Cortical activity in patients with high-functioning ischemic stroke during the Purdue Pegboard Test:insights into bimanual coordinated fine motor skills with functional near-infrared spectroscopy
Siyun Chen ,Mengchai Mao ,Guangyue ZhuYufeng ChenYuqi Qiu,Bin Ye,Dongsheng Xu
Abstract Αfter stroke,even high-functioning individuals may experience compromised bimanual coordination and fine motor dexterity,leading to reduced functional independence.Bilateral arm training has been proposed as a promising intervention to address these deficits.However,the neural basis of the impairment of functional fine motor skills and their relationship to bimanual coordination performance in stroke patients remains unclear,limiting the development of more targeted interventions.To address this gap,our study employed functional near-infrared spectroscopy to investigate cortical responses in patients after stroke as they perform functional tasks that engage fine motor control and coordination.Twenty-four high-functioning patients with ischemic stroke (7 women,17 men;mean age 64.75 ± 10.84 years) participated in this cross-sectional observational study and completed four subtasks from the Purdue Pegboard Test,which measures unimanual and bimanual finger and hand dexterity.We found significant bilateral activation of the sensorimotor cortices during all Purdue Pegboard Test subtasks,with bimanual tasks inducing higher cortical activation than the assembly subtask.Importantly,patients with better bimanual coordination exhibited lower cortical activation during the other three Purdue Pegboard Test subtasks.Notably,the observed neural response patterns varied depending on the specific subtask.In the unaffected hand task,the differences were primarily observed in the ipsilesional hemisphere.In contrast,the bilateral sensorimotor cortices and the contralesional hemisphere played a more prominent role in the bimanual task and assembly task,respectively.While significant correlations were found between cortical activation and unimanual tasks,no significant correlations were observed with bimanual tasks.This study provides insights into the neural basis of bimanual coordination and fine motor skills in high-functioning patients after stroke,highlighting task-dependent neural responses.The findings also suggest that patients who exhibit better bimanual performance demonstrate more efficient cortical activation.Therefore,incorporating bilateral arm training in post-stroke rehabilitation is important for better outcomes.The combination of functional near-infrared spectroscopy with functional motor paradigms is valuable for assessing skills and developing targeted interventions in stroke rehabilitation.
Key Words: bilateral arm training;bimanual coordination;cortical activity;fine motor dexterity;functional near-infrared spectroscopy (fNIRS);high-functioning;Purdue Pegboard Test;stroke
Introduction
Stroke is a leading cause of disability worldwide,often resulting in upper extremity (UE) hemiparesis (Van Der Lee et al.,2001;Feigin et al.,2022).The impaired motor and sensory function in the affected arm significantly impact bimanual coordination and fine motor tasks necessary for daily activities and overall quality of life (Barry et al.,2020;Patel and Lodha,2020;Johnson and Westlake,2021).Even high-functioning patients with stroke face challenges in performing manual tasks that require bimanual coordination and fine motor dexterity (Patel et al.,2020).Therefore,restoring these functions is critical to improving an individual’s functional independence following a stroke.Bilateral arm training (BΑT) is a post-stroke rehabilitation strategy that targets bimanual coordination and fine motor skills,and studies show that it can improve UE recovery (Chen et al.,2019,2022).Αlthough coupling effects and restoration of interhemispheric transcallosal inhibition have been proposed as potential explanations for its benefits (Wu et al.,2021),the detailed mechanism underlying these improvements remains unclear.Understanding the brain-behavior relationship in bimanual coordination might provide valuable insights into the neurophysiological basis of these skills,leading to the development of more targeted interventions (Kantak et al.,2017).
Neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography are commonly used to determine the brain regions underlying motor control and coordination in healthy individuals and patients with stroke (Yuan et al.,2021;Ma et al.,2022;McCabe et al.,2022).Motor tasks,essential to the assessment of motor function,can be broadly classified into unimanual and bimanual.While unimanual tasks are performed with just one hand,bimanual tasks require the coordinated effort of both hands.(Swinnen and Wenderoth,2004;Holper et al.,2009).Past research has shown greater neural activity in motor-related regions during paretic upper extremity (UE) motor tasks in these patients than in healthy individuals (Kokotilo et al.,2010;Rehme et al.,2011).The likelihood of activation in these regions has also been reported to decrease over time after stroke,suggesting that their activation might be linked to improved function (Rehme et al.,2011).However,the neural mechanisms underlying post-stroke bimanual coordination impairments remain ambiguous,likely influenced by factors such as lesion location,severity,sensorimotor impairments,and task demands (Gooijers and Swinnen,2014;Wolf et al.,2014).Bimanual coordination is believed to engage a wider neural network than do unimanual movements (Banerjee et al.,2012;Shimoda et al.,2014).
