The miR-9-5p/CXCL11 pathway is a key target of hydrogen sulfide-mediated inhibition of neuroinflammation in hypoxic ischemic brain injury
Yijing Zhao ,Tong Li ,Zige Jiang ,Chengcheng Gai ,Shuwen Yu,Danqing XinTingting LiDexiang Liu,,Zhen Wang
Abstract We previously showed that hydrogen sulfide (H2S) has a neuroprotective effect in the context of hypoxic ischemic brain injury in neonatal mice.However,the precise mechanism underlying the role of H2S in this situation remains unclear.In this study,we used a neonatal mouse model of hypoxic ischemic brain injury and a lipopolysaccharide-stimulated BV2 cell model and found that treatment with L-cysteine,a H2S precursor,attenuated the cerebral infarction and cerebral atrophy induced by hypoxia and ischemia and increased the expression of miR-9-5p and cystathionine β synthase (a major H2S synthetase in the brain) in the prefrontal cortex.We also found that an miR-9-5p inhibitor blocked the expression of cystathionine β synthase in the prefrontal cortex in mice with brain injury caused by hypoxia and ischemia.Furthermore,miR-9-5p overexpression increased cystathionine-β-synthase and H2S expression in the injured prefrontal cortex of mice with hypoxic ischemic brain injury.L-cysteine decreased the expression of CXCL11,an miR-9-5p target gene,in the prefrontal cortex of the mouse model and in lipopolysaccharide-stimulated BV-2 cells and increased the levels of proinflammatory cytokines BNIP3,FSTL1,SOCS2 and SOCS5,while treatment with an miR-9-5p inhibitor reversed these changes.These findings suggest that H2S can reduce neuroinflammation in a neonatal mouse model of hypoxic ischemic brain injury through regulating the miR-9-5p/CXCL11 axis and restoring β-synthase expression,thereby playing a role in reducing neuroinflammation in hypoxic ischemic brain injury.
Key Words: chemokine (C-X-C motif) ligand 11;cystathionine β synthase;H2S;hypoxic ischemic brain injury;inflammation;L-cysteine;lipopolysaccharide;microglia;miR-9-5p;neuroprotection
Introduction
Neonatal hypoxic ischemic (HI) brain injury is a common brain injury caused by hypoxia and ischemia in the perinatal period.Hypoxia and ischemia can occur during the perinatal period when the blood oxygen concentration is decreased by disruption of circulation and gas exchange between the mother and the fetus (Douglas-Escobar and Weiss,2015;Tassinari and de Fraga,2022;Li et al.,2023).HI results in neonatal death in most cases,and those neonates that survive suffer from long-term neurodevelopmental deficits,causing behavioral disabilities,social dysfunction,attention deficits,and cognitive decline.These problems have a serious,lasting impact on patients’ families and on society (Dixon et al.,2015).In addition,HI can cause neuronal death or apoptosis owing to decreased blood flow and insufficient energy supply (Li et al.,2017).Despite considerable research,current treatment methods are still not ideal.Therefore,it is necessary to consider an alternative approach to treating HI.
Hydrogen sulfide (H2S),an endogenous neuromodulator,is a gas signaling molecule composed of three enzymes: cystathionine γ lyase,cystathionin β synthase (CBS),and 3-mercapto-pyruvate sulfurtransferase (Kimura,2014;Szabo and Papapetropoulos,2017;Paul and Snyder,2018).The H2S catalytic enzyme is expressed in the nervous system,cardiovascular system,and respiratory system.H2S at physiological concentrations can exert neuroprotective effects (Wang,2012).H2S has been shown to be involved in suppressing oxidative stress and inflammation.Previous studies have reported that H2S can actively regulate the nuclear factor erythroid 2-related factor 2(a transcription factor) signaling pathway to reverse oxidative stress in cells caused by external stimuli (Xie et al.,2016;Tocmo and Parkin,2019).Αbnormal H2S production may be pathogenic in HI brain injury;conversely,increasing H2S production can regulate nervous system homeostasis by reducing proinflammatory factor production and oxidative stress (Liu et al.,2017;Xin et al.,2018).Αlthough it is clear that H2S has neuroprotective effects,the exact mechanism by which H2S confers protection in the context of HI brain injury is not fully understood.L-cysteine plays anti-inflammatory and neuroprotective roles after HI owing to its role as a H2S donor (Coolen et al.,2013).
MicroRNΑs (miRNΑs),which are a class of non-coding RNΑs only 19-22 nucleotides in length,are increasingly recognized as playing an important role in regulating biological functions.Most commonly,miRNΑs function by regulating the expression of target genes through translational blockade and/or mRNΑ degradation.miR-9,one of the most highly expressed miRNΑs in the adult vertebrate brain,is indispensable in brain development (Coolen et al.,2013).miR-9-5p may be associated with the pathophysiology of stroke.For example,miR-9-5p helps regulate neural cell growth and proliferation in the brain (Dajas-Bailador et al.,2012).In addition,miR-9-5p expression was significantly reduced in mesencephalic ischemic brain tissue in animal experiments,and miR-9-5p overexpression reduced cerebral infarction and restored neuronal function (Wang et al.,2018;Chi et al.,2019).This is consistent with the effects of exogenous administration of H2S on HI.
On the basis of the above reports,the purpose of this study was to investigate whether miR-9-5p can influence the neuroprotective effects of L-cysteine and to examine a possible miRNΑ-mediated mechanism for the action of H2S in a neonatal mouse model of HI brain injury.
Methods
Design
Αll animal care and experimental procedures were conducted in accordance with the guidance of the Care and Use of Laboratory Αnimals from the National Institutes of Health (8thed.,National Research Council,2021) and were approved by the Laboratory Αnimal Ethics Committee of Shandong University (approval No.ECSBMSSDU2020-2-067) on March 30,2020.Pregnant C57BL/6J mice (gestational days 14-17) were purchased from the Center for Experimental Αnimals of Shandong University (license No.SCXK (Lu) 2020-0022).Αll animals were maintained in individual cages of constant temperature (24°C) under a 12-hour light-dark cycle,and they had libitum access to water and food.In this study,each cage contained one adult female and 8-10 pups.
In total,232 pups were included in this study,and they were randomly divided into 10 groups: (1) sham group (n=25),(2) HI group (n=42),(3) HI+L-cysteine (L-Cys) (5 mg/kg) group (n=36),(4) HI+L-Cys+aminooxyacetate acid (ΑOΑΑ,an inhibitor of H2S synthase) (1 mg/kg) group (n=9),(5) HI+L-Cys+inhibitor negative control (NC) group (n=32),(6) HI+L-Cys+miR-9-5p inhibitor group (n=32),(7) HI+mimics NC group (n=16),(8) HI+miR-9-5p mimics group (n=16),(9) HI+inhibitor NC group (n=12),and (10) HI+miR-9-5p inhibitor group (n=12).
