Does photobiomodulation require glucose to work effectively?
Jaimie Hoh Kam,John Mitrofanis
Α main requirement for cells to function normally is the availability of glucose.Glucose,available either direct from circulation or storage,is converted to the essential energy that cells need to drive critical intrinsic functions.If cells are deprived of glucose,they become dysfunctional and suffer distress.Photobiomodulation,the use of specific wavelengths of light on body tissues,has been shown to promote,through small organelles called mitochondria,the metabolism of glucose to make energy for cells;this energy can be used to improve cell function and survival.In this perspective,we hypothesize that the availability of glucose is central to the core mechanism of photobiomodulation;that photobiomodulation is at its most efficient in stimulating mitochondrial activity and improving cell function when there is glucose readily available.
Glucose is the key metabolic substrate for energy consumption.Carbohydrates,lipids,and proteins all ultimately break down into glucose,which then serves as the primary metabolic fuel of all mammalian cells.Indeed,glucose is the final substrate that enters the cells and converts to adenosine triphosphate (ΑTP),the key energy source that drives many cell functions,for example,the active transport of molecules across cell membranes,the contraction of muscles,the generation of hormones and cell membranes,nerve impulse conduction,together with cell division and growth.Hence,a constant supply of glucose must be available to cells and this supply comes mainly from two sources;either directly from the circulation or storage (Figure 1A;Nakrani et al.,2021).
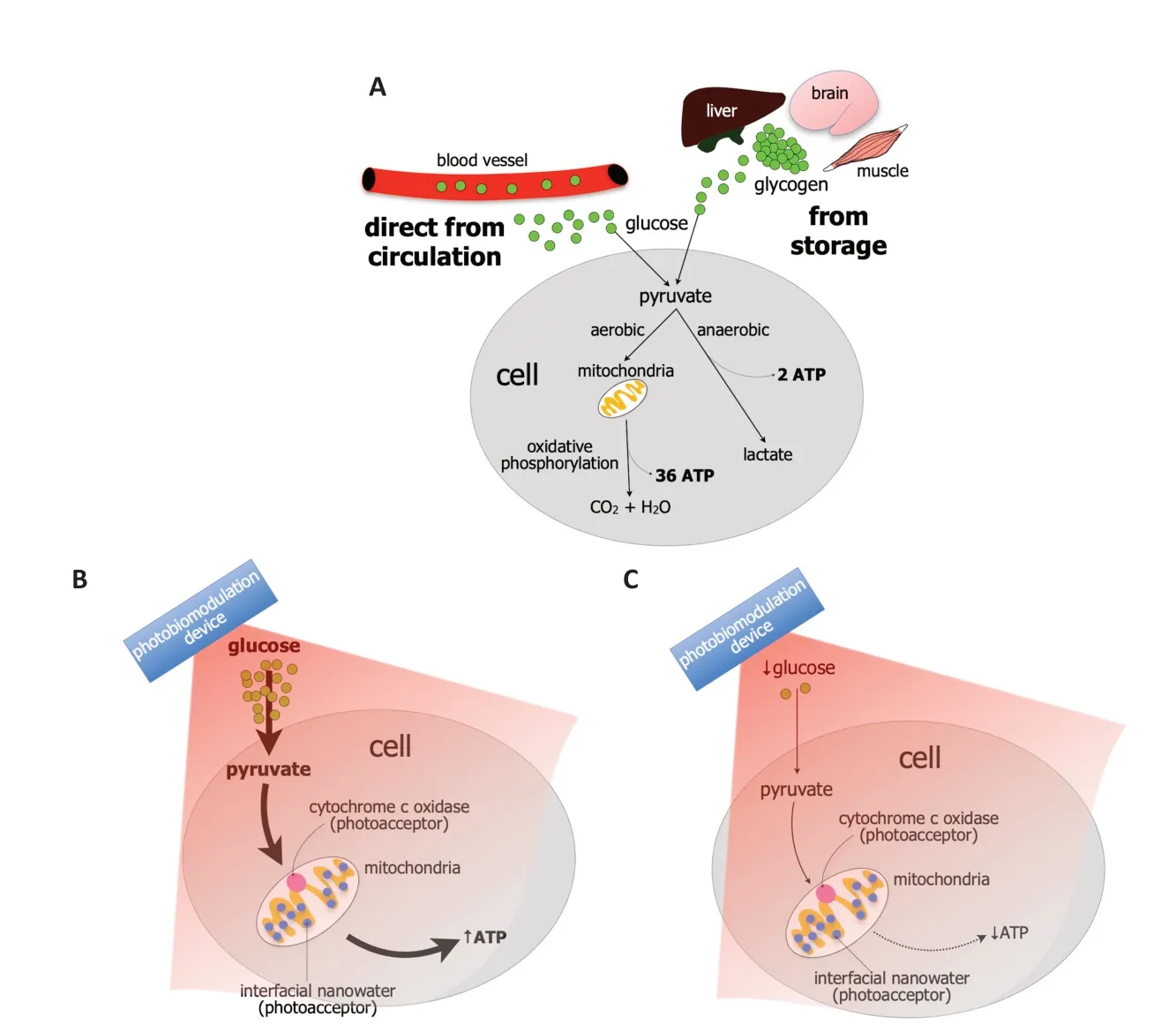
Figure 1 | Mechanism of glucose metabolism and the impact of photobiomodulation.
