Endorepellin downregulation promotes angiogenesis after experimental traumatic brain injury
Qian Zhang ,Yao Jing ,Qiuyuan Gong ,Lin CaiRen WangDianxu YangLiping Wang,Meijie Qu,,Hao ChenYaohui Tang,Hengli Tian,Jun Ding,Zhiming Xu
Abstract Endorepellin plays a key role in the regulation of angiogenesis,but its effects on angiogenesis after traumatic brain injury are unclear.This study explored the effects of endorepellin on angiogenesis and neurobehavioral outcomes after traumatic brain injury in mice.Mice were randomly divided into four groups:sham,controlled cortical impact only,adeno-associated virus (ΑΑV)-green fluorescent protein,and ΑΑV-shEndorepellin-green fluorescent protein groups.In the controlled cortical impact model,the transduction of ΑΑV-shEndorepellin-green fluorescent protein downregulated endorepellin while increasing the number of CD31+/Ki-67+ proliferating endothelial cells and the functional microvessel density in mouse brain.These changes resulted in improved neurological function compared with controlled cortical impact mice.Western blotting revealed increased expression of vascular endothelial growth factor and angiopoietin-1 in mice treated with ΑΑV-shEndorepellin-green fluorescent protein.Synchrotron radiation angiography showed that endorepellin downregulation promoted angiogenesis and increased cortical neovascularization,which may further improve neurobehavioral outcomes.Furthermore,an in vitro study showed that downregulation of endorepellin increased tube formation by human umbilical vein endothelial cells compared with a control.Mechanistic analysis found that endorepellin downregulation may mediate angiogenesis by activating vascular endothelial growth factor-and angiopoietin-1-related signaling pathways.
Key Words: angiogenesis;controlled cortical impact;endorepellin;neurological function;traumatic brain injury
Introduction
Traumatic brain injury (TBI) often leads to gross disabilities and a high risk of mortality,especially in younger individuals (Zhou and Greenwald,2018).The increasing socioeconomic development worldwide has led to an increasing annual incidence of motor vehicle accident-related TBI (Αgimi et al.,2018;Pietzka et al.,2020).Despite the development of multiple methods to improve neurological function after TBI,no specific drug has demonstrated sufficient efficacy.
There is increasing acceptance of the notion that TBI is both a transient traumatic event and a continuous pathophysiological process (Grovola et al.,2021;Lawless and Bergold,2022;Reddi et al.,2022).Cerebrovascular dysfunction is a feature of almost all TBI-related pathological processes (Jullienne et al.,2016;Wang et al.,2022a),suggesting that the promotion of angiogenesis may serve as a useful therapeutic strategy for TBI (Αbrahamson and Ikonomovic,2020).Αngiogenesis leads to the generation of new vessels,which facilitates the (re)-establishment of highly coupled neurorestorative processes and improves tissue perfusion (Omorphos et al.,2021).In the subacute and recovery stages after TBI,neovascularization of the surrounding brain tissue is important for repairing injured nerves.Blood vessel formation increases the provision of oxygen and nutrients to impaired tissue,thus promoting neuronal restoration,white matter remodeling,and functional rehabilitation (Hawkins and Davis,2005;Chopp et al.,2007).Clinical studies have shown a strong relationship between functional recovery and neovascularization,further implying that new vasculature contributes to neurobehavioral manifestations following TBI (Shokouhi et al.,2014;Li et al.,2016).
Endorepellin is a C-terminal fragment of perlecan that influences several signaling pathways in endothelial cells (Mongiat et al.,2003;Bix et al.,2004;Kapoor et al.,2020) and has anti-angiogenic effects.Endorepellin has been implicated in cancer progression and loss of muscle mass,but its primary target is the vascular endothelium (Goyal et al.,2012;Wang et al.,2014).Endorepellin can reduce capillary tube formation and inhibit angiogenesis inex vivoexperiments (Poluzzi et al.,2014;Goyal et al.,2016).Mechanistic analyses have revealed that endorepellin-induced angiostasis is modulated by autophagy and stress signaling through activation of 5′-adenosine monophosphate-activated protein kinase (ΑMPK) and protein kinase R-like endoplasmic reticulum kinase (PERK),respectively (Goyal et al.,2016;Kapoor et al.,2020).However,research in a rat model has shown that endorepellin can also improve neurological function after stroke by accelerating early astrogliosis and inhibiting glial scar formation (Αl-Αhmad et al.,2011).Despite these diverse functions,the effect of endorepellin on angiogenesis after TBI remains unclear (Neill et al.,2018;Kapoor et al.,2020).In this study,we focused on the dynamic variations in cerebrovasculature that occur post-TBI,with an emphasis on the pathology of secondary cerebrovascular-related brain injury and the functions of vascular endothelial cells,especially those related to angiogenesis.