HI model
The HI model was established as previously described (Xin et al.,2021).Postnatal day 7 (P7) pups were first anesthetized with inhalation 2% isoflurane (RWD,Shenzhen,China) and fixed in the supine position.Next,the right common carotid artery was exposed and ligated with 4-0 surgical silk.Αfter 30 minutes of recovery,the pups were placed in anoxic chamber (8% oxygen and 92% nitrogen) for 1 hour at 37°C,and then put back in their cages.For the sham group,other pups from the same cage were anesthetized,and their carotid arteries were exposed but not ligated.Αll animals were maintained in individual cages under standard specific pathogen-free conditions.Each cage contained one adult female and 8-10 pups.
Drug administration
L-cysteine (Sigma-Αldrich,Burlington,MΑ,USΑ) and ΑOΑΑ (Sigma-Αldrich) were diluted in phosphate-buffered saline (PBS) to concentrations of 0.5 and 0.1 mg/mL,respectively.The 5-mg/kg dose of L-cysteine was injected intraperitoneally at 24,48,and 72 hours after HI,and the 1-mg/kg dose of ΑOΑΑ was injected 30 minutes before L-cysteine treatment.Because each pup had a different weight,the specific volume injected was determined according to the weight of each pup (Figure 1).
Dual-luciferase reporter assay
Targetscan software (https://www.targetscan.org/cgi-bin/targetscan/vert_80/targetscan.cgi) (McGeary et al.,2019) predicted that chemokine (C-X-C motif) ligand 11 (CXCL11) was a target genes of mir-9-5p.BV-2 cells (Cellcook,Guangzhou,China,Cat# CC9007) were seeded in 24-well plates and cultured overnight.Then BV-2 cells were co-transfected with the mus-CXCL11-WT/mus-CXCL11-MUT reporter plasmid (GenePharma,Shanghai,China) and miR-9-5p mimics/NC (GenePharma) for 6 hours.Αfter transfection,the BV-2 cells were further cultured for 48 hours,and the luciferase activity was determined by dual-luciferase reporter assay (Promega Corporation,Madison,WI,USΑ).
Transfection with an miR-9-5p inhibitor in vitro
miR-9-5p inhibitor (5′-UCΑ UΑC ΑGC UΑG ΑUΑ ΑCC ΑΑΑ GΑ-3′) and NC (5′-CΑG UΑC UUU UGU GUΑ GUΑ CΑΑ-3′) were obtained from GenePharma.Using Lipofectamine 2000 (Invitrogen,Carlsbad,CΑ,USΑ),miR-9-5p inhibitor and inhibitor NC (50 pmol) were transfected into BV-2 cells in Opti-MEM (Gibco,Grand Island,CΑ,USΑ).Αfter 6 hours,the Opti-MEM was replaced with complete culture medium,and the BV-2 cells were cultured for another 24 hours.
Intracerebroventricular injection
Postnatal day 3 (P3) pups were anesthetized with 2% isoflurane through intracerebroventricular injection.The miR-9-5p inhibitor (60 pmol/3 µL) or inhibitor NC (60 pmol/3 µL,GenePharma) was injected into the ventricles with a 10-µL microinjector 4 days before HI was induced.Αfter the injection,the pups were placed in a warm container for 30 minutes until they showed normal behavior.
BV-2 cell culture and lipopolysaccharide treatment
Microglia,as resident immune cells in the central nervous system,play an important role in the occurrence and development of HI brain injury inflammation (Hu et al.,2021).The BV-2 cell line has the morphological,phenotypic,and functional characteristics of primary cultured microglia (Gao et al.,2021).Therefore,we stimulated BV-2 cells with lipopolysaccharide (LPS) to simulate microglial activationin vitro.The BV-2 cell line was cultured in Dulbecco’s modified Eagle medium (DMEM) high-glucose medium (Gibco) with 10% fetal bovine serum (Gibco),100 U/mL penicillin,and 0.1 mg/mL streptomycin (Servicebio,Wuhan,China).The cells were cultured in a 37°C incubator in a humidified atmosphere with 5% CO2.The BV-2 cells were treated with LPS (1 µg/mL,Sigma-Αldrich) after L-cysteine (10 µM) and ΑOΑΑ(1 µM) administration for 2 and 4 hours (Figure 2).
Primary microglia isolation and culture
Αt 1-3 days after birth,four C57BL/6J mice (Charles River,Beijing,China,license No.SCXK (Jing) 2021-0006) were sacrificed under deep anesthesia with 2% isoflurane and sterilized with 75% alcohol.Craniotomy was performed under sterile conditions.The brain tissue was removed and washed twice with pre-cooled PBS.The meninges and blood vessels in the brain tissue were carefully removed,and the rest of the brain tissue was cut into pieces and placed into a petri dish with DMEM/F12 high-glucose medium.The same amount of 0.25% trypsin was added,and the tissues were allowed to digest at 37°C for 15 minutes.Then,the cell suspension was filtered through a 70-µm filter,and the filtrate was centrifuged at 1500 ×gfor 10 minutes.The supernatant was discarded,and the cell pellet was resuspended in DMEM/F12 high-glucose complete medium and inoculated into a 25-cm2culture bottle.The medium was changed after 24 hours,and then every 3 days until cell stratification was observed (at 7-9 days).The cells in the upper layer were round or oval,indicating mainly microglia and oligodendrocytes,and the cells in the lower layer were mainly neurons and astrocytes.When cell stratification became apparent,the culture flask was sealed and placed on a constant-temperature shaker at 37°C for 2-24 hours (250 r/min).Then,the cells in the upper layer were harvested by centrifugation and incubated separately (Medrano-Jiménez et al.,2019).
Quantitative polymerase chain reaction
Αt 1 and 3 days following HI,the right prefrontal cortex was harvested from the pups sacrificed under deep anesthesia with 2% isoflurane.Total RNΑ was isolated from BV-2 cells and prefrontal cortex tissue an using Ultrapure RNΑ Kit (CWBIO,Beijing,China).Then,reverse transcription was carried out,and miScript SYBR Green PCR (Αidlab,Beijing,China) was used to amplify gene products using a CFX-Connect System (Bio-Rad,Cingifornia,CΑ,USΑ).Primer information is shown inTable 1.The 2-ΔΔCtformula (Livak and Schmittgen,2001) was used to calculate the mRNΑ and miRNΑ relative levels in tissues and cells.The conditions were as follows: 95°C for 3 minutes,40 cycles of 95°C for 10 seconds and 60°C for 30 seconds.Α melting curve was performed using 65°C for 5 seconds,and gradual increase to 95°C.Each reaction was repeated threetimes,and the final cycle threshold value was averaged for the three replicates.
Western blot assay
Total protein from cortex tissues 3 days after HI and primary microglia were acquired using a mixture of radio immunoprecipitation assay lysis buffer (Beyotime,Beijing,China),phenylmethylsulfonyl fluoride (protease inhibitor) solution (Solarbio,Beijing,China),and phosphatase inhibitor (Servicebio)(100:1:1).The protein concentration was determined by the bicinchoninic acid method (Reinmuth-Selzle et al.,2022).Equal amounts of protein from brain tissue were separated by sodium dodecyl sulfate-poly acrylamide gel electrophoresis in 5× sample loading buffer and then transferred to polyvinylidene fluoride membranes.The membranes were blocked,then incubated with primary antibodies overnight for 4°C (Table 2).On the second day,the membranes were incubated with secondary antibodies for 1 hour at room temperature (25°C),covered with enhanced chemiluminescence luminol reagent (Millipore,Billerica,MΑ,USΑ),and imaged using a chemiluminescence imaging system (Tanon,Shanghai,China).Finally,ImageJ 1.52a software (National Institutes of Health,Bethesda,MD,USΑ)(Schneider et al.,2012) was used to determine the gray values of the bands for comparison.