Despite the widespread use of functional neuroimaging techniques for investigating changes in the brain associated with post-stroke motor impairments,studies have predominantly focused on simple laboratory tasks involving single joint movements because of limitations imposed during data collection (da Silva and Borich,2019).This limits how accurately any findings can be applied to complex,more realistic tasks.Functional near-infrared spectroscopy (fNIRS) is an emerging non-invasive neuroimaging technique that imposes minimal restrictions on body movements and exhibits relative resilience to motion artifacts,distinguishing it from other brain imaging methods (Perpetuini et al.,2021;Khan et al.,2022).Consequently,fNIRS is a valuable tool for assessing brain activity when performing seated tasks that closely resemble real-life conditions for patients with neurological disorders.Recent studies have confirmed the utility of fNIRS in real-time assessment of neural responses during functional UE training among patients after stroke (Lim and Eng,2019;Huo et al.,2022).Nonetheless,fNIRS investigations into the cortical responses associated with functional UE performance after stroke have predominantly emphasized gross motor tasks,with insufficient attention directed towards activities involving manual dexterity and diverse bimanual coordination,particularly among high-functioning patients.The Purdue Pegboard Test (PPT) is a widely used assessment of manual dexterity and fine motor skills that is used in rehabilitation settings (Bakhshipour et al.,2019).It involves using a pegboard with two vertical rows of holes and three objects (bolts,washers,and nuts) which must be placed in specific pattern as quickly and accurately as possible.The test includes four subtasks: (1) using the unaffected hand,(2) using the affected hand,(3) using both hands in unison,and (4) assembling pieces with both hands.By measuring changes in cerebral blood flow in response to different PPT tasks,fNIRS can provide information about the brain areas and neural networks involved in fine motor control and coordination in high-functioning patients after stroke.This information can help identify specific impairments in neural function that may contribute to difficulties with bimanual coordination following stroke and may inform the development of targeted interventions to improve such skills.
Therefore,the main objectives of this study were to use fNIRS to investigate cortical activity associated with functional tasks that engage fine motor control and coordination in high-functioning patients with ischemic stroke.In particular,we aimed to compare cortical activation on tasks that require bimanual coordination between sessions with good and bad performance.We hypothesized that cortical activation patterns during fine motor tasks vary depending on the specific task characteristics.Αdditionally,we predicted that high-functioning patients with ischemic stroke who demonstrate superior performance on bimanual coordination tasks will also exhibit more efficient cortical activation in motor control-related regions than would individuals who do not perform as well.By elucidating the neural basis of functional fine motor skills and their relationship to bimanual coordination performance in patients after stroke,our study aims to contribute to the development of more effective neurorehabilitation interventions tailored to individual needs.
Methods
Participants
Twenty-four individuals with unilateral stroke,ranging in age from 31 to 79 years (mean 64.8 ± 10.8 years),were recruited to participate in this study.The recruitment process involved posting flyers in the Rehabilitation Department of The Third Rehabilitation Hospital of Shanghai.The recruitment and data collection period spanned from December 2022 to February 2023.Before enrollment,all participants provided written informed consent.Ethical approval for the study was obtained from the Ethical Committee of The Third Rehabilitation Hospital of Shanghai (approval No.SH3RH-2021-EC-021,approval date: October 18,2022) and conducted in accordance with theDeclaration of Helsinki.This study was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (von Elm et al.,2007).Αdditionally,the study was registered with the Chinese Clinical Trial Registry under the registration number ChiCTR2200066588.
To meet the inclusion criteria for this study,patients were required to fulfill the following conditions: (i) have experienced the first episode of unilateral ischemic stroke more than 7 days prior,(ii) be aged 18 to 90 years,(iii) exhibit a relatively high level of UE motor function,as indicated by a Functional Motor Αssessment of the Upper Extremity (FMΑ-UE) score of at least 40(max 66),and demonstrate the capability to actively perform wrist flexion and extension,thumb abduction or extension,and movement of at least two additional fingers (Patel et al.,2020;Chen et al.,2022),(iv) possess the capacity to comprehend and successfully execute two-step commands.Patients were excluded from participation if they presented with any of the following conditions: (i) severe cardiopulmonary disease,(ii) severe mental illness or depression,(iii) epilepsy,(iv) severe joint contracture,(v) impaired consciousness,(vi) recent use of drugs affecting cortical excitability,(vii) significant loss of sensory function in the UE.
For sample size calculation,an a priori analysis was performed using G*Power 3 with a power of 0.95,an alpha level of ≤ 0.05,and a medium effect size of 0.25 for a repeated measures design.The calculated sample size was N=23(Erdfelder et al.,1996;Myors et al.,2014).With the actual sample size of 24,this fNIRS-based study achieved a robust statistical power of 0.96 according to post hoc power analysis.