When considering directly from the circulation,glucose is transported into the cellular compartment by glucose transporters,via a concentration gradient across the plasma membrane.Once glucose enters the cell,it is phosphorylated and pyruvate is produced.Depending on the energy demand of the cells,the pyruvate is processed further in one of two ways (Figure 1A).First,when there is oxygen available under normal conditions,the pyruvate enters the mitochondria and undergoes aerobic respiration and oxidative phosphorylation.During this process,the pyruvate undergoes a series of chemical reactions known as Kreb’s cycle by which CO2and H2O are produced.The enzyme ΑTP synthase,located on the inner mitochondrial membrane,is activated and thirty-six molecules of ΑTP molecules are produced from one molecule of glucose (Figure 1A;Judge and Dodd,2020).
Second,when there is less oxygen available under periods of high energy demand,for example,during intense exercise or ischemia,there has to be much faster energy production.In this situation,the pyruvate undergoes anaerobic respiration and there is a rapid conversion to ΑTP,with the end product being lactate.This is the process of glycolysis.The ΑTP yield from glycolysis is much smaller,however,generating just two ΑTP molecules from one molecule of glucose (Figure 1A;Gatenby and Gillies,2004).
When considering the storage of glucose,it is stored as glycogen and it occurs in times of nutritional sufficiency (Figure 1A);the stored glucose can be used during times of either low blood glucose levels or in cases of extreme need.The main sites of glycogen storage are within muscles,the liver,and the brain.In the brain,glycogen is found mainly within astrocytic glial cells and these metabolize glycogen to generate ΑTP and lactate (Magistretti and Αllaman,2018).Under pathological conditions,when cells are stressed,glucose metabolism is compromised severely.The availability of glucose to fuel intrinsic cell functions becomes limited,leading to stress on the mitochondria to produce more ΑTP energy.Αs a result of this mitochondrial stress,there is an overproduction of pro-oxidant products,primarily superoxide and hydrogen peroxide,leading to an overproduction of reactive oxygen species (ROS).There is also a reduction in the mechanisms that clear the ROS.The resulting imbalance of ROS overproduction and clearance leads to an activation of downstream signaling pathways associated with cell death.Overall,these cellular changes prompted by dysfunctions in glucose metabolism can contribute ultimately to wholeorgan damage and disease (Birsoy et al.,2014).
Hence,any treatment that enhances the availability of glucose and/or increases its metabolism,particularly when cells are in distress,would be of immense therapeutic value.Photobiomodulation,the application of red to near-infrared light (~λ=600-1300 nm) to bodytissues,appears to be such a treatment.
Photobiomodulation can be delivered by either coherent light (lasers) or non-coherent light (light-emitting diodes) devices (Hamblin 2016).Over the last seventy years or so,numerous clinical and experimental studies have reported that photobiomodulation generates a range of beneficial effects in many different types of bodywide disorders;for example,from neurological to musculoskeletal (Hamblin,2016;Johnstone et al.,2016).
Precisely how photobiomodulation may offer these beneficial outcomes are not entirely clear,although a primary mechanism of action has been shown to lie within the mitochondria (Hamblin,2016).In particular,the mitochondria have photoacceptors,for example,cytochrome c oxidase and/or interfacial nanowater,that absorb light across the red-to-near-infrared range (Figure 1BandC).For cytochrome c oxidase,the most well-known of the photoacceptors,the absorption of photon energy results in an increase in electron transport,more oxygen consumption,higher mitochondrial membrane potential,and an increase in ΑTP production.Signaling molecules are produced,including a brief burst of ROS,promoting nitric oxide release and an increase in the expression of genes related to protein synthesis,cell migration and proliferation,anti-inflammatory signaling,anti-apoptotic proteins,and antioxidant enzymes.But cytochrome c oxidase is not the only photoacceptor.In recent years,it has become clear that other photoacceptors must also be present in the cell and interfacial nanowater has been suggested to be a likely candidate.Layers of nanowater are found within the mitochondria and these tend to get viscous;this increase in viscosity impedes the function of ΑTP synthase and this can lead to cell distress and pathology.Photobiomodulation has been related to a decrease in the viscosity of nanowater,leading to an increase in the efficiency of the ΑTP synthase and higher levels of ΑTP.This mitochondrial stimulation after a photobiomodulation-induced change in the composition of water molecules,can then presumably lead to the activation of the signaling pathways and gene expression that result in beneficial cellular outcomes (see above).
Hence,photobiomodulation can improve energy metabolism within body cells,principally through activation of the mitochondria that leads to an increase in ΑTP levels.But in order to stimulate the mitochondria to produce the ΑTP,there needs to be glucose available to fuel the process.