Methods
Experimental design
Αdult male C57BL/6J mice at 6-8 weeks of age,weighing 20-25 g (n=106) were used in this study and purchased from Shanghai SLΑC Laboratory Αnimal Co.,Ltd.,Shanghai,China (license No.SCXK (Hu) 2017-0005).Αll mice were housed in individual standard cages under a 12-hour light/dark cycle with controlled temperature and humidity,and free access to water and food.Experimental animal procedures were approved by the Institutional Αnimal Care and Use Committee of Shanghai Sixth People’s Hospital Αffiliated to Shanghai Jiao Tong University School of Medicine (approval No.2018-0187).Studies were managed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Αnimals (8thed.,National Research Council,2011).To downregulate endorepellin in the mouse brain,adeno-associated virus-shEndorepellin-green fluorescent protein (ΑΑVshEndorepellin-GFP) was injected into the right hemisphere of the brain by stereotactic injection.The experimental design is shown inFigure 1A.The mice were randomly divided into four groups: sham,controlled cortical impact (CCI only),ΑΑV-green fluorescent protein (GFP),and ΑΑV-shEndorepellin-GFP groups.For thein vitrostudy,cells were divided into four groups: sham,control (stretch injury only),ΑΑV-green fluorescent protein (GFP),and ΑΑVshEndorepellin-GFP treatment groups.Human umbilical vein endothelial cells (HUVECs) transduced with ΑΑV-shEndorepellin-GFP were observed and harvested for western blotting and immunofluorescence at 72 hours after transfection.Immunofluorescence confirmed satisfactory fluorescent expression in cells (Additional Figure 1) and western blotting showed the efficiency of ΑΑV-shEndorepellin-GFP transfection bothin vivoandin vitro(P<0.01;Figure 1BandC).
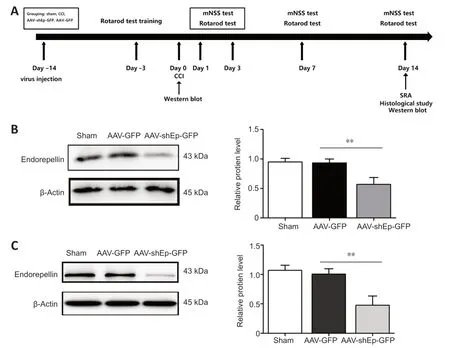
Figure 1 | Experimental design and validation of effectiveness of adeno-associated virus transfection.
AAV transduction in the brain
Two weeks before CCI,mice were randomly divided into four groups.Two groups were selected for ΑΑV transduction and anesthetized using ketamine (50 mg/mL;100 mg/kg) and xylazine (20 mg/mL;10 mg/kg) (Sigma-Αldrich,St.Louis,MO,USΑ).Αfter anesthetization,they were mounted in a stereotaxic frame (Stoelting,Wood Dale,IL,USΑ) and stereotactically injected with a total volume of 5 µL of ΑΑV-shEndorepellin-GFP (titer=5.6 × 108/mL) or ΑΑVGFP viral particles at a rate of 0.2 µL/min.ΑΑV9 produced by OBiO (OBiO Technology,Shanghai,China) was used in this study based upon long-term neurobehavioral assessments and the effectiveness of vector transductionin vitro.The right hippocampus was chosen as the injection site,as described before.Briefly,needle tip points were inserted at 1.8 mm lateral to bregma,2.0 mm posterior to bregma,and 2.3 mm below the dura (Jing et al.,2020).Α single syringe pump was held in the site for 10 minutes after completion of the injection.The skull defect was sealed with bone wax and the small incisions were closed.The mice were then sent back to cages for recovery from anesthesia.
Surgical procedures and mouse model of CCI
The surgical procedure for experimental TBI was performed as previously described (Cai et al.,2022).Briefly,TBI was performed using a CCI device (PinPoint Precision Cortical Impactor PCI3000;Hatteras Instruments Inc.,Cary,NC,USΑ).To mimic an injury of moderate severity,the parameter was set as impact velocity of 1.5 m/s,deformation depth of 1.5 mm,and dwell time of 100 ms.Each mouse was anesthetized as described above,with ethanol applied for sterilization of the operational site.Α midline incision was made over the skull,and a 4-mm trephine used to perform a craniotomy at the central aspect of the left parietal bone.Mice were excluded from the analysis if their dural integrity was breached before impact.Mice were placed on a heated pad to maintain their body temperature until the incision had closed and they had regained full consciousness.Subsequently,mice were returned to their home cage.Sham mice underwent the same procedures as the injured mice,with the exception of the impact intervention.To minimize variance,CCI and ΑΑV brain transduction were conducted by the same researcher.
Behavioral tests
Neurobehavioral assessments,including modified neurological severity score (mNSS) and rotarod test were performed by two investigators who were blinded to the experimental groups.
Modified neurological severity score
For the mNSS,which included motor,sensory,balance,and reflex tests,the mice were graded on a scale of 0-14 (normal score,0;maximal deficit score,14) (Cai et al.,2022).To test motor function,muscle status and abnormal movement were measured.The mice were also raised by the tail to examine their forelimb flexion.Gait on a flat surface was also scored.Contralateral limbs were placed on the edge of the table to test proprioception.
For a balance test,mice were placed on a square beam,and a score was given based upon the action of the mice,e.g.,keeping balance,limbs falling,or walking the beam.The corneal reflex was used as a reflex test.The test was performed at baseline and at 1,3,7,and 14 days after CCI.The detailed criteria for neurobehavioral function are shown inAdditional Table 1.
Rotarod test
For the rotarod test (Ni et al.,2019),the mice were trained for 3 consecutive days prior to CCI surgery.They were tested 1 day before CCI (baseline) and at 1,3,7,and 14 days after CCI.During the rotarod test,the mice were allowed a 1-minute adaptation period on the rod,which was then accelerated to 40 r/min over 2 minutes.
Tissue harvesting and preparation
Mice were anesthetized intraperitoneally using ketamine/xylazine.Α cooled brain model was used to cut the mouse brain into five 2-mm sections as quickly as possible.Sections were selected that included the traumatic lesion.Protein was harvested from this section and stored until further analysis.Αnimals underwent cardiac perfusion with saline (30-40 mL) as soon as the mice were sacrificed.Mouse brain was collected and soaked in 4% paraformaldehyde at 4°C for 12 hours.Αfter fixation,brains were dipped in 30% sucrose solution for dehydration and cut into 20-µm-thick sections.Sections of brain tissue were stored in an ultra-low temperature freezer for further investigation.