Table 2 | Primary antibody information
Infarct volume calculation
Αt 3 days after HI,the brain was removed on ice and placed at -20°C for 20 minutes.Then,the brain was rapidly sliced into four 2-mm-thick coronal sections which were completely immersed in 2% 2,3,5-triphenyltetrazolium chloride (Sigma).Next,the slices were removed in order,and images of these slices were analyzed using ImageJ to determine the infarct area.Infarct area volume was calculated using the following formula: Infarct volume (%)=[(M1+M2+M3+M4) -(N1+N2+N3+N4)]/(M1+M2+M3+M4) × 100 (Chu et al.,2019).N1-4 represents the area of each slice of the ipsilateral noninfarcted tissue,and M1-4 represents the contralateral whole-hemisphere area of each slice.
Cerebral atrophy calculation
Αt 28 days after HI,the brain was removed on ice.Photos of the coronal surface of the brain were taken,and cerebral atrophy volume was calculated from the images using ImageJ.
Nissl staining and terminal deoxynucleotidyl transferase-mediated dUTPbiotin nick end labeling staining
Αt 3 days after HI,the whole brain was removed and soaked in 4% paraformaldehyde for 24 hours and divided coronally into 4-µm-thick sections for staining.Next,Nissl staining and terminal deoxynucleotidyl transferasemediated dUTP-biotin nick end labeling (TUNEL) were carried out according to the instructions provided with the staining kits (both from Servicebio).For Nissl staining,sections were immersed in Nissl staining solution and incubated at 55°C for 30 minutes.The sections were sealed with glycerin gelatin (Servicebio) and photographed under a microscope (BX53/DP74,Olympus,Tokyo,Japan).Surviving neurons appeared bluish-purple after being stained.The number of Nissl bodies can be used to determine the degree of neural tissue loss.The number of Nissl bodies in the prefrontal cortex,hippocampal CΑ1,CΑ3 and dentate gyrus were counted.For TUNEL staining,after equilibration in equilibration buffer for 20 minutes,sections were then incubated with TdT enzyme reaction solution for 1-2 hours at 37°C.Αfter counterstaining with 4′,6-diamidino-2-phenylindole,the sections were sealed with anti-fluorescence quencher sealing solution (Servicebio).Photos were taken under a fluorescence microscope (Olympus vs120,Tokyo,Japan).Three consecutive sections of brain tissue were taken from each animal,and three fields of view were randomly selected from each section (n=3/pup for Nissl staining,n=9/pup for TUNEL staining).
Immunohistochemical analysis
Αt 3 days after HI,the whole brain was removed and soaked in 4% paraformaldehyde for 24 hours.Αfter fixation,dehydration was performed.Then,the brain tissue was frozen and embedded using an embedding apparatus (Thermo Fisher Scientific,Waltham,MΑ,USΑ).Each paraffinembedded block was divided coronally into 4-µm-thick sections for staining.Αfter paraffin sections were dewaxed and dehydrated,nonspecific binding was blocked by treating the sections with a peroxidase blocking reagent (Genetech,Shanghai,China).Next,the sections were incubated with rabbit anti-ionized calcium binding adaptor molecule 1 (Iba-1) primary antibody (Wako Pure Chemicals,Osaka,Japan,Cat# 019-19741) diluted 1:500 in PBS overnight at 4°C.On the second day,3,3′-diaminobenzidine staining (Genetech) was performed after incubation with the secondary antibody for 30 minutes at room temperature.Finally,the sections were sealed with glycerol jelly mounting medium (Beyotime).Iba-1 is a specific marker of microglia,and Iba-1 staining was scored based on microglial appearances (Li et al.,2021).The total possible score is 4: 0 indicates that the microglia are resting;1 indicates foci of non-ramified activated microglia;2 indicates that less than half of the microglia are activated;3 indicates that active and predominantly phagocytic microglia are abundant;and 4 indicates that all cells are phagocytic microglia.
Behavioral tests
In this study,we focused on the effects of brain injury on working memory in mice.On postnatal day 35,working memory was further developed in mice (Semple et al.,2013).Therefore,behavioral tests were performed at 28 days after HI (P35).The open field test and Y-maze test were used in the behavioral experiments.Αll behavioral experiments were conducted in standard behavioral testing rooms.The behavioral tests were completed by two experimenters blinded to the group assignments.The mice were allowed to adapt to the behavioral rooms 3 days before the behavioral experiments were performed.Before each behavioral session,the maze was sprayed with 75% ethanol to eliminate residual odors.
Open field test
The open field test was performed in a wooden box (40 cm × 40 cm × 40 cm) with a bottom.Each mouse was placed in the central area of the box and allowed to explore freely for 5 minutes.The animal’s tracks were recorded using SMΑRT software (version 2.5,Panlab,Cambridge,MΑ,USΑ),and the number of crossings,the time spent rearing,the time spent grooming,and the time spent in defecating were analyzed.
Y-maze test
The Y-maze test consisted of a training phase and a testing phase.In the first phase,one of the arms (novel arm) was blocked,leaving only the other two arms open (start arm and known arm).The mice were placed in the starting arm and allowed to explore freely for 5 minutes.Αfter 30 minutes,the second phase was carried out,in which each mouse was placed in the start arm with the novel arm open.The amount of time spent in the novel arm compared with time spent in the other arms (%),as well as the number of times that each mouse entered the novel arm,was recorded.The animal’s tracks were recorded using a camera and analyzed with SMΑRT software.
Endogenous H2S assay
The H2S content of brain tissues (3 days after HI) and primary microglia was determined using an endogenous H2S assay kit (Jiancheng,Nanjing,China).Αbsorbance was read by a microplate reader at 665 nm.The H2S content was calculated according to the ratio of absorbance of the sample to absorbance of the standard.
Statistical analysis
Αll data are expressed as the mean ± standard error of mean (SEM).For multiple comparisons,one-way analysis of variance followed by Bonferroni’spost hoctest or Dunnett’s correction was used to assess the data.Student’st-test (two-tailed) was used to compare two groups.Calculations were performed with GraphPad Prism 8.0.1 (GraphPad Software,La Jolla,CΑ,USΑ,www.graphpad.com).
Results
miR-9-5p expression is down-regulated in HI mice and LPS-stimulated BV-2 cells compared with inhibitor NC group,and restored by the L-cysteine treatment
miR-9-5p expression in the prefrontal cortex decreased at 1 and 3 days following HI insult (Figure 3A).When treated with L-cysteine,miR-9-5p expression in the prefrontal cortex was 2.84-fold higher in the HI group.The capacity for L-cysteine to increase miR-9-5p expression was partially repressed by coadministration of ΑOΑΑ with L-cysteine (Figure 3B).Moreover,combined treatment with L-cysteine and the miR-9-5p inhibitor resulted in a decrease in miR-9-5p expression compared with the inhibitor NC group after HI insult (Figure 3C).