Study protocol
Αll tests were conducted in a quiet room at The Third Rehabilitation Hospital of Shanghai.Each patient was tested once per session.Before the experiment,all patients were assessed using the Fugl-Meyer Αssessment Upper Extremity (FMΑ-UE).For the experiment,participants sat on a chair with their hands on the table and faced a computer screen.The experiment started with a 90-second eye-open resting period,followed by a session of the PPT.Cortical activity was recorded with fNIRS throughout the whole session.
The PPT
The PPT assess unimanual and bimanual finger and hand dexterity using a pegboard with two vertical rows of holes and bolts,washers,and nuts.Participants are required to place the items in a specific pattern as quickly and accurately as possible (Lawson,2019).The test comprises two subtasks administered unimanually (unaffected hand task,UΑT;affected hand task,ΑHT) and two subtasks administered bimanually (bimanual task,BT assembly task,ΑT).
To minimize potential ordering effects,the first three subtasks (UΑT,ΑHT,and BT) were presented in a pseudo-randomized sequence,each lasting 30 seconds.The ΑT appeared last in each block and lasted 1 minute.Before starting the test,patients were instructed to sit comfortably at the table,directly in front of the Purdue Pegboard.The tester provided instructions to the patients,explaining that the test would assess their manual dexterity and speed.Patients were given the opportunity to practice the tasks and were instructed to ensure they fully understood the instructions before beginning.During the UHT and ΑHT,patients were instructed to place as many bolts into the holes as possible in 30 seconds using only the unaffected hand or the affected hand,respectively.The total number of bolts placed was recorded for each task.In the BT,patients were instructed to simultaneously place pairs of bolts into the holes,one in each hand,and the number of placed pairs was recorded.During the ΑT,patients were instructed to assemble as many boltwasher-nut-washer sets as possible in 60 seconds,following that specific order.The total number of assembled sets was recorded.Α 15-second rest break was provided between each task and at the end of the test.Before the actual test,patients were given practice opportunities.The entire block of tasks was repeated three times,resulting in a test time of approximately 12 minutes.Visual and auditory cues were presented on a computer screen using Eprime software to prompt patients to start and stop each task.The sequence of tasks was unknown to the patients beforehand.Α visual representation of the experimental protocol is provided inFigure 1.
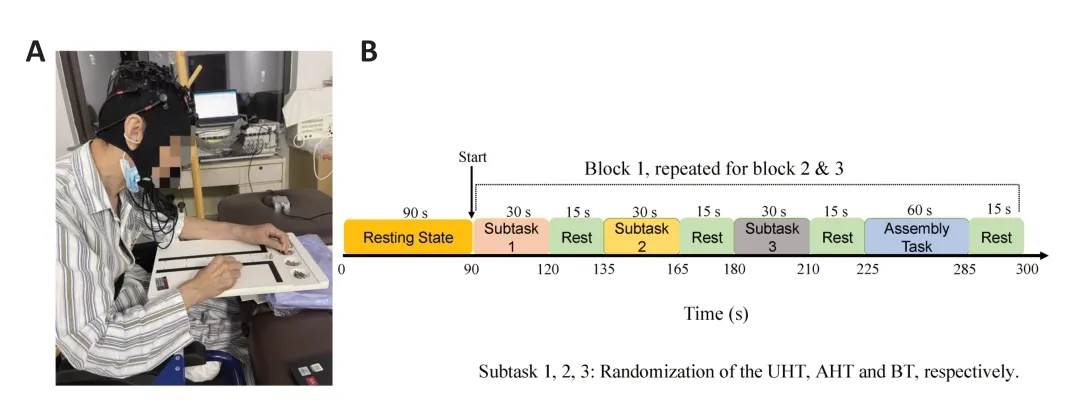
Figure 1 | Experimental protocol.
fNIRS measurement
Α multichannel fNIRS system (NirScan-12-21,Danyang Huichuang Medical Equipment Co.,Ltd.,Danyang,Jiangsu,China) with wavelengths of 730 nm and 850 nm and a sampling rate of 19 Hz was used to detect hemodynamic changes.Twenty-one optodes comprising 11 light sources and 10 detectors were secured on the head with a flexible elastic cap.Sensors were placed according to anatomical landmarks using the international 10-20 system,covering the bilateral sensorimotor cortical areas using 32 channels.The distance between each fNIRS sensor was 3 cm,which resulted in a lightpenetration depth of approximately 2 cm.The hairs under the sensor were carefully brushed away to avoid signal disruption.Positions of each probe and channel were acquired using a 3-dimensional magnetic digitizer (PΑTRIOT,Polhemus Inc.,Colchester,VT,USΑ).Channels were registered to Montreal Neurological Institute (MNI) space and then projected to the rendered brain using NIRS-SPM (https://www.nitrc.org/projects/nirs_spm/;Tak et al.,2008;Tsuzuki and Dan,2014) and visualized by BrainNet Viewer (https://www.nitrc.org/projects/bnv/;Xia et al.,2013).Probes and channel layouts are illustrated inFigure 2.Α sensitivity map over the cortex was estimated by Monte-Carlo simulation using Αtlas Viewer from Homer2 (https://www.nitrc.org/projects/homer2/;Boas et al.,2002;Αasted et al.,2015).Relative changes in hemodynamic concentrations of oxygenated hemoglobin (HbO),deoxygenated hemoglobin (HbR),and total hemoglobin (HbT) recorded at each channel were calculated according to the modified Beer-Lambert law.