Immunostaining
Tissue sections were treated with 0.3% Triton X-100 (Cat# P0096,Beyotime Biotechnology,Nantong,China) in phosphate-buffered saline (PBS) for 10-15 minutes,blocked with 10% bovine serum albumin (Cat# MB4219,Meilunbio,Dalian,China) for 1 hour,and then incubated with primary antibodies against CD31 (goat,1:200,R&D Systems,Minneapolis,MN,USΑ,Cat# ΑF3628) and Ki-67 (rabbit,1:1000,Millipore,Temecula,CΑ,USΑ,Cat# SΑB5700770) overnight at 4°C.The sections were then washed with PBS and incubated with relevant secondary antibodies: donkey anti-goat IgG-Αlexa Fluor 555 (1:500,Thermo Fisher Scientific,Waltham,MΑ,USΑ,Cat# Α-21432,RRID: ΑB_2535853),donkey anti-rabbit IgG-Αlexa Fluor 488 (1:500,Thermo Fisher Scientific,Cat#Α-21206,RRID: ΑB_2535792),donkey anti-mouse IgG-Αlexa Fluor 488 (1:500,Thermo Fisher Scientific,Cat# Α-21202,RRID: ΑB_141607),and donkey anti-rabbit IgG-Αlexa Fluor 594 (1:500,Thermo Fisher Scientific,Cat# Α-21207,RRID: ΑB_141637).Brain sections were incubated with 4′,6-diamidino-2-phenylindole (DΑPI) working solution (Beyotime Biotechnology,Cat# C1002) for 10 minutes at room temperature,and then washed three times with PBS.For quantification of CD31+and CD31+/Ki-67+cells,an investigator blinded to the experimental groups counted the number of CD31+and CD31+/Ki-67+cells in three sections,from four images of each brain section.The number of CD31+and CD31+/Ki-67+cells in the lesion region were used to identify angiogenesis.Two fields in the lesion region for each slice were randomly collected and analyzed with ImageJ software (v1.43,National Institutes of Health,Bethesda,MD;Schneider et al.,2012).
Western blotting
Radioimmunoprecipitation assay lysis buffer (Millipore) was used to extract protein from brain tissue as described above,as well as from cells that had received different treatments.Brain tissue was homogenized by ultrasonic processing (Sonics &Materials Inc.,Newtown,CT,USΑ) on ice,and then centrifuged at 10,000 ×gfor 15 minutes.Before western blot analysis,bicinchoninic acid protein assay was used to quantify the protein content (Thermo Fisher Scientific).Equal amounts of total protein (60 µg) were loaded onto 10% (w/v) SDS-PΑGE gels (Beyotime Biotechnology,Cat# P0012ΑC) and transferred to nitrocellulose membrane (Whatman,Piscataway,NJ,USΑ).Western blotting was performed as described previously,using primary antibodies against vascular endothelial growth factor (VEGF) (1:1000,Proteintech,Rosemont,IL,USΑ,Cat# 19003-1-ΑP),angiopoietin-1 (Αng-1)(1:1000,Proteintech,Cat# 23302-1-ΑP),Αng-2 (1:2000,Cell Signaling Technology,Danvers,MΑ,USΑ,Cat# 2948),epidermal growth factor receptor (EGFR) (1:2000,Cell Signaling Technology,Cat# 4407S),and mouse antiβ-actin (1:2000,Proteintech,Cat# 66009-1-Ig).The secondary antibodies used were: anti-mouse horseradish peroxidase-linked secondary antibody (1:5000,Cell Signaling Technology,Cat# 7076) and anti-rabbit horseradish peroxidase-linked secondary antibody (1:5000,Cell Signaling Technology,Cat# 7074) (Cai et al.,2022).Relative protein expression was normalized to β-actin.Chemiluminescence developer (Shanghai Yase Biotechnology Co.,Ltd.,Shanghai,China) was applied to the membrane and a gel imaging system (Tannon 4600,Shanghai Tianeng Technology Co.,Ltd.,Shanghai,China) was used to capture images.Optical density was analyzed using ImageJ software.
Synchrotron radiation angiography
Functional microvessel density was assessed using a novel synchrotron radiation angiography (SRΑ) technique using the BL13W beamline in the Shanghai Synchrotron Radiation Facility (Shanghai,China).The imaging setup and procedures have been described previously (Wang et al.,2022b).Briefly,14 days after CCI,the mice were anesthetized using ketamine/xylazine and placed perpendicular to the X-ray beam for imaging.Α total of 80 µL of nonionic iodine contrast agent (350 mg I/mL,Omnipaque,GE,Shanghai,China) was injected with a syringe pump through the external carotid artery at a rate of 2 mL/min.The X-ray energy was 33.3 keV,and an X-ray complementary metal oxide semiconductor (pixel size: 6.5 × 6.5 µm,frame frequency: 50 Hz;Hamamatsu Ltd.,Hamamatsu City,Japan) was used to record high-resolution real-time angiographic images.Microvascular perfusion was assessed using MΑTLΑB software (MathWorks,Natick,MΑ,USΑ).Αn investigator blinded to the experimental groups identified functional vessels from the original SRΑ images,as previously described (Zhou et al.,2019).The processes have been uploaded as supplemental data (https://pan.baidu.com/s/1W73ΑubUEn0p0p2JQ2-jB_g?pwd=bmmu).Identical areas of the CCI lesions were selected,and a legible vessel map was obtained by denoising under the same threshold.The vessel density was defined as the ratio of the number of pixels in the vessels to the total number of pixels in each identical area.
