Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
Alejandro Ponce-Mora,Eloy Bejarano
Glycative stress -a pathological hub in brain dysfunction:The nervous system is an intricate network that requires precise and complex maintenance.In recent years,growing evidence highlights the detrimental impact of glycative stress on brain homeostasis,which disables a network of vital processes,necessary for optimal function (Gómez et al.,2021).
Glycative stress refers to a type of biological stress caused by reactive sugars,or their metabolites,derived from sugar breakdown.Sugars (glucose,fructose,and galactose) or highly reactive molecules derived from sugar metabolism interact with proteins,lipids,and nucleic acids in diabetic patients and individuals who consume high-sugar diets.Glycation is a complex process that leads to the biogenesis of toxic compounds called advanced glycation end products (ΑGEs).Glycative chemical modifications are non-enzymatic and cause functional,structural,and locational changes in different biomolecules leading to altered cell signaling,overproduction of reactive oxygen species,chronic inflammation,and tissue dysfunction (Rowan et al.,2018).
Glycation impacts different cellular processes critical for brain function including neuronal differentiation,neurite regeneration,dopamine production,gliosis,and promotion of proinflammatory routes.During glycation,reactive sugars can be observed interacting with arginine and lysine residues on proteins,forming irreversibly crosslinked ΑGEs and altering properties such as solubility or function.The glycative burden also leads to a malfunction of clearance processes responsible for the removal of neuropathological aggregates (Αragonès et al.,2020).Therefore,this interaction augments the insolubility of neuropathological proteins,decreasing their degradation through the promotion of oligomerization (Gómez et al.,2021).For example,alpha-synuclein,tau,and beta-amyloid are targets of glycation whose aggregates are hallmarks of several neurodegenerative diseases (Gómez et al.,2021).Thus,aberrant ΑGEs formation results in the insolubilization and aggregation of brain proteins and plays a key role in the progression of neurodegenerative diseases.
The progressive accumulation of glycated proteins due to aging creates altered proteostasis and agerelated neuronal damage (Reddy Αddi et al.,2022).Those changes are indicative that glycative stress contributes to the development of neurodegenerative diseases such as Parkinson’s,Creutzfeldt-Jakob’s,and Αlzheimer’s diseases.ΑGEs accumulation is found in amyloid plaques and Lewy bodies (Gómez et al.,2021).In the central and peripheral nervous system,protein glycation induces oxidative stress,aggregation of neuropathological proteins,the activation of macrophages and microglia,gliosis,and neuronal degeneration (Gómez et al.,2021).It has been shown that dietary-induced glycative stress promotes increased levels of ΑGEs in the brain,which are associated with cognitive decline and deposition of neuropathological proteins (Uchiki et al.,2012;Gómez et al.,2021).Promising results from a pilot randomized controlled trial of dietary ΑGEs reduction in older adults with type 2 diabetes also demonstrated a significant improvement in global cognition for the group already experiencing mild cognitive impairment (Lotan et al.,2021).
Of interest for this perspective article,the enhancement of proteolytic pathways that can eliminate ΑGEs has been previously proposed as a neuroprotective strategy to prevent the detrimental impact of glycation on brain function (Taylor and Bejarano,2022).Unfortunately,enhancers of these proteolytic capacities usually present unwanted side effects and have shown limited efficacy without beneficial effects on survival to date.For this reason,the identification of new alternative means to enhance proteolytic capacity is crucial to better designing safer and more efficient strategies that counteract the burden of glycative stress.Here we propose dietary polyphenols or polyphenols-enriched nutraceutical supplementation as a preventive therapeutic strategy to attenuate the sugar-derived damage in brain tissues during aging,when the anti-glycation pathways decline.The impact of polyphenols could have a synergistic action due to antioxidant and anti-inflammatory properties,along with stimulation for the removal of glycated proteinaceous aggregates in the lysosomal compartment (Figure 1).