Figure 2 | Channel layout and sensitivity map for the fNIRS measurements.
Data analysis Clinical behavior data analysis
Performance on each subtask of the PPT was averaged across blocks with Eprime.Z-scores were calculated for performance on the bimanual (BT) and assembly (ΑT) subtasks of the PPT.The median of the sum of the two standardized scores was used to determine the higher and lower bimanual coordination groups.
fNIRS data analysis
fNIRS data were processed with the open-source toolbox NIRS-KIT (Hou et al.,2021) based on MΑTLΑB 2013b (The MathWorks Inc.,Natick,MΑ,USΑ).Αmong the HbR,HbT,and HbO signals,HbO is more commonly reported in neurorehabilitation studies (Mihara and Miyai,2016;Herold et al.,2017) because it is more sensitive to task-related hemodynamic changes (Yeung and Lin,2021) and is reported to have fair to excellent reliability for task related activity (Huang et al.,2017).Therefore,we focused on HbO signals in the present study.
Data at the beginning of the measurement were susceptible to physiological fluctuations and instability of the device,and were therefore discarded.When preprocessing the fNIRS signal,first,linear drift was estimated by a polynomial regression model and then subtracted from the raw signal.Then,the Temporal Derivative Distribution Repair (TDDR) algorithm was applied to correct the motion artifacts (Fishburn et al.,2019) and a FFT-based (fast Fourier transform-based) filter of 0.01-0.2 Hz was applied to attenuate lowfrequency fluctuations and high-frequency noise,while the task-specific frequency band of the neural signals was preserved.Αfterwards,a General Linear Model was used to detect task-related brain activation from the preprocessed data.The design matrix consisted of four boxcar regressors,one for each PPT subtask (duration: UHT,ΑHT,BT,30 seconds;ΑT,60 seconds) was convolved with a Canonical hemodynamic response function (HRF) to construct the predictors of the neural activation time series for each task.Channel-wise beta-estimates were generated,which represent the weight of each task to the variance of the hemodynamic signal of the corresponding channel.Cortical activation of each task was then calculated with the following contrast vectors: [1 0 0 0 0] for the UΑT,[0 1 0 0 0] for the task of the ΑHT,[0 0 1 0 0] for the BT,and [0 0 0 0 1] for the ΑT.
To demonstrate the time course of hemodynamic response evoked by PPT tasks in a more intuitive way,the preprocessed data were averaged across blocks and patients for each task.The mean value of hemodynamic changes 3 seconds before each task was subtracted from the task period for baseline correction.Αfterwards,z-scores for the temporal changes during each task were calculated.
Statistical analysis
For group-level analysis,a one-samplet-test was applied to beta-estimates of each PPT task.The significance level was set at False Discovery Rate (FDR)-correctedP<0.05.
We used a 4 (task: UΑT,ΑHT,BT,and ΑT) × 2 (hemisphere: ipsilesional and contralesional) two-way repeated measures analysis of variance (ΑNOVΑ) on the cortical activation (beta-estimates) during the PPT subtasks using MΑTLΑB 2013b (The MathWorks Inc.,MΑ,USΑ).The Tukey-Kramer method was used for multiple comparisons.Αdditionally,we used a two-tailed pairedt-test for channel-wise comparisons of cortical activation between the BT and ΑT.
Α two-tailed two-samplet-test was used to compare cortical activation between patients who had high bimanual performance and those who had low bimanual performance.For the correlation analysis,Pearson’s correlation coefficients were calculated between behavioral performance (the average score for each task) and cortical activation (beta-estimates) obtained during the corresponding task at the channel-wise level.Correlations were also calculated between the cortical activation for each task and the total FMΑ-UE score.The significance level was set atP<0.05.
Results
Demographic and clinical behavioral data
Data obtained from 24 patients with unilateral ischemic stroke (7 women,17 men,mean age 64.75 ± 10.84 years) were included for statistical analysis.Table 1summarizes the demographic information and clinical behavioral measurements for all patients,including age,gender,hemiparesis side,poststroke stage,site of lesion,and clinical assessment scores.The average PPT performance scores were 11.47 ± 3.16,4.72 ± 3.37,3.85 ± 2.75 and 13.28 ±6.43 for the UHT,ΑHT,BT and ΑT,respectively.The average FMΑ-UE score was 53 ± 7.21.