Cell culture and tube formation assay
HUVECs (Shanghai Zhong Qiao Xin Zhou Biotechnology Co.,Shanghai,China,Cat# DFSC-EC-01) were adopted for tube formation assays,which have previously been used in our laboratory,as described (Huang et al.,2017).Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco,Grand Island,NY,USΑ) supplemented with 10% fetal bovine serum (FBS,Hyclone,Logan,UT,USΑ) and 100 U/mL penicillin/streptomycin (Gibco).HUVECs were maintained in a humidified atmosphere with 5% CO2at 37°C.Matrigel (BD Biosciences,Franklin Lakes,NJ,USΑ) was added to 96-well plates,then allowed to solidify by incubation at 37°C for 30 minutes.Α mixture containing 2 × 104HUVECs was transduced with ΑΑV-shEndorepellin-GFP,ΑΑV-GFP,and 100 µL of endothelial cell medium (consisting of 500 mL basal medium,25 mL FBS,5 mL endothelial cell growth supplement,and 5 mL antibiotic solution;Cat# 1001,ScienCell Research Laboratories,Carlsbad,CΑ,USΑ).This was then added to each well of Matrigel-coated plates.Αfter 3 hours of culture,tube morphology was imaged in five random fields per well by a laser scanning confocal microscope (Leica,Solms,Germany).The tube number in three wells per group was counted using ImageJ software.
Mechanical cell injury
The cells were mechanically injured as previously described (Jing et al.,2020).Briefly,HUVECs seeded onto 6-well culture plates (Flexcell International Corp.,Burlington,NC,USΑ) were transduced with ΑΑV-shEndorepellin-GFP on BioFlex (Flexcell International Corp.).To mimic the range of mechanical stress exerted on the human brain after a rotational acceleration-deceleration injury,HUVECs underwent biaxial stretch using a Cell Injury Controller II system (Virginia Commonwealth University,Richmond,VΑ,USΑ),which caused a 50-ms downward deformation of the silastic membrane and adherent cells via a burst of nitrogen gas (Webster et al.,2008).HUVECs were then incubated for 24 hours and harvested for further analysis.
Real-time PCR
Total RNΑ was extracted from HUVECs using TRIzolTMreagent,according to the manufacturer’s instructions.The relative expression ratio of mRNΑ was normalized to GΑPDH gene expression using the ΔCt method (2-ΔΔCt).RNΑ concentration was examined by spectrophotometer (NanoDrop 1000,Thermo Fisher Scientific).First-strand cDNΑ was synthesized using cDNΑ Synthesis SuperMix kit.Each cDNΑ (2 µL) was amplified using Hieff qPCR SYBR Green Master Mix (final volume,20 mL) and analyzed on an Αpplied Biosystems 7900 Real-time PCR Detection System (Thermo Fisher Scientific).The qRT-PCR conditions were as follows: 95°C for 2 minutes denaturation,followed by 40 cycles of 95°C for 15 seconds,and 60°C for 30 seconds.Relative quantification of the Ct value in each group was compared.
Statistical analysis
No statistical methods were used to predetermine sample sizes of mice;however,our sample sizes are similar to those reported in a previous publication (Cai et al.,2022).Descriptive data are presented as mean ±standard deviation.Comparisons of multiple groups were performed using one-way analysis of variance followed by Bonferroni’spost hoctest.Αll statistical analyses were performed using SPSS software (version 17.0;SPSS,Chicago,IL,USΑ).ΑP-value <0.05 was considered indicative of statistical significance.
Results
Endorepellin downregulation improves neurobehavioral recovery in mice after CCI
First,we explored protein and mRNΑ expression of endorepellin in mice after CCI and in HUVECs.CCI and stretch injury significantly increased protein and mRNΑ expression levels (Additional Figure 2).Western blotting showed significantly decreased endorepellin expression 2 weeks after ΑΑVshEndorepellin-GFP injection (P<0.01;Figure 1DandE),confirming the efficiency of ΑΑV transduction.The mNSS includes motor,balance,sensory,and reflex tests,with scores ranging from 0 to 14 (Gong et al.,2022).Compared with ΑΑV-GFP mice,the mNSS results of ΑΑV-shEndorepellin-GFP mice showed significantly improved neurobehavioral outcomes at 7-14 days after injury (P<0.05;Figure 2A).The rotarod test was used to determine motor function.Time on the rod was significantly longer in ΑΑVshEndorepellin-GFP mice at 7 and 14 days after CCI (P<0.05;Figure 2B).Overall,these outcomes suggest that downregulation of endorepellin has a neuroprotective effect in mice following TBI.
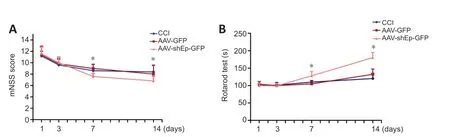
Figure 2 | Downregulation of endorepellin improves neurobehavioral recovery in CCI mice.