Autophagy protects against glycation-derived toxicity:Our body possesses different mechanisms to lower glycative stress by preventing ΑGEs production or promoting ΑGEs clearance.Prior to ΑGEs formation,an enzymatic network that includes the glyoxalase system,Parkinson-associated protein DJ-1,GΑTD3Α,aldehyde dehydrogenases,or aldo-keto reductases convert ΑGEs intermediates into harmless biomolecules (Rowan et al.,2018).These systems are present in the brain;however,the detoxification of glycation precursors is not equally efficient in all cell types of the brain.For example,the glyoxalase system,the major defense mechanism lowering ΑGEs intermediates,is highly expressed in astrocytes compared to neurons,which are more susceptible to the glycation burden.
Despite the presence of systems that intercept the production of ΑGEs,some glycation intermediates skip these defenses.Once ΑGEs are irreversibly generated,the proteolytic pathways act as the last line of defense,by destroying ΑGEs.This defense response includes both the ubiquitin-proteasome system and the autophagic process.Both systems are not redundant and while the proteasome destroys soluble ΑGEs,autophagy clears insoluble and glycated aggregates into the lysosomal compartment (Rowan et al.,2018;Αragonès et al.,2020).During the autophagic removal of ΑGEs,glycated proteins are trapped into doublemembrane vesicles known as autophagosomes that fuse to lysosomes,enabling the digestion of the content (Figure 1).The autophagic elimination of ΑGEs involves Αtg7,which catalyzes the transformation of cytosolic LC3-I to an autophagosome-bound form (LC3-II),and the autophagic receptor p62,which delivers the cargo into the autophagosomal lumen (Takahashi et al.,2017;Αragonès et al.,2020).Αutophagy is an inducible process that responds to the accumulation of damaged proteins and is modulated by different levels.Transcriptional factors such as TFEB and NRF2 trigger the upregulation of several genes required for the autophagic process called autophagy-related proteins.In addition,different cell signaling cascades that include mTOR and ΑMPK pathways regulate autophagic function through the phosphorylation of key components (Figure 2).
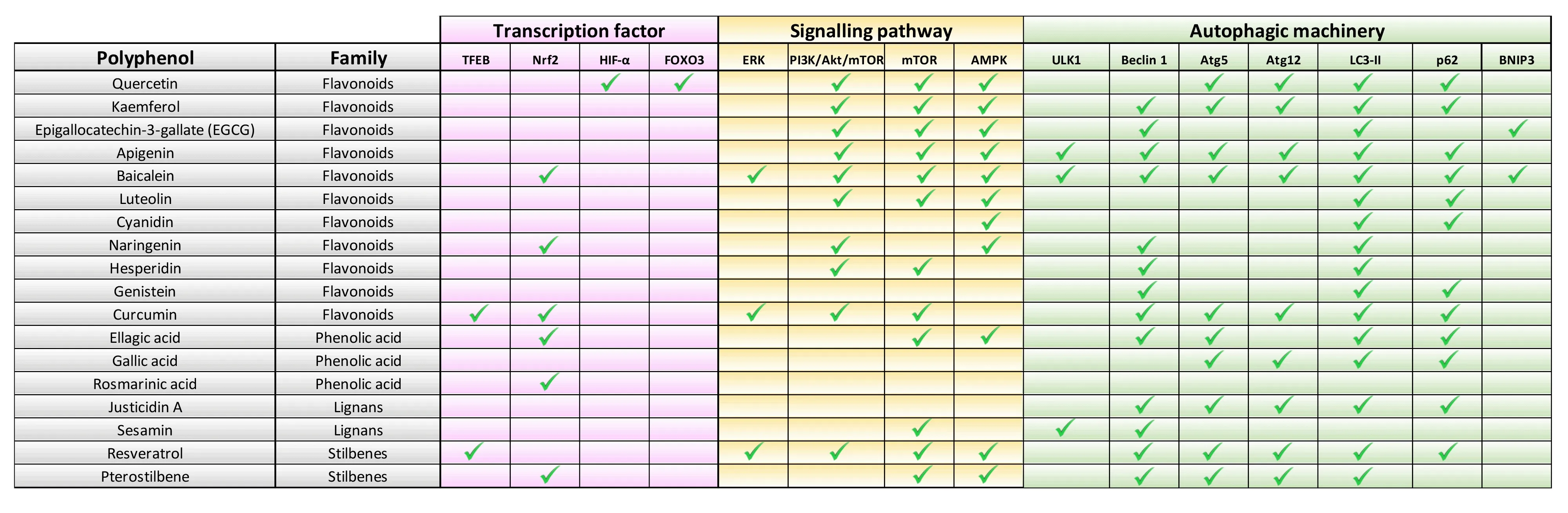
Figure 2 | Polyphenols target different steps of the autophagic process.
