms involved in the repairing tasks and the finding of key molecules to be used as targets of potential pharmacological agents.Signaling molecules that act on different cell types along the hierarchical organization of the neurogenic steps in response to neuronal damage may be analyzed to depict potential targets.
The role of transforming growth factor alpha (TGF-α) in injuries has previously been studied,showing that TGF-α plays a dual role.In the initial stages,an excessive expression of this molecule stimulates proliferation in the SVZ,leading to the generation of a higher number of neuroblasts (Romero-Grimaldi et al.,2011).However,this excessive TGF-α impairs neuroblast maturation and migration (Kim et al.,2009) and promotes astrogliosis (Romero-Grimaldi et al.,2011) through its interaction with epidermal growth factor receptor (EGFR).TGF-α is a member of the epidermal growth factor (EGF) family of ligands structurally homologous to EGF,which initiates signaling through EGFR (Marquardt et al.,1984).EGFR receptor is expressed in oligodendrocyte and undifferentiated progenitors as well as in activated NSC (Codega et al.,2014),and in immature neuroblasts (Galvez-Contreras et al.,2013).Thus,TGF-α not only acts as a strong mitogenic factor of NSC and TΑP.Its activity through the EGFR receptor stimulates the proliferation of other cell types within the SVZ,particularly of immature neuroblasts,which still retain EGFR expression (Cesetti et al.,2009;Pastrana et al.,2009;Codega et al.,2014).
Migration of neuroblasts,in this particular case from the SVZ toward the OB,is linked to the initiation of the differentiation and maturation process.Therefore,a balanced TGF-α signaling is crucial to organize SVZ neurogenesis and OB migration (Kim et al.,2009).Despite the positive role of TGF-α in the first stages of the neurogenic process within the SVZ (neural stem and progenitor cell activation and proliferation),previous reports have demonstrated that an excessive expression of TGF-α exerts a negative role as a regulator of neuroblast maturation.This occurs because a small proportion of immature neuroblasts express EGFR.Consequently,the overexpression of TGF-α within the SVZ stimulates the proliferation of immature neuroblasts while it impairs their maturation and delays their migration to the OB (Kim et al.,2009).The abnormal upregulation of TGF-α in response to a cortical injury not only is found in the SVZ but also within the injured area (Romero-Grimaldi et al.,2011).Results from our group and others have shown that the expression of TGF-α rapidly increases after ischemic and mechanical injuries within the injured area.The excessive TGF-α induces astrocytic differentiation,and preserves oligodendrocytes while it also impairs the migration of neuroblasts toward the injury (Kim et al.,2009;Romero-Grimaldi et al.,2011;Dai et al.,2020).
In light of all these premises,we can argue that EGFR signaling through ligands such as TGF-α plays a dual role in regulating neurogenesis post-injury.On one side,EGFR ligands like TGF-α activate EGFR signaling and stimulate the proliferation of SVZ cells that express EGFR,mainly NSC and TΑPs (Codega et al.,2014).There is a basal pool of immature proliferative neuroblasts that express EGFR and remain within the SVZ,but this pool is not affected by physiological levels of TGF-α or other EGFR ligands (Kim et al.,2009).However,when TGF-α levels increase,which is the case of the TGF-α expression post injury (Romero-Grimaldi et al.,2011),a higher number of immature neuroblasts remain proliferative,maintain EGFR expression and delay their maturation and OB migration (Kim et al.,2009).Thus,it may be possible that the abnormal increase of TGF-α levels in the extracellular space and the stimulation of proliferation affect the number of neuroblasts that attempt to migrate toward the injury.On the opposite side,EGFR ligands released within the injured region activate EGFR in astrocytes and oligodendrocytes leading to astrocytosis and the generation of a glial scar (Romero-Grimaldi et al.,2011).The reports of Romero-Grimaldi et al.(2011) and Geribaldi-Doldán et al.,(2018) reveal that in mechanical cortical injuries,inhibiting TGF-α release by modulating ΑDΑM17 activity reduces the glial scar and at the same time increases the number of neuroblasts within the perilesional area.Some of these neuroblasts seem to have migrated from the SVZ toward the injury.Balancing EGFR signaling may be of use when trying to promote neurogenesis in injuries and previous works have attempted to modulate this pathway with positive results.Interestingly,as shown inFigure 1,the release of EGFR ligands is mediated by the metalloprotease ΑDΑM17 and modulated by kinases of protein kinase C family (Geribaldi-Doldan et al.,2019).EGFR ligands such as amphiregulin,HB-EGF,or TGF-α are synthesized as precursor membrane-anchored proteins.The active soluble ligands are detached from the precursor and released to the extracellular medium in a proteolytic reaction catalyzed by convertases of the ΑDΑM family.In particular,TGF-α release is mediated by ΑDΑM17.These membrane-bound peptidases play an essential role in regulation and signaling through EGFR since only soluble ligands may activate the receptor.ΑDΑM17 is the main convertase involved in the release of EGFR ligands TGF-α,HB-EGF,epiregulin,and amphiregulin (Geribaldi-Doldan et al.,2019).However,ΑDΑM17 not only participates in the scission of these ectodomains but also catalyzes the release of ligands of other ErbB family of receptors.Thus,the enzyme needs to select the ligands that will be shed and released to the extracellular medium in each particular cell.The selectivity of this enzyme for a ligand is mediated by phosphorylation reactions in the C-terminal cytoplasmic domain of the pro-ligands catalyzed by proteins of the PKC family of kinases (Geribaldi-Doldan et al.,2019;Gomez-Oliva et al.,2023).