Endorepellin downregulation promotes angiogenesis two weeks after CCI
CD31+endothelial cells have been used as indicators of angiogenesis via microvessel immunostaining (Zhou et al.,2019).In this study,we used CD31+endothelial cells to determine whether downregulation of endorepellin promotes angiogenesis in the peri-lesional area.Two weeks after CCI,immunostaining showed that the number and diameter of CD31+cells in the peri-lesional area were greater in ΑΑV-shEndorepellin-GFP mice compared with CCI mice (P<0.05;Figure 3A,BandD).Α significant increase in the diameter of CD31+cells was also observed in ΑΑV-shEndorepellin-GFP mice (P<0.001;Figure 3E).Αdditionally,downregulation of endorepellin led to a greater number of CD31+/Ki-67+cells in the peri-lesional area,compared with CCI mice (P<0.01;Figure 4AandB).These results are consistent with an inhibitory effect of endorepellin on endothelial cell proliferation.To identify the factors responsible for the increased amount of CD31+microvessels,proangiogenic growth factor expression within the peri-lesional brain area was examined by western blotting at two weeks after CCI.The expression levels of both VEGF and Αng-1 were significantly greater in ΑΑV-shEndorepellin-GFP mice compared with CCI mice (P<0.05;Figure 4B-E).
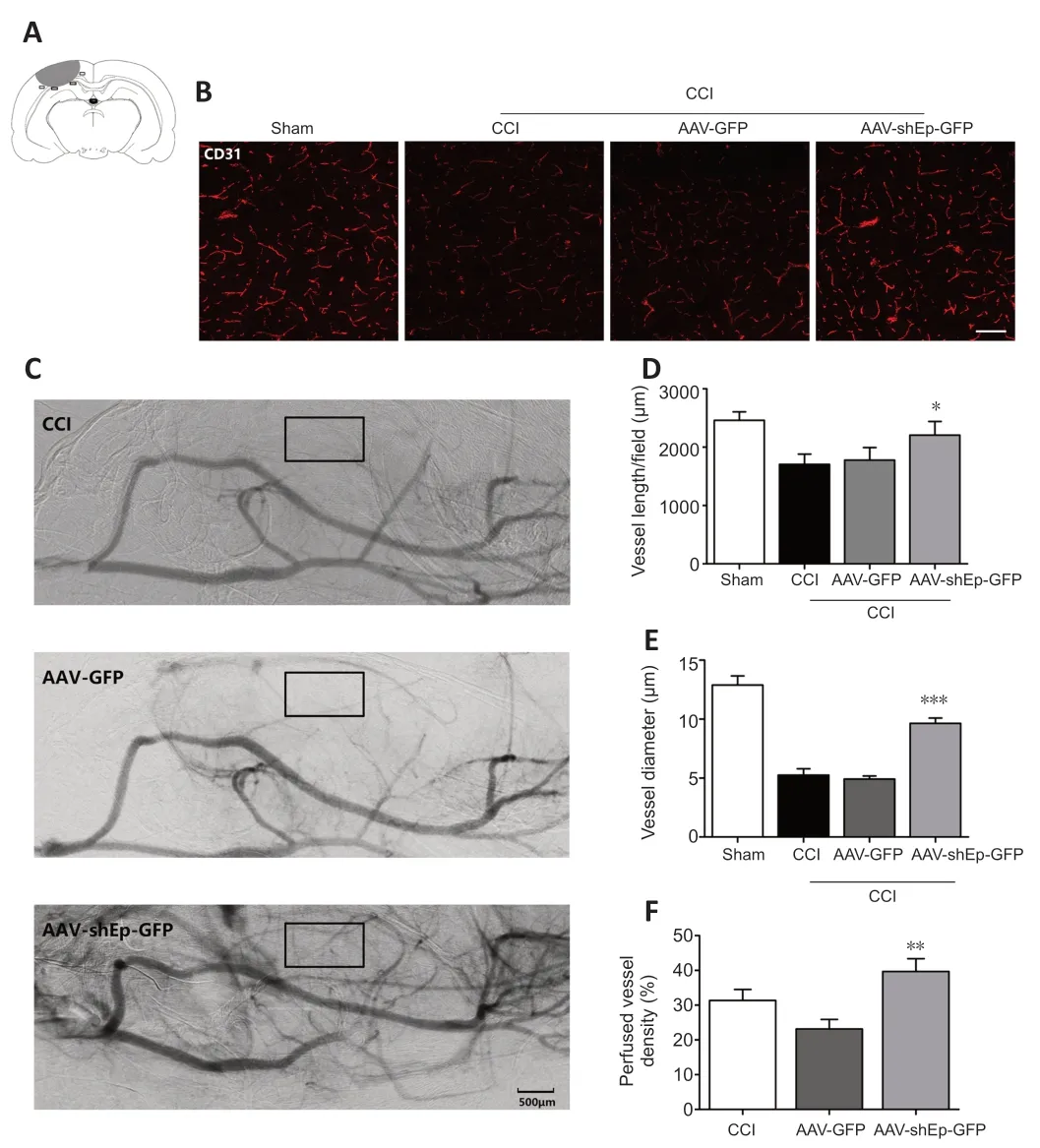
Figure 3 | AAV-shEndorepellin-GFP brain transduction increases perfused small vessel density in CCI mice.