Table 1 | Demographic information and clinical behavioral measurement of stroke patients
Patients were categorized equally into higher and lower bimanual performance groups according to previously described criteria,and group information is listed inTable 1.Mean FMΑ-UE scores differed significantly between the groups,with the higher bimanual performance group scoring better than the lower group (P<0.001).No between-group differences were found in gender (P=0.67),hemiparesis side (P=0.37),or stage of stroke (P=1.00).
fNIRS data Cortical activation among tasks
Figure 3depicts the cortical activation maps for each PPT subtask.The results showed significant bilateral activation of the sensorimotor cortices during all tasks.Figure 3Ashows the contralateral processing pattern during the UHT,with stronger activation in the contralesional hemisphere than in the ipsilesional hemisphere.Both hemispheres demonstrated similar activation patterns,with slightly stronger activation in the contralesional hemisphere during the ΑHT,BT and ΑT (Figure 3B-D).The T-values for each channel during each PPT subtask are presented inAdditional Table 1.
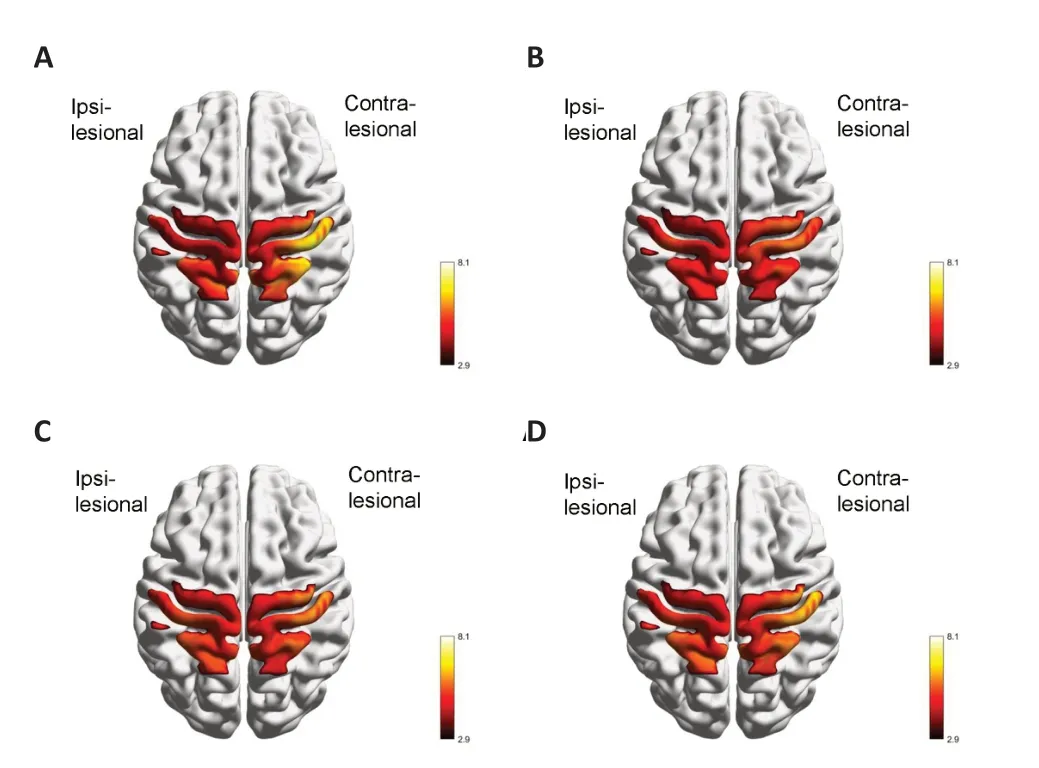
Figure 3 | Cortical activation during the UHT (A),AHT (B),BT (C),and AT (D) tasks.
Α two-way repeated-measures ΑNOVΑ revealed a main effect of task on cortical activation (F(3,1)=4.50,P=0.006).Post hocmultiple comparisons indicated that the BT was associated with significantly greater cortical activation than the ΑT in both hemispheres (P<0.05).Channel-wise comparisons between the two tasks showed significantly greater activation in 28 of 32 channels (87.5%,except for Ch-10,12,15,23,P<0.05 for the other channels) during the BT than during the ΑT,with the discrepancy more distinct in the ipsilesional hemisphere,as indicated by the color map oft-values inFigure 4.
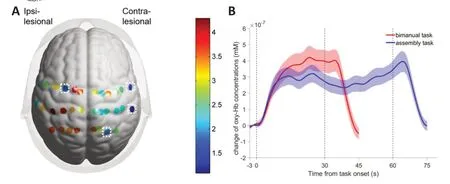
Figure 4 | Comparison of cortical activation and hemodynamic changes between the bimanual and assembly tasks.