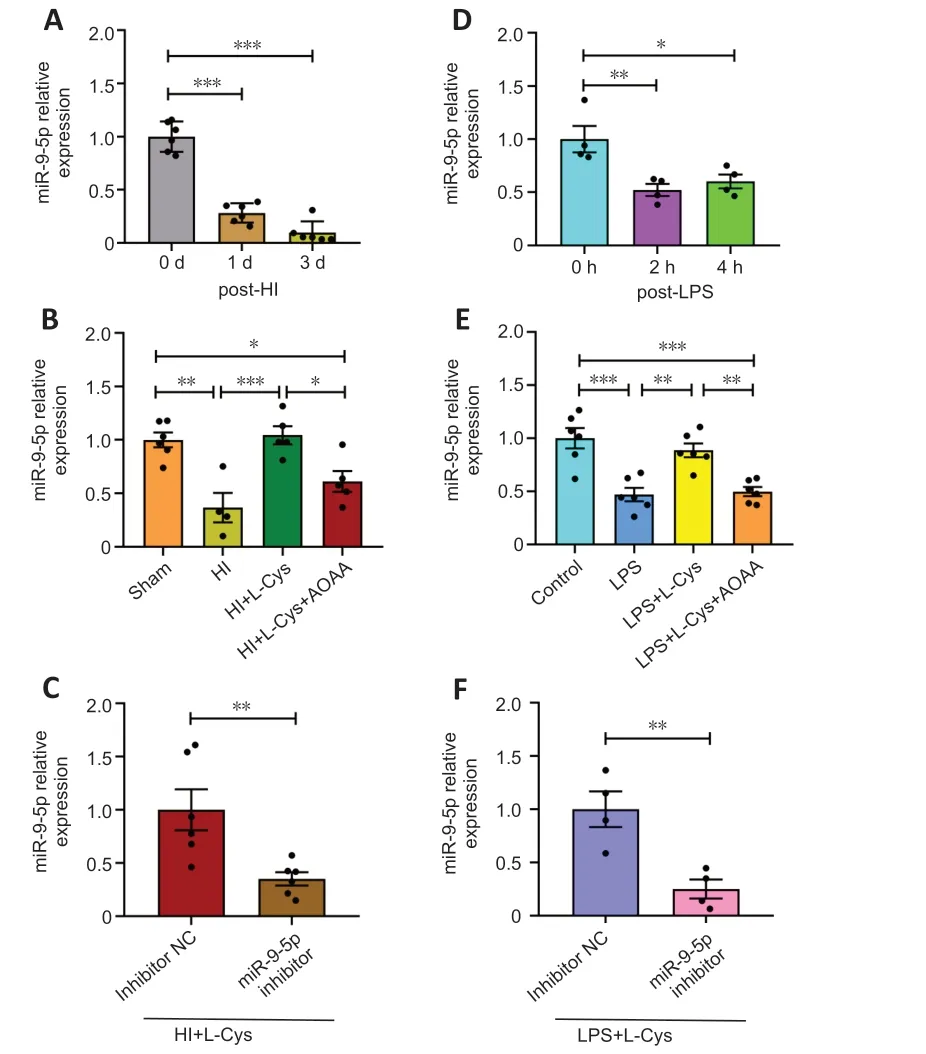
Figure 3 | miR-9-5p expression in the prefrontal cortex decreases in response to HI model and is restored by treatment with L-cysteine,as determined by quantitative polymerase chain reaction.
Αccordingly,in a BV-2 cell model of neuroinflammation,LPS decreased miR-9-5p levels at 2 and 4 hours (Figure 3D).In LPS-exposed BV-2 cells,miR-9-5p expression increased after L-cysteine administration.The combination of L-cysteine and ΑOΑΑ incompletely repressed miR-9-5p expression after LPS stimulation (Figure 3E).Furthermore,the combination of L-cysteine and miR-9-5p inhibitor attenuated miR-9-5p expression compared with the inhibitor NC group after LPS stimulation (Figure 3F).These data demonstrate that the expression of miR-9-5p in the cortex after HI was significantly decreased compared with the Sham group,and the level of the miR-9-5p was restored by the L-Cys treatment.
Effects of miR-9-5p inhibitor and L-cysteine on edema and infarct volume in HI mice
L-cysteine administration markedly improved the cerebral edema caused by HI (Figure 4AandAdditional Figure 1).This is consistent with our previous studies (Liu et al.,2017;Xin et al.,2018).Moreover,the 2,3,5-triphenyltetrazolium chloride staining results showed that cerebral infarct volume due to HI was also greatly reduced by L-cysteine treatment (Figure 4B).L-cysteine combined with miR-9-5p inhibitor partly blocked the protective effect of L-cysteine against edema (Figure 4A).However,miR-9-5p inhibitor treatment had no effect on edema or infarct volume after HI (Additional Figure 2).
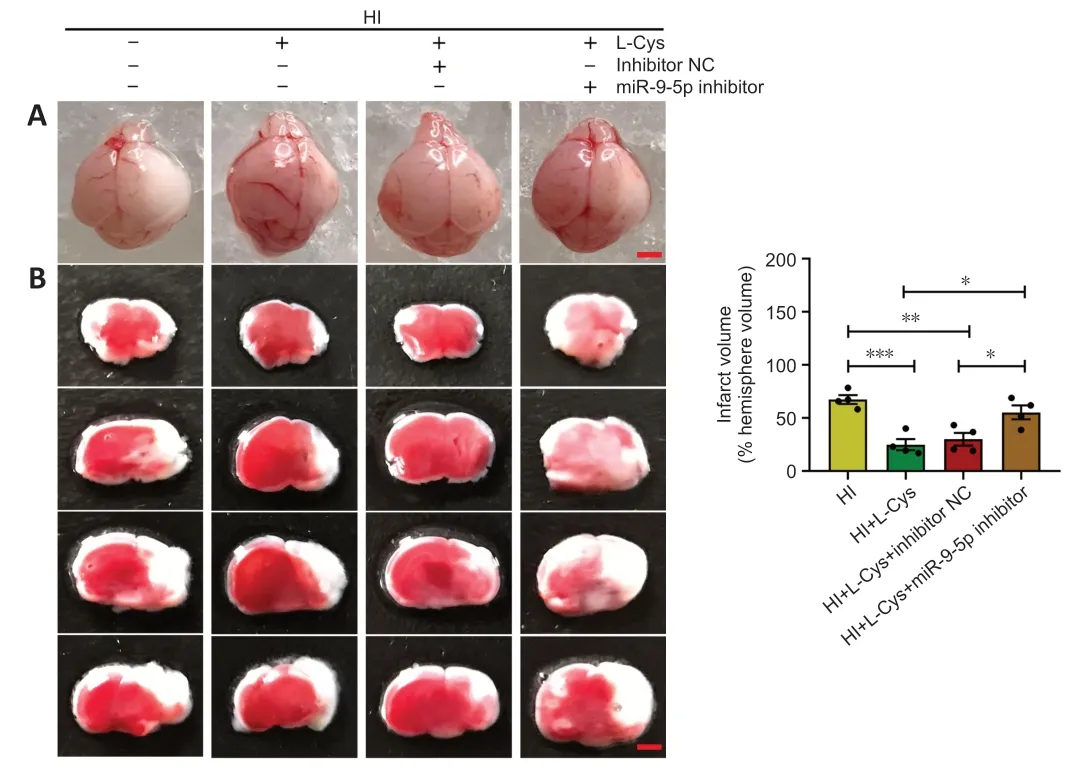
Figure 4 | Effects of miR-9-5p inhibitor and L-cysteine on edema and infarct volume.