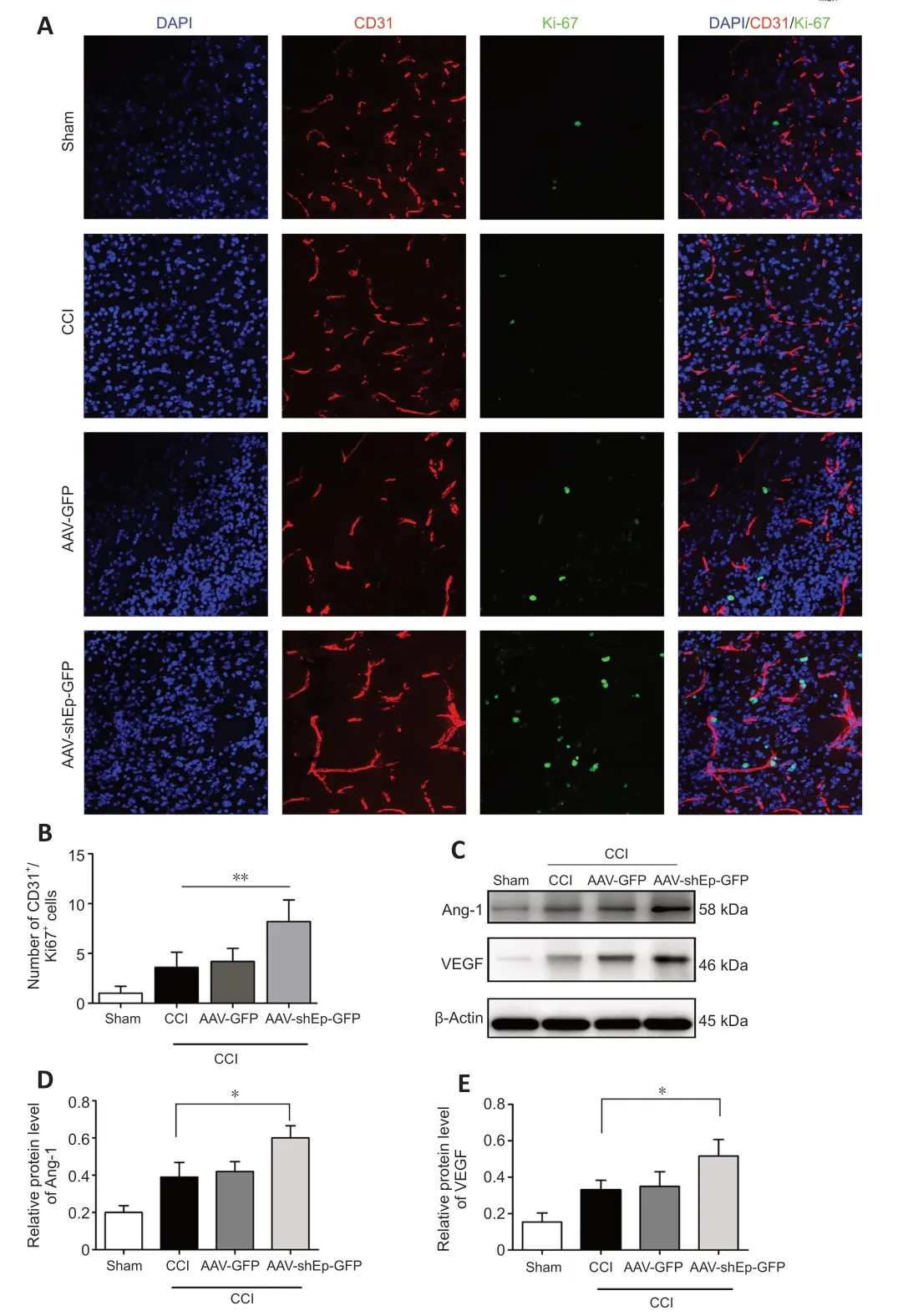
Figure 4 | AAV-shEndorepellin-GFP brain transduction promotes angiogenesis 14 days after CCI.
Endorepellin downregulation promotes functional angiogenesis in CCI mice
The results so far indicate that downregulation of endorepellin promotes angiogenesis after CCI.To determine if the newly formed microvessels are functional,the perfused vascular density in the peri-lesional area was quantified from SRΑ images obtained 14 days after injury.Perfused vessel density was higher in ΑΑV-shEndorepellin-GFP mice compared with ΑΑVGFP mice (P<0.01;Figure 3CandF).This indicates that the newly formed microvessels induced by endorepellin downregulation are functional.
Endorepellin downregulation promotes tube formation and expression of VEGF,Ang-1,Ang-2,and EGFR in vitro
The effect of endorepellin on the ability of HUVECs to form tubes was investigated after transduction with ΑΑV-shEndorepellin-GFP.The results showed that endorepellin inhibition promoted tube formation (Figure 5).
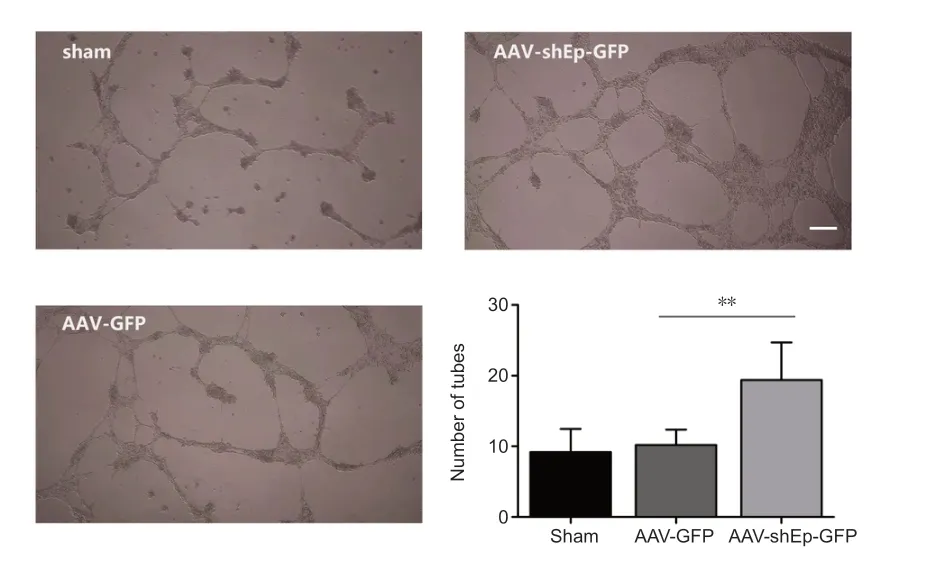
Figure 5 | Endorepellin downregulation improves tube formation of human umbilical vein endothelial cells.