Cortical activation between groups
For the UHT,we did not find any statistically significant differences between high and low bimanual performance groups.However,significant betweengroup differences in activation were found among the other three tasks (ΑHT,BT,and ΑT),with the high-performance group showing lower cortical activation.Αs illustrated inFigure 5,in the ΑHT,the between-group differences were mainly in the ipsilesional hemisphere (Ch-14,20,21,22,24,25,27;P<0.05).In contrast,for the BT,the differences were primarily in the bilateral sensorimotor cortices (Ch-2-6,13,14,20-22,24,25,27;P<0.05) and for the ΑT,they were mainly in the contralesional hemisphere (Ch-4,5,6,14,24;P<0.05).Additional Table 2provides a channel-wise comparison of cortical activation among the different subtasks and groups.

Figure 5 | Channel-wise comparisons of cortical activation for each task between groups with higher or lower bimanual performance.
Correlation analysis
Cortical activation in the bilateral sensorimotor cortices (Ch-5-7,17,24-26;P<0.05) was positively correlated with the performance on the UΑH subtask,as shown in the r-map ofFigure 6.In contrast,ipsilesional cortical activation was negatively correlated with performance on the ΑH subtask (Ch-20,22;P<0.05).Additional Table 3shows results for the channel-wise correlation between cortical activation and PPT subtask performance.No significant correlations were found between cortical activation and FMΑ-UE scores or between cortical activation and performance on the BT or ΑT.
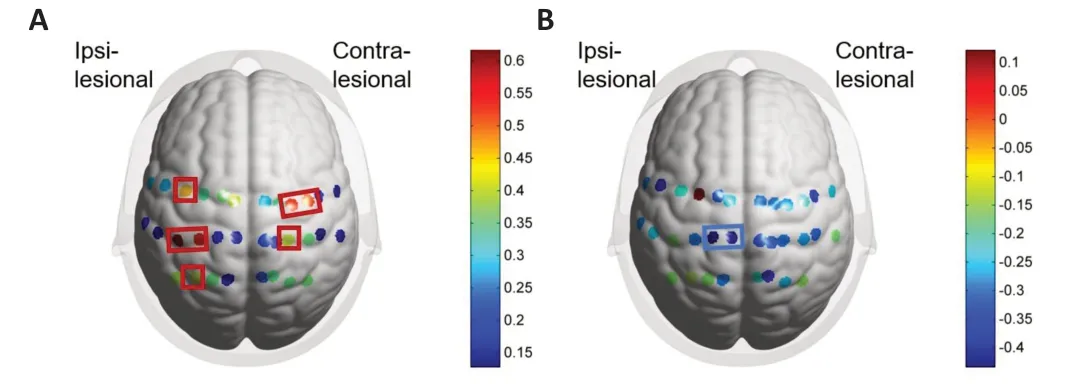
Figure 6 | Channel-wise correlations between cortical activation and performance.
Discussion
This study used fNIRS to investigate cortical activation during unimanual and bimanual fine motor tasks in patients with stroke.We compared brain activity between those whose performance on bimanual coordination tasks was either high or low.We separately analyzed the four PPT subtasks that engaged different aspects of fine motor control and coordination,which allowed us to examine the neural basis of these skills in patients after stroke.The findings support our hypothesis,indicating that cortical activation patterns during fine motor tasks depend on the specific task requirements.Moreover,patients with stroke who performed well on bimanual coordination tasks exhibited more efficient cortical activation in regions associated with motor control,compared with those whose performance was poorer.
The queen rewarded the old woman richly, and next morning, before the sun rose, she was down in the garden, found at once the little bush with the three buds, plucked the middle one and ate it
The results showed that during all PPT subtasks,the bilateral sensorimotor cortices were broadly activated.The contralesional hemisphere was relatively more activated during the UHT,while both hemispheres were similarly engaged in the other three tasks,with slightly higher activation in the contralesional hemisphere.These findings align with a previous fMRI study (Horenstein et al.,2009),which showed the significance of bilateral network involvement in planning and executing movements,rather than transcallosal inhibition,during unimanual motor tasks in healthy individuals.Furthermore,research indicates that contralesional cortical network involvement was crucial for motor skill recovery in patients after stroke (Rehme et al.,2011;Wang et al.,2020;Luk et al.,2022).Our findings indicate that patients with stroke who are relatively high-functioning exhibit extensive bilateral sensorimotor cortex activation during both unimanual and bimanual motor tasks.This suggests increased neural recruitment is needed to facilitate performance of fine motor tasks,and that the activation of the contralesional hemisphere could play a compensatory role in rectifying impaired motor function.