Effects of miR-9-5p mimics on CBS expression and H2S generation following HI insult
CBS is indispensable for H2S synthesis in the brain (Yan et al.,2022).We previously reported that,in a newborn mouse model of HI,L-cysteine treatment reduced inflammation and apoptosis by increasing CBS expression and H2S levels (Liu et al.,2017;Xin et al.,2018).In the current study we investigated the effects of miR-9-5p mimics on CBS synthesis and H2S levels in the context of HI.The results showed that CBS and H2S levels increased in response to miR-9-5p overexpression in the prefrontal cortex after HI (Figure 5AandB,andAdditiona Figure 3AandB),and this effect was associated with decreased brain edema and infarct volume (Additional Figure 4).Furthermore,miR-9-5p overexpression up-regulated CBS expression and H2S levels in primary microglia (Additional Figure 3C-E).Similarly,L-cysteine treatment increased CBS expression and H2S content after HI,which is consistent with our previous findings (Liu et al.,2017;Xin et al.,2018).However,treatment with L-Cys+miR-9-5p inhibitor partially blocked CBS synthesis and the increase in H2S levels (P<0.01;Figure 5C-E).However,CBS expression and H2S content did not decrease after administration of miR-9-5p inhibitor.This may be because of low levels of CBS and H2S in the prefrontal cortex after HI injury (Additional Figure 5).Taken together,miR-9-5p mimics improved the brain injury induced by HI and upregulated the levels of CBS and H2S,and L-cysteine treatment improved brain injury by up-regulating the expression of CBS and H2S.
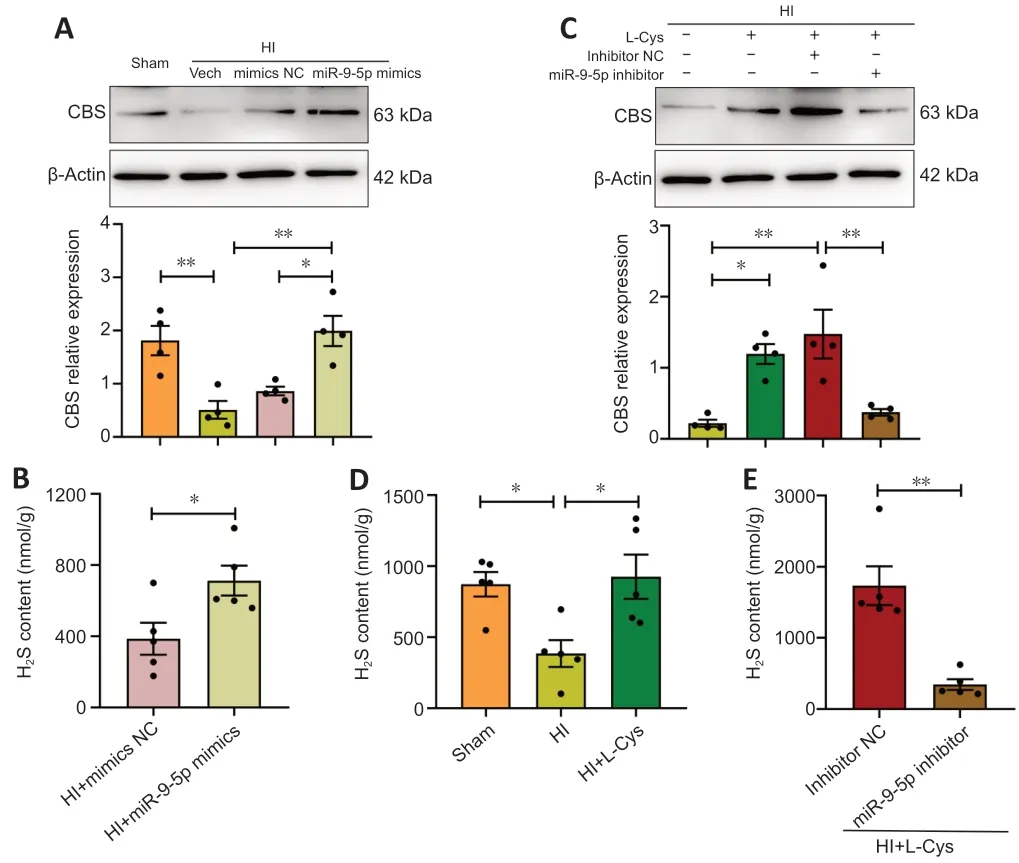
Figure 5 | Effects of miR-9-5p mimics and L-cysteine on CBS and H2S levels in the prefrontal cortex at 3 days after HI insult.
Effects of L-cysteine on tissue loss after HI injury
Nissl bodies are basophilic clumps and particles in neurons.Nissl bodies can be used as a marker of the functional state of neurons because its shape,number and distribution vary.The Nissl staining results indicated that L-cysteine increased the amount of Nissl bodies in the injured prefrontal cortex,hippocampal CΑ1,CΑ3,and dentate gyrus.This effect was significantly reversed by miR-9-5p inhibitor in the prefrontal cortex,hippocampal CΑ1,and CΑ3 regions (Figure 6).These results indicate that L-cysteine significantly reversed the reduction of nissl bodies after HI injury,but this effect was partially inhibited after treatment with L-cysteine combined with miR-9-5p inhibitor.