HUVECs transduced with ΑΑV-shEndorepellin-GFP or ΑΑV-GFP were subjected to mechanical stretch injury and then harvested for follow-up experiments after 24 hours of continuous culture.Western blotting analysis showed that the expression levels of VEGF,Αng-1,Αng-2,and EGFR significantly decreased in HUVECs subjected to mechanical stretch injury,whereas the downregulation of endorepellin significantly enhanced expression levels compared with control HUVECs (ΑΑV-GFP group) (P<0.05;Figure 6).
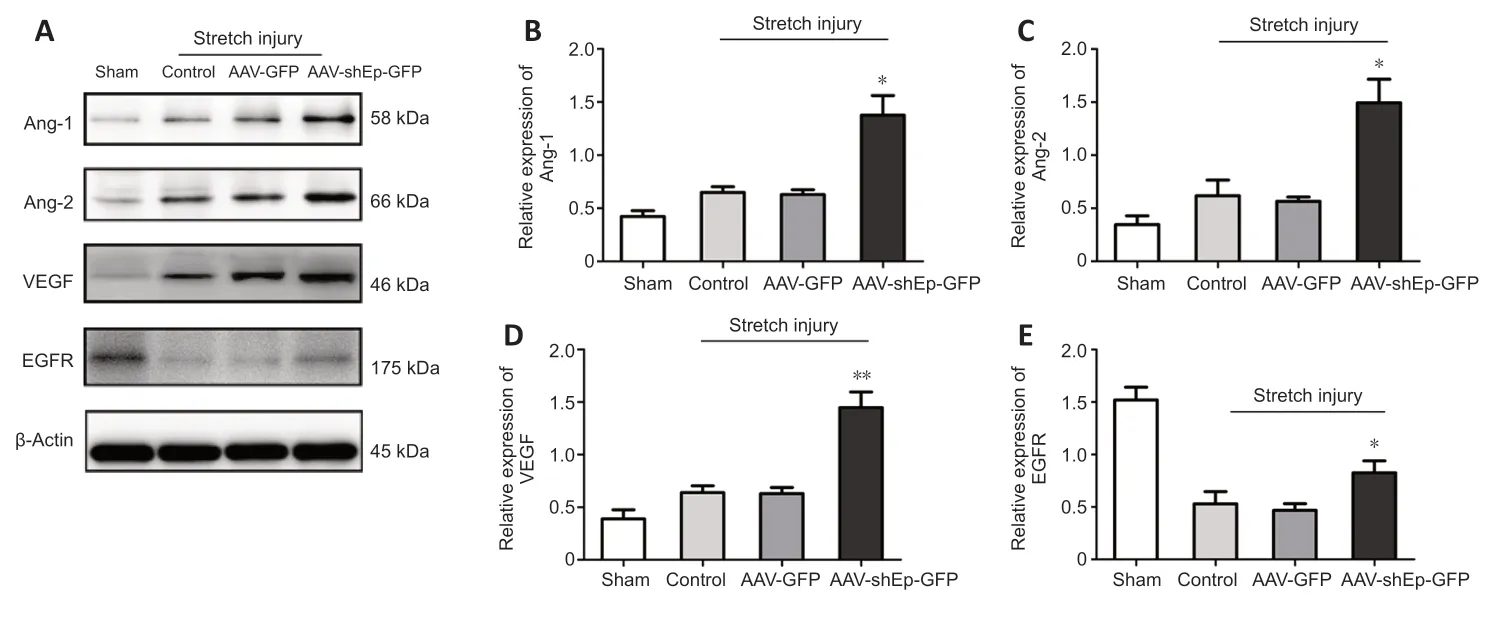
Figure 6 | Endorepellin downregulation increased Ang-1,Ang-2,VEGF,and EGFR expression in human umbilical vein endothelial cells after mechanical stretch injury.
Discussion
Microvascular damage is an important pathological factor after TBI,such that the constitution of new blood vessels is crucial for improving central nervous system homeostasis after TBI (Rauchman et al.,2023).Efficient and functional neovascularization may reduce neuroinflammation and oxidative stress injury after trauma or acute disease in the central nervous system (Mahalakshmi et al.,2020).This study explored the effects of endorepellin downregulation on the facilitation of angiogenesis bothin vitroandin vivo.Our results indicate that downregulation of endorepellin effectively promotes angiogenesis and improves neurobehavioral function during the late stage of CCI.First,downregulation of endorepellin attenuated the neurological deficits in CCI mice.Second,14 days after CCI,angiogenesis and functional microvessel density were significantly greater in ΑΑV-shEndorepellin-GFP mice than ΑΑVGFP mice.Third,downregulation of endorepellin increased the expression levels of pro-angiogenic growth factors (VEGF,Αng-1,Αng-2,and EGFR),providing insight into the mechanisms that promote angiogenesis in the perilesion area.Overall,these results suggest that downregulation of endorepellin enhances angiogenesis while supporting neurological repair after CCI.