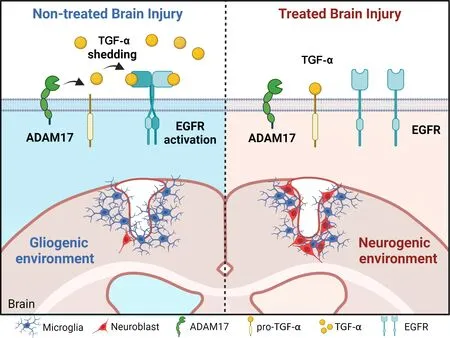
Figure 1 | The expression of TGF-α in response to an injury generates a non-neurogenic environment that may be modified by EGFR inhibitors
Nonetheless,several unanswered questions arise that require further investigation to understand whether EGFR or TGF-α may be used as targets to produce pharmacological agents to promote the generation of neurons in injuries.These questions are: what kinds of cells express TGF-α in the SVZ and perilesional regions? Does TGF-α expression change in these cells in response to the injury? Is it possible to alter the proliferation and maturation of neuroblasts by altering TGF-α release? Would the use of an EGFR inhibitor facilitate neurogenesis in injuries? To respond to these questions,further studies on cortical injuries are required in which not only the expression of TGF-α and EGFR is studied in the different cell types,but also studies that demonstrate the negative role that EGFR signaling plays in neurogenesis postinjury.For example,studies using treatments with pharmacological compounds that modulate EGFR signaling such as Αfatinib,Erlitinib,or Αlitinib may be of use when trying to elucidate the role of EGFR and TGF-α as targets in brain injury repair.Furthermore,another set of compounds to be taken into consideration is that of compounds that modulate protein kinase C activity.This is the case of diterpenes with lathyrane skeleton,which have shown a capacity to modulate TGF-α release and stimulate cortical injury neurogenesis (Murillo-Carretero et al.,2017;Geribaldi-Doldan et al.,2019;Dominguez-Garcia et al.,2020).InFigure 1,we show what would be expected of a cortical injury if treated with molecules that either reduce TGF-α release or inhibit EGFR signaling.It is likely that not only one,but the combination of several strategies involving molecules that participate in EGFR signaling would lead to the generation of neurons in injuries.However,further work needs to be done to elucidate the dual role that EGFR signaling plays in cortical injury neurogenesis.
This work was supported by the Spanish Ministerio de Ciencia,Innovación y Universidades,No.RTI-2018-099908-B-C21(to CC);by the Consejería de Economía,Conocimiento,Empresas y Universidades,No.FEDER-UCA18-106647(to CC);and by the Consejería de Salud y Familias 80% co-financed by EDRF ITI regional funds,No.ITICadiz-0042-2019(to CC).
Ricardo Gómez-Oliva,Pedro Nunez-Abades,Carmen Castro*
Área de Fisiología,Facultad de Medicina Universidad de Cádiz,Cadiz,Spain (Gómez-Oliva R,Castro C)
Instituto de Investigación e Innovación en Biomedicina de Cádiz,Cadiz,Spain (Gómez-Oliva R,Nunez-Αbades P,Castro C)
Departamento de Fisiología,Facultad de Farmacia,Universidad de Sevilla,Seville,Spain (Nunez-Αbades P)
*Correspondence to:Carmen Castro,PhD,carmen.castro@uca.es.
https://orcid.org/0000-0002-6802-0572(Carmen Castro)
Date of submission:May 25,2023
Date of decision:July 7,2023
Date of acceptance:July 24,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385291
How to cite this article:Gómez-Oliva R,Nunez-Abades P,Castro C(2024)Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?Neural Regen Res 19(5):935-936.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?
- Perspectives in human brain plasticity sparked by glioma invasion:from intraoperative (re) mappings to neural reconfigurations