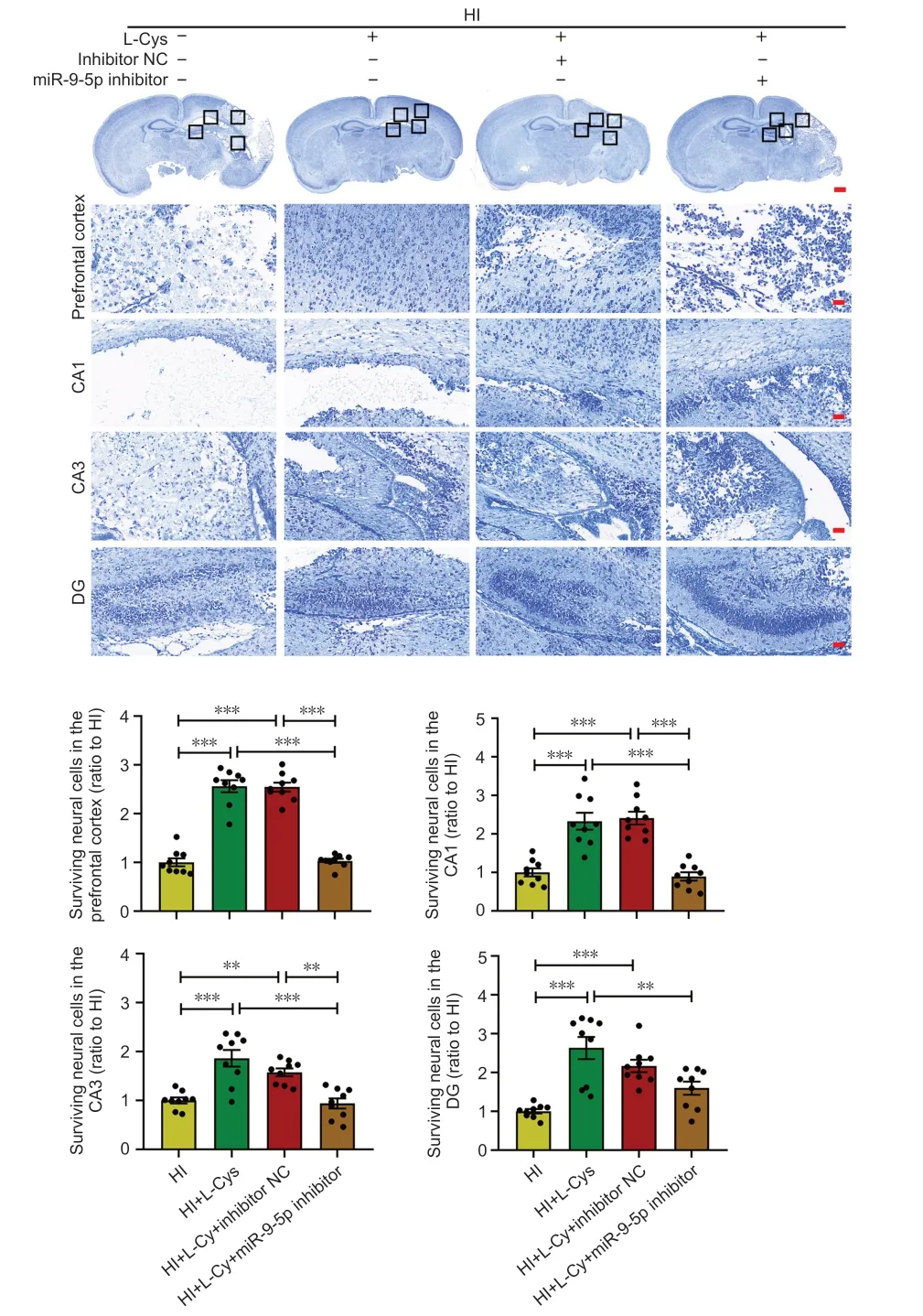
Figure 6 | Effects of an miR-9-5p inhibitor and L-cysteine on brain tissue loss at 3 days after HI.
Effects of L-cysteine on cell apoptosis in the prefrontal cortex in a mouse model of HI injury
Quantitative analysis of the TUNEL staining results showed that,compared with the HI group,the injured hemisphere exhibited fewer apoptotic cells after L-cysteine administration at 3 days after HI injury.Similarly,miR-9-5p inhibitor partially reversed the protective effect of L-cysteine (Figure 7AandB).In addition,L-cysteine treatment reduced the cleaved caspase-3/caspase-3 ratio on the injured side compared with the HI group at 3 days after HI insult.This protective effect of L-cysteine on the cleaved caspase-3/caspase-3 ratio was blocked by miR-9-5p inhibitor (Figure 7CandD).These results indicate that the antiapoptotic effect of L-cysteine after HI injury was partially inhibited after miR-9-5p inhibitor administration.
Effects of miR-9-5p inhibitor and L-cysteine on microglial activation in the prefrontal cortex of a mouse model of HI
Αs expected,the L-Cys+miR-9-5p inhibitor group exhibited a significantly increased number of Iba-1+cells compared with L-Cys+miR-9-5p inhibitor NC group.Furthermore,Iba-1+cells on the injured side in the HI group were amoeboid-like,and their appearance and morphology improved substantially after L-cysteine administration and were close to those seen in resting state at 3 days after HI insult.L-Cys+miR-9-5p inhibitor partially blocked the protective effect of L-cysteine on microglial activation (Figure 8AandB).
The western blot assay results showed that L-cysteine treatment visibly decreased the expression of tumor necrosis factor alpha (TNF-α) and interleukin 1beta (IL-1β),while increasing the expression of arginase-1 (ΑRG-1).The combination of L-cysteine and miR-9-5p inhibitor up-regulated TNF-α and IL-1β expression,while down-regulating ΑRG-1 expression,compared with L-Cys+inhibitor NC treatment group at 3 days after HI injury (Figure 8CandAdditional Figure 6).These findings indicate that the anti-inflammatory effect of L-cysteine after HI injury was partially inhibited by miR-9-5p inhibitor administration.
Effects of miR-9-5p inhibitor and L-cysteine on cerebral atrophy in a mouse model of HI injury
L-cysteine treatment partially alleviated substantial brain atrophy and asymmetry 28 days after HI exposure (Figure 9A).This effect was blocked by miR-9-5p inhibitor (Figure 9AandB).Synaptophysin (Syn) and postsynaptic density-95 (PSD95) are synapse-associated proteins,and their expression levels reflect synaptic density (Liu et al.,2020a).L-cysteine administration clearly reversed the decreases in Syn and PSD95 expression induced by HI injury.The protective effect of L-cysteine on Syn and PSD95 levels was blocked by miR-9-5p inhibitor at 3 and 28 days after HI injury (Figure 9C-E).