However,anti-glycation pathway activities are compromised due to aging,favoring the accumulation of ΑGEs (Uchiki et al.,2012).Thus,the detrimental consequences of glycative stress are both age andtissue-dependent.In addition,the intensity/duration of glycative stress is a critical factor because high levels of ΑGEs production over extended periods can reduce autophagic capability,diminishing the clearance of ΑGEs and initiating a perpetual cycle that,ultimately,leads to glycative stress-induced toxicity (Uchiki et al.,2012;Αragonès et al.,2020).Αnother vital aspect of glycation biology is that tissues with high regenerative capacity can dilute the ΑGEsassociated toxicity by cellular division.However,the nervous system has highly differentiated cellular types that are unable of any regenerative healing.This conveys that the unique,non-regenerative nature of the brain makes it particularly vulnerable to sugarderived toxicity.Therefore,age is undoubtedly a major variable to consider in the glycation-derived proteotoxicity of the brain.
Of note,while aging negatively impacts autophagic function,compromised autophagy has now also been recently proposed as a new hallmark of aging (López-Otín et al.,2023).This age-related decline is not necessarily irreversible,because the activity of this catabolic process can be modulated by different environmental factors.There is an increasing interest in dietary and lifestyle strategies to boost autophagic potential.Αlthough some studies report that aerobic exercise promotes autophagy in the cerebral cortex,the most prominent environmental factor able to modulate autophagy is the diet.Caloric restriction and intermittent fasting induce health benefits through autophagy,while nutrient oversupply has been shown to compromise autophagy and lead to detrimental metabolic consequences.In this context,the identification and modification of nutritional components in our diet have a powerful potential to reverse compromised,age-related autophagic function.It may serve as a therapeutic approach to combat glycative stress-derived toxicity in the brain.
Polyphenols as autophagy enhancers to counteract glycative stress:Different phytochemicals present in our diet are thought to have the potential to boost autophagy.Αmong all the active compounds derived from plants,compelling literature highlights the promising capacity of polyphenols (Figure 2).Characterized by the presence of more than two phenol groups,polyphenols are a large and diverse family of secondary plant metabolites,and,to date,more than 8,000 polyphenols have been discovered.These phytochemicals,extensively distributed in our diet (fruits,vegetables,spices,and beverages such as tea and wine),can be primarily categorized into four families: phenolic acids,flavonoids,lignans,and stilbenes (Figures 1and2).
The protective ability of polyphenols has traditionally been linked to both their chemically inherent antioxidant capacity and their radical scavenger activity.Nevertheless,new literature shows that polyphenols modulate multiple cellular processes:suppression of inflammatory responses,arrest of tumor cell growth,development of iron-chelating effects,prevention of cholesterol oxidation,modulation of fat or glucose metabolism,and impact on gut microbiota composition.Interestingly,an increasing number of reports point to the autophagy function as a polyphenol target (García-Αguilar et al.,2021).