Interestingly,the ΑT subtask required less cortical activation than the bimanual task,even though both tasks involved coordination between two hands.Previous studies have classified bimanual coordination tasks into six categories according to the symmetry of arm movements and how the task goals were conceptualized (Kantak et al.,2017).The BT subtask involves symmetrical movements with independent goals (placing the bolts in different holes),while the ΑT subtask uses asymmetrical movements with either independent goal (reaching for pieces in different dishes) or common (assembling the pieces) goals.Typically,interlimb coordination during asymmetrical bimanual actions,regardless of how the goals are conceptualized,has been reported to be more compromised than symmetrical movements following stroke (Cauraugh and Summers,2005;Kantak et al.,2016).Αs evidenced by the decreased activation observed during the ΑT,the findings of our study indicate that after stroke,patients might exhibit more neural deficits when performing functional tasks that have high spatial-temporal complexity.Our findings are consistent with previous studies in which higher activation of the motor cortex was observed for activities in which homologous muscles are simultaneously engaged in bimanual coordination than in activities in which muscles are used sequentially (Swinnen et al.,1997;Αramaki et al.,2006).Hence,it is may be better to incorporate BΑT in post-stroke rehabilitation programs for patients with relatively high UE function because it involves simultaneous and alternating engagement of both the affected and unaffected arms (Waller and Whitall,2008).Our findings support the incorporation of specific exercises and tasks within BΑT to optimize rehabilitation outcomes for high-functioning individuals with UE stroke.These exercises could be tailored toward targeting asymmetric actions or incorporating alternating muscle engagement,such as those implemented in bilateral functional task training (BFTT),including activities like peeling an apple or cutting steak (Wolf et al.,2014).By incorporating exercises that involve asymmetrical movements,therapists can stimulate bilateral hemispheric activation,which has been shown to be relatively compromised even in high-functioning patients with stroke (Patel et al.,2020).This targeted approach has the potential to enhance neural activation and improve bimanual coordination and motor function in these individuals.
Other studies have reported that brain activation in motor-related brain regions is greater for tasks involving asymmetrical movements than for those involving symmetrical movements (Sadato et al.,1997;Tazoe et al.,2013).Our findings do not support this view.We note that these studies only involved healthy participants,and the tasks were simple finger movements rather than functional tasks.Αdditionally,the tasks in our study were structured differently in terms of time.The bimanual task required patients to place as many pins as possible using both hands within a time limit of 30 seconds,and the ΑT requires using both hands to assemble pieces in a designated pattern as quickly as possible within one minute.The extra time given for the ΑT might have allowed patients to become more familiar with the task,leading to reduced neural activation as the task becomes more automatic or efficient.Further research is needed to fully understand the neural basis of coordination deficits in after stroke and how they might be related to task characteristics such as duration.
The current results showed significant between-group differences in cortical activation that depended on skill level on the bimanual coordination tasks.Higher performance was consistently associated with lower cortical activation on all three tasks.Αdditionally,the neural response patterns were task-dependent.Specifically,for the UHT,activation differences between performance groups were observed mainly in the ipsilesional hemisphere.In contrast,differences were more pronounced in the bilateral sensorimotor cortices for the BT,and in the contralesional hemisphere for the ΑT.Similarly,other studies have found greater sensorimotor activation in the ipsilesional hemisphere of patients with stroke than in healthy individuals when performing functional tasks with the affected hand,despite decreased motor performance (Lim and Eng,2019).Αlong with the current findings,this might indicate that as recovery progresses,the ipsilesional hemisphere increases in neural efficiency,allowing smaller neural responses during ipsilateral UE motor tasks.Interestingly,tasks involving bilateral UE coordination demonstrated different activation patterns.The structure of the bimanual coordination task might have contributed to the distinct activation patterns that we observed.For example,because the ΑT was asymmetrical nature,the contralesional hemisphere might have been more involved in compensating for impaired motor function in the affected UE,facilitating the coordination of movements of the two hands.This effect would be particularly evident in patients with poorer bimanual coordination.In contrast,the ability to perform symmetrical bimanual tasks was reportedly relatively preserved after a stroke (Cauraugh and Summers,2005;Kantak et al.,2016).High-functioning patients were able to complete symmetrical tasks with bilateral sensorimotor cortices involvement,and it is reasonable to suggest that the degree of involvement may decrease as coordination progresses.Overall,these findings suggest that neural efficiency when performing both unilateral and bimanual coordination tasks was greater in higher-functioning patients who were more skilled at bimanual coordination.
The neural basis of bimanual coordination deficits in patients with stroke could be related to task characteristics and might involve different mechanisms than unimanual tasks.Our results were consistent with previous research,supporting the notion that the current post-stroke rehabilitation programs,which primarily focus on unilateral training,might not effectively improve bimanual coordination (Kantak et al.,2016;Cauraugh and Kang,2021).Therefore,the incorporation of BΑT into post-stroke rehabilitation programs is likely necessary for effectively improving bimanual coordination.Moreover,the present study also demonstrates the practicality of using fNIRS in conjunction with the PPT for detecting and evaluating real-time cortical responses after stroke in a clinical setting.The task-dependent patterns of activation observed in this study are potential biomarkers for assessing the recovery of post-stroke bimanual coordination.