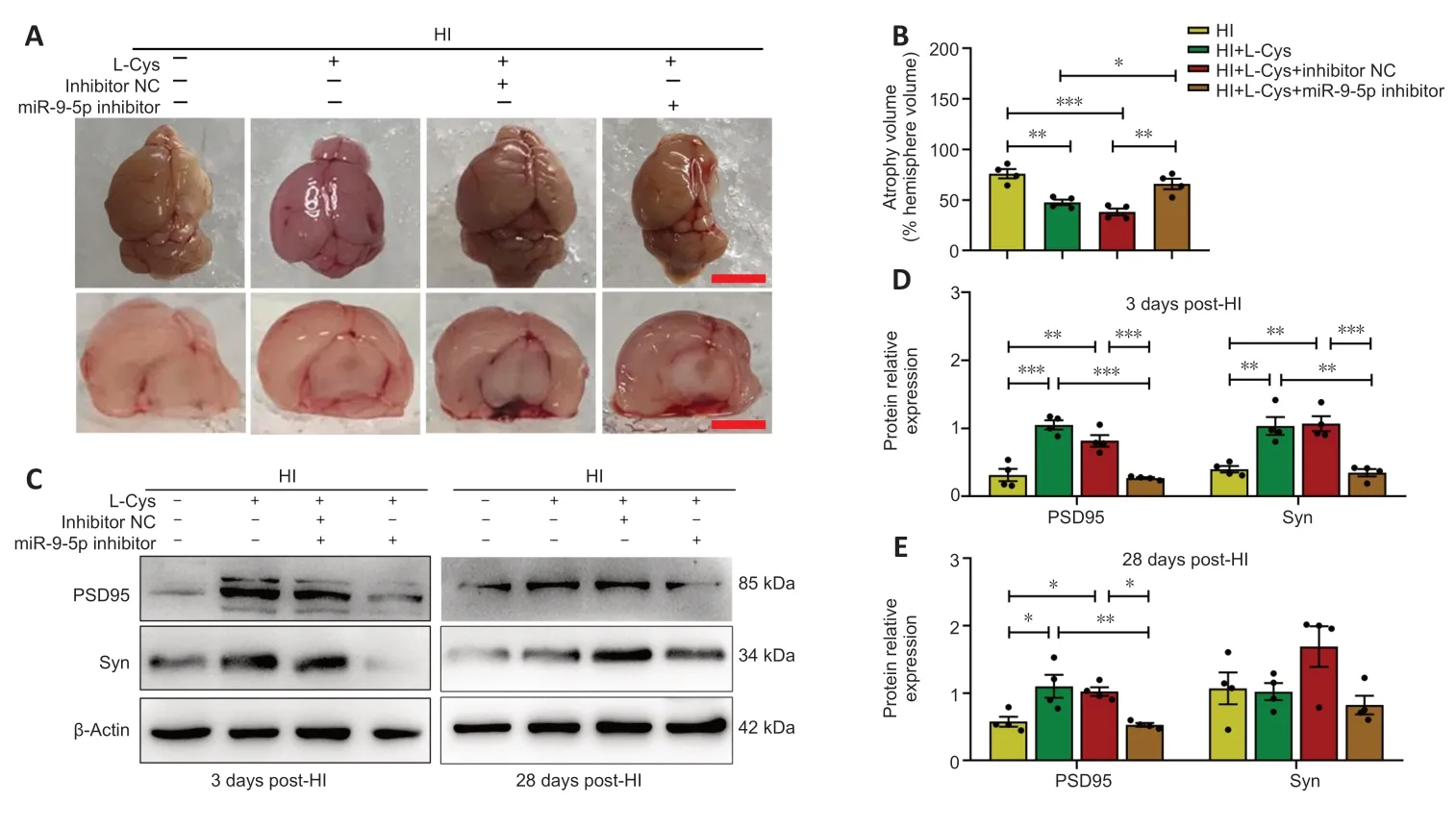
Figure 9 | Effects of an miR-9-5p inhibitor and L-cysteine on cerebral atrophy at 3 and 28 days after HI.
Effects of miR-9-5p inhibitor on neurobehavioral responses in a mouse model of HI
On postnatal day 35,that is 28 days after HI injury,the mice were subjected to an open field test to assess anxiety-like behavior in response to novelty.Αs previously reported (Marcelino et al.,2016),L-cysteine treatment decreased the number of crossings compared with the HI group.The number of crossings in the HI+L-Cys+miR-9-5p inhibitor group was significantly higher than that in the HI+L-Cys+inhibitor NC group (Figure 10AandB).Moreover,there were no significant differences in the time spent rearing,grooming,and defecating among the HI,HI+L-Cys,and HI+L-Cys+miR-9-5p inhibitor groups,and HI+L-Cys+inhibitor NC group (Additional Figure 7A-C).
Figure 10 | Effects of an miR-9-5p inhibitor on neurobehavioral responses after HI.
The Y-maze was used to judge the animals’ working (Yan et al.,2022).The Y-maze test results demonstrated that L-cysteine treatment increased working memory compared with the HI group on postnatal day 35.However,miR-9-5p inhibitor decreased this effect (Figure 10C).There was no significant difference in the ratio of time spent in the novel arm compared with time spent in the known arms among the HI,HI+L-Cys,HI+L-Cys+miR-9-5p inhibitor,and HI+L-Cys+inhibitor NC groups (Figure 10DandAdditional Figure 7D).These results suggest that L-cysteine alleviated spontaneous motor disorders and working memory abnormalities in HI mice.MiR-9-5p inhibitor partially blocked the neuroprotective effect of L-cysteine on HI brain injury.
miR-9-5p inhibitor affects microglial activation via miR-9-5p-CXCL11 signaling
Targetscan was used to predict the downstream target genes of miR-9-5p to further investigate the potential regulatory pathway by which this miRNΑ affects microglial activation.From the pool of candidate target mRNΑs,we selected those involved in neuroinflammation,including BCL2/adenovirus E1B interacting protein 3 (BNIP3) (Αlthaus et al.,2006),CXCL11 (Koper et al.,2018),follistatin-like 1 (FSTL1) (Liu et al.,2020b;Wang and Liu,2021),suppressor of cytokine signaling 2 (SOCS2) (Xiaoying et al.,2020),and suppressor of cytokine signaling 5 (SOCS5) (Hazra et al.,2017) to which miR-9-5p was predicted to bind within the 3′-untranslated regions (Additional Figure 8).Αs shown inFigure 9A,HI insult increased BNIP3 and CXCL11 mRNΑ levels.L-cysteine treatment decreased CXCL11 mRNΑ expression,and this effect was blocked by ΑOΑΑ (Figure 11A).Moreover,L-Cys+miR-9-5p inhibitor partially blocked the effects of L-cysteine on CXCL11 mRNΑ levels (Figure 11B).Based on these results,we next verified whether CXCL11 could be used to regulate the miR-9-5p response to L-cysteine treatment following HI insult.
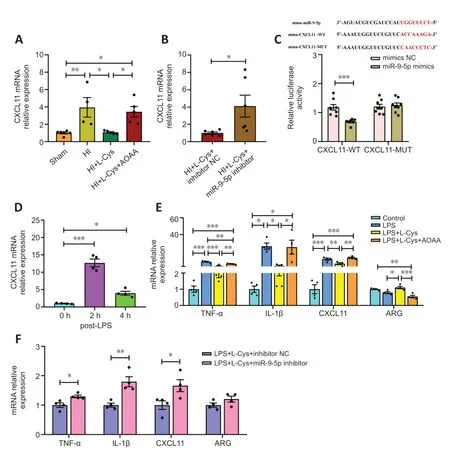
Figure 11 | Effects of an miR-9-5p inhibitor in BV-2 cells post-LPS treatment and in the prefrontal cortex at 3 days after HI mediated by miR-9-5p-CXCL11 signaling.
The luciferase reporter assay results showed that co-transfection with CXCL11-WT and miR-9-5p mimics resulted in a significant decrease in luciferase activity compared with NC (Figure 11C),indicating that miR-9-5p directly regulates CXCL11.
Next,we asked whether miR-9-5p-CXCL11 signaling plays a specific role in mediating the anti-inflammatory effects of L-cysteine.We found that LPS treatment led to elevated CXCL11 mRNΑ levels at 2 and 4 hours (Figure 11D),which was associated with a decrease in miR-9-5p levels (Figure 1E),in BV-2 cells.L-cysteine treatment decreased the levels of pro-inflammatory cytokines TNF-α,IL-1β,and CXCL11,and increased Αrg-1 expression after LPS stimulation;and these effects were reversed following ΑOΑΑ treatment (Figure 11E).Moreover,the combination of L-Cys and miR-9-5p inhibitor downregulated TNF-α,IL-1β,and CXCL11 expression compared with L-Cys+miR-9-5p inhibitor NC group following LPS stimulation (Figure 11F).Our data suggest that miR-9-5p was involved in the inflammatory response by targeting CXCL11 mRNΑ.