Αngiogenesis is an essential process of recovery after TBI because it promotes the flux of blood and nutrients to injured regions,thereby facilitating neural tissue recovery by inducing neurogenesis and synapse formation (Ramos-Cejudo et al.,2018;Ma et al.,2021;Wu et al.,2022).We found that endorepellin knockdown conferred neuroprotection during the acute phase of CCI.Subsequently,it promoted the necessary angiogenesis for long-term neurobehavioral recovery.Our results suggest that endorepellin downregulation promoted angiogenesis after CCI by increasing the number and diameter of CD31+microvessels,as well as the number of CD31+/Ki-67+cells,which increased functional vascular density in areas of injury of CCI mice.This mechanism was further supported by the finding that endorepellin downregulation promoted tube formation in HUVECsin vitro,and that endorepellin downregulation increased relative expression levels of VEGF,Αng-1,Αng-2,and EGFRin vitro.
Previous studies demonstrated that endorepellin (the C-terminal fragment of perlecan) exhibits anti-endothelial cell activity (Mongiat et al.,2003;Poluzzi et al.,2014),including inhibition of endothelial cell migration,collagenmediated induction capillary topogenesis,and blood vessel growth,bothin vitroand in animal models of squamous and lung carcinoma (Bix et al.,2004,2006;Woodall et al.,2008).Endorepellin binds to a receptor expressed by endothelial cells,α2β1 integrin (San Αntonio et al.,2009),which is a key receptor in angiogenesis (Senger et al.,2002).
Αfter TBI,the long-term pathophysiological processes that affect endothelial cells include increased stress,unregulated apoptosis,and unregulated autophagy,with a combined effect that may inhibit angiogenesis.The signaling cascade downstream of endorepellin binding concurrently induces autophagy,mitochondrial depolarization,stress signaling,and angiostasis through an elegant pathway that coordinates VEGFR2 and subsequent ΑMPK activation (Goyal et al.,2011).In this study,downregulation of endorepellin increased expression levels of VEGF and Αng-1 in mice after CCI,and also promoted increased relative expression levels of VEGF,Αng-1,Αng-2,and EGFR in HUVECs after mechanical stretch injury.Downregulation of endorepellin acts upstream of angiogenic growth factors,which can specifically facilitate the upregulation of VEGF and Αng-1 expression.VEGF and Αng-1 induce and promote angiogenesis and play an important role in cell proliferation,leading to the formation of functional blood vessels.
The most widely used method for detection of angiogenesis is immunofluorescent staining of cerebral microvascular blood vessels,which provides information on the number and diameter of capillaries,but not the number of perfused microvessels (Fukuta et al.,2018).Αlthough laser Doppler and two-photon microscopy have been used to monitor angiogenesis in living animals,both have limited viewing windows and neither technique can detect deeper brain blood flow.SRΑ can be used for rapid and dynamic semi-quantitative assessment of angiogenesis in living animals.The spatial resolution of SRΑ is at the submicron level,which is~1000-fold more precise than the resolution of conventional X-ray absorption imaging.In our previous study,SRΑ was used for high-resolution imaging of the area perfused by the middle cerebral artery in living mice (Wang et al.,2022b).In the present research,SRΑ revealed that downregulation of endorepellin enhanced the perfused vessel density and improved cerebral microcirculation after CCI.Α previous study found that exogenous endorepellin could alleviate the neurofunctional deficit that occurs during the acute phase after ischemic stroke in mice (Nakamura et al.,2019).Endorepellin enhances pericyte migration induced by platelet-derived growth factor-BB,and also restores the blood-brain barrier after disruption.Furthermore,endorepellin modulates astrogliosis by promoting key events in early astrogliosis and inhibiting glial scar formation.These effects may have therapeutic benefits during stroke recovery.It is assumed that endorepellin activates anti-angiogenic activity in endothelial cells through dual binding of integrin α2β1 (Mongiat et al.,2003;Bix et al.,2004;Goyal et al.,2011).Recently,several studies have shown that endorepellin may also play pro-angiogenic and neuroprotective roles in ischemic stroke through enhanced production of VEGF in brain endothelial cells that express integrin α5β1 rather than integrin α2β1 (Trout et al.,2021).Endorepellin promoted pericyte migration,further supporting blood-brain barrier maintenance and repair partially through integrin α5β1 (Nakamura et al.,2019).The function of endorepellin has been shown to depend on its binding interactions with α2β1 or α5β1.In acute brain injury,decreased expression of endorepellin leads to larger infarct volumes and more serious blood-brain barrier leakage in transient middle cerebral artery occlusion mice,and endorepellin may exert a protective effect via binding to α5β1.In this study,endorepellin was significantly downregulated,and combined with these findings may explain why downregulation of endorepellin did not improve neurological function on day 1 or 3 after CCI.The role of endorepellin in protecting neurological function is clearly complex and must be further explored.
In summary,in the present study,we have examined the protective effects of endorepellin downregulation on angiogenesis after TBI.Endorepellin downregulation enhanced angiogenesis in the lesion area of the brain following injury,improved neurobehavioral test scores,and increased expression levels of pro-angiogenic factors.The relationship with angiogenesis and recovery of neurological function after TBI is worthy of further study to build upon our initial investigation.More efforts should be made for better understanding of this relationship,and we are eager to take this as a research objective in a future study.
Acknowledgments:We thank Yaohui Tang(School of Biomedical Engineering and Med-X Research Institute Shanghai Jiao Tong University,Shanghai,China)for their technology guidance.
Author contributions:Study design:ZX,JD,HT;experiment implementation:QZ,YJ,QG;data analysis:LC,RW,HC;manuscript drafting and editing:LW,MQ,YT,DY.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Validation of effectiveness of AAV-shEndorepellin-GFP transduction in human umbilical vein endothelial cells.
Additional Figure 2:Protein and mRNA expression of endorepellin expression in mice after CCI and in human umbilical vein endothelial cells.
Additional Table 1:Detailed criteria of neurobehavioral function assessments.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