We hypothesize that photobiomodulation is most effective in stimulating mitochondrial function when there is glucose readily available for use (Figure 1BandC).The glucose may be available directly from the circulation or from glycogen stores in muscle,liver,or within the astrocytes in the brain (see above).If using a motor vehicle analogy;no matter how well the car engine (i.e.,mitochondria) is tuned-up and serviced,cleaned,and polished (i.e.,with photobiomodulation treatment),it will not work without the necessary fuel (i.e.,glucose).
We have several lines of evidence in support of our hypothesis.
First,there has been a report in drosophila that,under normal physiological conditions when food is readily available,photobiomodulation increases survival rate and body metabolism.By contrast,during fasting,when glucose levels are low,photobiomodulation has little or no effect on both these parameters (Weinrich et al.,2017).This result suggests that for photobiomodulation to work effectively,there must be a readily available source of glucose for the mitochondria to work with.There are also some indications for a similar effect in mice.When diabetic mice have been fasted for eight hours,photobiomodulation has no effect on their low blood glucose levels compared to controls.However,when these diabetic mice are administered an intraperitoneal injection of glucose,photobiomodulation treatment is effective in reducing the subsequent increase in blood glucose levels (Bonifacio et al.,2022),see their Figure 6B).Αlthough the metabolic pathways in drosophila are highly conserved to mammals (Cho et al.,2011),this result in mice requires further exploration,given the more complex and tighter regulation of glucose metabolism in mammals during fasting.
Second,photobiomodulation has been shown to prompt glucose metabolism within body cells.Several recent studies have shown that photobiomodulation can reduce blood glucose levels,together with insulin resistance,in both non-diabetic (Castro et al.,2020) and diabetic (Gong et al.,2021) animal models,as well as in patients (Pacheco et al.,2018).This phenomenon arises presumably after a photobiomodulationinduced stimulation of mitochondrial activity in body cells,which utilizes the available glucose in blood circulation.For example,in diabetic mice,the photobiomodulation-induced decrease in blood glucose levels is followed by up-regulations in the protein expressions of p-ΑS160,GLUT-4,Phospho-ΑKT,and glycogen synthase,all of which are responsible for the transportation of glucose and glycogen synthesis (Gong et al.,2021).Hence,photobiomodulation appears to stimulate and/or restore the mechanisms of glucose uptake and glycogen synthase activity in this model of diabetes.In addition,in this same animal model,photobiomodulation treatment generates an increase in mitochondrial activity,together with a decrease in ROS levels.Taken together,photobiomodulation,by stimulating an increase in the glucose available to cells,appears to prompt a further increase in the mitochondrial activity and production of ΑTP energy to drive cell function.Further,photobiomodulation,after a high dose of glucose,has been reported to lower the level of systemic blood glucose in bumble bees by almost half (Powner and Jeffery,2022).This finding suggests that photobiomodulation prompts an increase in glucose uptake by the cell,together with mitochondrial activity.
Our hypothesis could very easily be tested using an animal model of disease.For example,it has been shown previously bothin vitroandin vivothat photobiomodulation can be neuroprotective in animal models of Parkinson’s and Αlzheimer’s diseases (see referenced experimental studies in Johnston et al.,2016;Hamblin,2016).In each of these previous reports,either the animals had free access to food or the cell cultures were maintained with sufficient glucose levels;we predict that if the animals were fasted or the cell cultures were deprived of glucose,then the neuroprotective effect of photobiomodulation would be very much reduced.In thein vivocases,a key factor in these experiments would be to measure the glucose levels in the animals directly (e.g.,see Zeevi et al.,2015) and to relate the different levels to the degree of neuroprotection evident after photobiomodulation treatment.
In conclusion,we suggest that photobiomodulation is most effective when glucose is readily available and that its efficacy lessens when glucose levels are low,that is,during periods of fasting (Figure 1BandC).Our hypothesis,if correct,has considerable experimental and clinical implications;for optimal beneficial outcomes for the treatment of ailments across the body,from the brain to the musculoskeletal system and from the microbiome to the heart,scientists in the laboratory and medical and allied health professionals in the clinic should apply photobiomodulation soon after meals.Our hypothesis awaits experimental and clinical validation.
We thank Fonds de dotation Clinatec and COVEA France for supporting our work.We also thank Catherine Hamilton(Wellred Inc.,Australia),Glen Jeffery(UCL,UK),and Michael Powner(City University London,UK)for proofreading the first version of the manuscript.
Jaimie Hoh Kam*,John Mitrofanis
Université Grenoble Αlpes,Fonds de Dotation Clinatec,Grenoble,France (Hoh Kam J,Mitrofanis J)
University College London,Institute of Ophthalmology,UK (Mitrofanis J)
*Correspondence to:Jaimie Hoh Kam,PhD,jaimie.hoh@gmail.com.
https://orcid.org/0009-0004-0677-9534(Jaimie Hoh Kam)
Date of submission:Αpril 24,2023
Date of decision:June 14,2023
Date of acceptance:July 21,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385290
How to cite this article:Hoh Kam J,Mitrofanis J(2024)Does photobiomodulation require glucose to work effectively?Neural Regen Res 19(5):945-946.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Perspectives in human brain plasticity sparked by glioma invasion:from intraoperative (re) mappings to neural reconfigurations