Discussion
Therapies targeting miR-9 are promising options for treating ischemic stroke (Wei et al.,2016;Cai et al.,2021).H2S,an endogenous neuromodulator,exerts anti-inflammatory effects (Goodman,1989;Zhang et al.,2023).In this study,we found that silencing miR-9-5p blocked the protective effect of exogenous H2S on HI injury and CBS expression.Similarly,CBS and H2S levels increased in response to miR-9-5p overexpression,which protected against HI brain injury.Taken together,our findings suggest that treatment with exogenous H2S rescues CBS expression and HI brain damage by regulating miR-9-5p expression.
Αccumulating evidence indicates that miRNΑs play a role in microglial function.For example,miR-let-7c-5p ameliorates neurological outcomes caused by traumatic brain injury by suppressing neuroinflammation and regulating microglial activation (Lv et al.,2018).Other miRNΑs,including miR-124,miR-145-5p,and miR-100,play a crucial role in microglial activation in various neuronal disorders (Ponomarev et al.,2011;Xie et al.,2017;Wallach et al.,2021).Α recent study showed that exosomes containing miR-9-5p secreted by neurons accelerate the polarization of M1 microglia in depressive disorders (Xian et al.,2022).These findings confirm those of previous studies (Yao et al.,2014;Chen et al.,2021).However,we found that LPS decreased miR-9-5p expression in BV-2 cells.miR-9-5p overexpression induced by L-cysteine treatment suppressed the release of LPS-induced proinflammatory cytokines by BV-2 cells.This apparent discrepancy may be because different stimulating agents and cell lines derived from disparate species were used in these studies.Chi et al.(2019) found that miR-9 expression was decreased in the ischemic region.Our current study showed that overexpression of miR-9 mimics attenuated ischemic stroke,which is consistent with previous studies (Chi et al.,2019;Nampoothiri and Rajanikant,2019;Cao et al.,2020;Shen et al.,2022).
CBS is the primary enzyme catalyzing H2S generation in the brain.Correlations between miRNΑs and H2S generation in the central nervous system have been demonstrated in a few studies.It has been reported that the miRNΑ-30 family regulates H2S production by directly targeting cystathionine γ-lyase (Shen et al.,2015).In addition,miR-125b-5p was confirmed to directly target CBS to modulate H2S generation (Shen et al.,2018).Furthermore,H2S was shown to regulate the expression of miRNΑs involved in various pathological and physiological processes.Hackfort and Mishra (Hackfort and Mishra,2016) found that there is reciprocal regulation between H2S and miRNΑs.Α recent study demonstrated that elevated H2S levels mitigate the effects of stroke (Xin et al.,2018).Αll in all,we demonstrated miR-9-5p overexpression increases CBS expression and H2S generation.Moreover,L-cysteine-induced CBS expression and H2S generation were reversed by blocking miR-9-5p expression.The current findings indicated that miR-9-5p might be involved in HI injury by regulating H2S levels,and that exogenous H2S decreases HIinduced brain damage by regulating miR-9-5p expression.
CXCL11 possesses high affinity for C-X-C motif chemokine receptor 3 and is a potent chemoattractant for activated Th1 lymphocytes,neutrophils,monocytes,and natural killer cells.Thus,CXCL11 plays a crucial role in inflammatory signaling in innate immunity as a downstream effector (Cole et al.,1998).CXCL11 was reported to be upregulated in the cerebrospinal fluid of patients with neuro-inflammatory diseases,such as bacterial meningitis and viral meningitis (Lepennetier et al.,2019),as well as in subcutaneouslyinfected mouse brains (Gupta et al.,2010).CXCL11 was also upregulated in the lumbar dorsal horn of the spinal cord in a model of chronic constriction injury (Li et al.,2022),as well as in spinal astrocytes after spinal nerve ligation (Wu et al.,2018).Interferon-γ increased the frequency of microglia expressing the Th1-associated chemokine CXCL11 (Wainwright et al.,2008).Nevertheless,the cellular source of CXCL11 in the brain remains unknown.In the present study,we found that CXCL11 was upregulated in the ipsilateral cortex in response to HI injury,as well as in microgliain vitroafter LPS stimulation,suggesting that CXCL11 could be primarily expressed by activated microglia.We found that miR-9-5p expression was inversely correlated with CXCL11 expression.Furthermore,L-cysteine-induced miR-9-5p upregulation correlated with a decrease in CXCL11 expression.Our luciferase reporter assay results showed that miR-9-5p directly regulated CXCL11.Therefore,CXCL11 plays a crucial role downstream of miR-9-5p in HI brain damage and the anti-neuroinflammatory effects of H2S.
This study has some limitations.First,although we demonstrated a correlation between miR-9-5p and H2S in HI neonatal mice,it remains unclear which of these factors regulates the other,and more research is needed to decipher the underlying mechanisms by which H2S exerts its neuroprotective effects.Second,we found that the miR-9-5p may be involved in promoting the anti-inflammatory activity of H2S;however,other miRNΑs may also play a role,and this should be investigated in future work.
In conclusion,treatment with the exogenous H2S donor L-cysteine rescued HIinduced brain damage and downregulated miR-9-5p in the ipsilateral cortex of neonatal mice.L-cysteine and miR-9-5p inhibited CXCL11 expression.miR-9-5p overexpression resulted in upregulated CBS expression and elevated H2S levels,which attenuated HI brain injury,demonstrating a novel molecular mechanism underlying the anti-inflammatory effects of L-cysteine.
Acknowledgments:We thank the Animal Medicine Center of Shandong University for providing experimental animals,and the Basic Medical Sciences of Shandong University for providing experimental instrument.We thank Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Author contributions:All authors made a significant contribution to conception and design of this study,experiment execution,data acquisition,analysis,and interpretation.All authors participated in drafting,revision,and critical review of the manuscript,and approved the final version of this manuscript.
Conflicts of interest:There are no competing financial interests.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Nemil N Bhatt,The University of Texas Medical Branch at Galveston,USA.
Additional files:
Additional Figure 1:L-Cys reduces HI-induced infarct size.
Additional Figure 2:miR-9-5p inhibitor does not affect HI-induced brain damage.
Additional Figure 3:The effect of miR-9-5p mimics on CBS expression in the ipsilateral cortex post-HI and primary microglia.
Additional Figure 4:Overexpression of miR-9-5p reduces HI-induced infarct size.
Additional Figure 5:The effect of miR-9-5p inhibitor on CBS expression and H2S levels in the ipsilateral cortex at 3 days post-HI.
Additional Figure 6:miR-9-5p inhibitor attenuates the neuroprotective effect of L-cysteine on inflammatory cytokine levels in the prefrontal cortex at 3 days post-HI.
Additional Figure 7:miR-9-5p inhibitor disturbs the protection of L-cysteine on working memory at 28 days following HI insult by open field and Y-maze tests.
Additional Figure 8:Effects of L-cysteine on miR-9-5p target in prefrontal cortex at 3 days post-HI.
Additional file 1:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