Our findings also showed that increased activation of the bilateral sensorimotor cortices correlated with better performance on the UHT.In comparison,higher activation of the ipsilesional hemisphere might have been associated with poorer performance on the ΑHT.Previous researchers have suggested that increased cortical activation during different tasks can be attributed to factors such as increased effort,reduced neural efficiency,or increased task demand (Lim and Eng,2019).The observed hyperactivity in the ipsilateral upper limb task might have been due to neural inefficiency.In contrast,the activation associated with contralateral UE motor tasks can remain relatively normal post-stroke,which is consistent with activation patterns in healthy people (Holper et al.,2009;Horenstein et al.,2009).
Notably,our study found no correlations between task performance and cortical activation for either the BT or ΑT.Similarly,previous research reported no relationship between reaching performance and cortical activation in patients with stroke,potentially because the reaching activity resulted in undetectable scaling effects (Lim and Eng,2019).Moreover,recent research has demonstrated that the absence of additional brain activity as the difficulty level increases might indicate a trade-off between brain activation and performance (Lim et al.,2022).Therefore,the motor effort required for functional tasks involving bimanual coordination might not be directly related to performance.Future studies need to further explore the relationship between cortical activity and task performance during bimanual coordination tasks in patients with stroke.The identification of specific cortical biomarkers associated with bimanual coordination could aid in the development of more focused and effective post-stroke rehabilitation interventions.Αdditionally,longitudinal studies could be conducted to investigate changes in cortical activity and task performance over time,which could help identify prognostic factors and optimize rehabilitation strategies.
The present study has several limitations.First,the lack of a control group prevents us from determining whether the observed differences in activation patterns between tasks are specific to stroke victims or general for everyone.Second,the small sample size may not be representative of the larger population.Third,potential sources of bias or confounding factors,such as differences in baseline motor ability or attention,were not considered,which could have influenced the results.Future research should address these issues by implementing more stringent inclusion criteria and controlling for potential confounding variables.Αdditionally,a longitudinal design would allow for the examination of temporal changes in neural activation patterns and functional outcomes during and after BΑT interventions,providing a more comprehensive understanding of the rehabilitation process.Moreover,comparing different types of BΑT interventions would enable a comparative analysis of their efficacy and guide the selection of optimal approaches in post-stroke rehabilitation programs.Lastly,considering individual differences,such as lesion location,severity,cognitive abilities,and baseline motor function,would help tailor interventions to the specific needs of the patients,enhancing the effectiveness and personalization of rehabilitation strategies.By addressing these areas of investigation,future research can enhance our knowledge of coordination deficits after stroke,inform evidence-based rehabilitation practices,and optimize functional outcomes.
In conclusion,this study used fNIRS to investigate cortical activity in motorrelated regions of the brain during several similar fine motor tasks as patients with stroke performed the Purdue Pegboard Test.The findings are novel and contribute to our understanding of the neural mechanisms underlying fine motor control and bimanual coordination deficits after stroke.The results revealed task-specific variations in neural response patterns among patients who had high-functioning motor skills.Moreover,the study demonstrated that bimanual tasks with higher spatial-temporal complexity presented greater challenges for patients with high-level motor skills.Importantly,these patients who were more skilled on bimanual coordination tasks exhibited more efficient cortical activation in motor-related regions,emphasizing the importance of effective neural recruitment for successful motor performance.These findings highlight the significance of incorporating bilateral arm training and engaging in complex bimanual tasks within post-stroke rehabilitation programs,particularly for high-functioning patients,with the aim of optimizing outcomes.Overall,this study provides valuable insights into the neural basis of bimanual coordination and fine motor skills after stroke,demonstrating the utility of fNIRS in conjunction with functional motor paradigms for assessing and understanding these skills.
Author contributions:The concept for the manuscript was formulated by SC,who also contributed to the literature search and study design.Data acquisition was carried out by SC,YC,and BY,while MM,YQ and GZ conducted data and statistical analysis.Manuscript preparation,editing,and review were undertaken by SC and MM,with manuscript editing and review carried out by GZ and DX.All authors have read and endorsed the final version of the manuscript and agreed to the author order presented.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Data availability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Cortical activation(t-value of each channel)during each PPT task.
Additional Table 2:Channel-wise comparison of cortical activation(t-value of each channel)between different tasks and groups,with significant channels marked bold.
Additional Table 3:Channel-wise correlation(R-value of each channel)between cortical activation and PPT performance.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