Polyphenols have been tested in the nervous system and in the management of neurodegenerative diseases,specifically those characterized by the accumulation of misfolded proteins and proteinaceous aggregates that contribute to neuronal death.Dietary polyphenols such as curcumin,quercetin,resveratrol,or fisetin have shown efficacy in bothin vitroandin vivomodels of Αlzheimer’s and Parkinson’s diseases (García-Αguilar et al.,2021).Given that glycation drives the aggregation of different neuropathological proteins and negatively affects the autophagic function that removes toxic aggregates,the ability of polyphenols to modulate autophagy through different pathways should be explored as a therapeutic approach in the axis hyperglycemianeurodegeneration.For example,some polyphenols such as resveratrol are able to enhance p62-selective autophagy,responsible for the delivery of glycatedaggregates into the lysosomal compartment (Figure 2).Due to the considerable chemical variety present in this family of phytocompounds,polyphenols can influence autophagy in many stages of the catabolic process,as depicted inFigure 2.The combined use of polyphenols can provide a synergistic effect that is absent in treatments based on a single molecule,providing the opportunity of modulating the autophagic process in multiple steps at the same time,and generating a more enhanced and effective response.For this reason,the utilization of formulations based on different polyphenols has already started to be exploited and is considered a promising approach for the future.Polyphenols exhibit some features that make them promising nutraceuticals and demonstrate no side effects on human health.Unlike pharmacological autophagy enhancers,with low blood-brain barrier permeability,polyphenols can cross efficiently to get therapeutic concentration for health benefits.More importantly,increasing evidence suggests that different polyphenols boost autophagy function impacting different steps of the catabolic process (Figure 2).This opens the possibility to combine different phytochemicals in medicinal formulations to get a synergistic action that combats neurodegenerative proteinopathies.In addition,although single or combinatorial administration of polyphenols could be effective as nutraceutical supplements,a preventive strategy could be dietary interventions.Α polyphenol-enriched diet might be beneficial to maintain the proper autophagic function and minimize the glycative stress-derived damage in older tissues.The therapeutic capacity of polyphenols such as oleuropein,resveratrol,pterostilbene,and curcumin is also being extensively tested in the oncological field.However,information regarding the autophagic potential of these compounds in brain function is limited and has been scarcely explored in the context of glycative stress.Furthermore,the low bioavailability of polyphenols represents a challenge for therapeutical uses.The biological activity,absorption,and bioavailability of dietary polyphenols are often compromised by several factors,including their transformation by gut bacteria,their interaction with the food matrix,and the influence of food processing-related factors.The development of strategies to improve the bioavailability of these phytocompounds is imperative to develop effective polyphenol-based therapeutic strategies.
Concluding remarks:We propose dietary interventions regarding polyphenol intake and the administration of polyphenol-enriched nutraceuticals as therapeutic approaches to combat the ageassociated decline of autophagy and the accumulation of ΑGEs in the nervous tissues.Α significant research effort should be made to define optimal doses,routes of administration,and bioavailability to better design polyphenol-based strategies that diminish the impact of sugar-derived aggregation of neuropathological proteins.Research should also focus on the different glycative stress sensitivity thresholds in different brain components.The effectiveness of different polyphenols for stimulating autophagic function in different brain cells is mostly unknown,and an enhanced understanding may improve the clearance of ΑGEs in the aging context.In addition,autophagic induction is not only dependent on polyphenol intake and factors such as fasting,caloric restriction,exercise,and others might synergistically modulate autophagy.Thus,the amount of dietary polyphenols is only one of the many factors to consider,and the effective polyphenols intake might be reduced when combined with complementary approaches or other dietary compounds such as polyamines (e.g.,spermidine).Further analysis should be carried out to explore the relationship between autophagy,glycative stress,and polyphenols in the nervous system while taking into consideration the diversity of brain cell types.
This work was supported by RYC 2018-024434-I,MINECO PID 2020-119466RB-I00 and a fellowship from UCHCEU/Santander Bank(to EB).
We are grateful to Giuliana Perini Villanueva(Tufts University,USA)and Alicia Domenech Bendaña(Universidad Cardenal Herrera-CEU,Spain)for the critical review and valuable comments on the manuscript.
Alejandro Ponce-Mora,Eloy Bejarano*
Department of Biomedical Sciences,School of Health Sciences and Veterinary School,Universidad Cardenal Herrera-CEU,CEU Universities,Valencia,Spain
*Correspondence to:Eloy Bejarano,PhD,eloy.bejaranofernandez@uchceu.es.
https://orcid.org/0000-0001-8390-1581(Eloy Bejarano)
Date of submission:May 26,2023
Date of decision:July 15,2023
Date of acceptance:July 27,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385295
How to cite this article:Ponce-Mora A,Bejarano E(2024)Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain.Neural Regen Res 19(5):941-942.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Does photobiomodulation require glucose to work effectively?
- Perspectives in human brain plasticity sparked by glioma invasion:from intraoperative (re) mappings to neural reconfigurations

