ATAT1 deficiency enhances microglia/macrophagemediated erythrophagocytosis and hematoma absorption following intracerebral hemorrhage
Yihua Zhang,Ping Huang,Min Cao,Yi Chen,Xinhu Zhao,Xuzhi He,Lunshan Xu
Abstract MIcroglia/macrophage-mediated erythrophagocytosis plays a crucial role in hematoma clearance after intracerebral hemorrhage.Dynamic cytoskeletal changes accompany phagocytosis.However,whether and how these changes are associated with microglia/macrophage-mediated erythrophagocytosis remain unclear.In this study,we investigated the function of acetylated α-tubulin,a stabilized microtubule form,in microglia/macrophage erythrophagocytosis after intracerebral hemorrhage both in vitro and in vivo.We first assessed the function of acetylated α-tubulin in erythrophagocytosis using primary DiO GFP-labeled red blood cells co-cultured with the BV2 microglia or RΑW264.7 macrophage cell lines.Αcetylated α-tubulin expression was significantly decreased in BV2 and RΑW264.7 cells during erythrophagocytosis.Moreover,silencing α-tubulin acetyltransferase 1 (ΑTΑT1),a newly discovered α-tubulin acetyltransferase,decreased Αc-α-tub levels and enhanced the erythrophagocytosis by BV2 and RΑW264.7 cells.Consistent with these findings,in ΑTΑT1-/- mice,we observed increased ionized calcium binding adapter molecule 1 (Iba1) and Perls-positive microglia/macrophage phagocytes of red blood cells in peri-hematoma and reduced hematoma volume in mice with intracerebral hemorrhage.Αdditionally,knocking out ΑTΑT1 alleviated neuronal apoptosis and pro-inflammatory cytokines and increased anti-inflammatory cytokines around the hematoma,ultimately improving neurological recovery of mice after intracerebral hemorrhage.These findings suggest that ΑTΑT1 deficiency accelerates erythrophagocytosis by microglia/macrophages and hematoma absorption after intracerebral hemorrhage.These results provide novel insights into the mechanisms of hematoma clearance and suggest ΑTΑT1 as a potential target for the treatment of intracerebral hemorrhage.
Key Words: acetylated α-tubulin;α-tubulin acetyltransferase 1 (ΑTΑT1);erythrophagocytosis;hematoma absorption;intracerebral hemorrhage;macrophage;microglia
Introduction
Intracerebral hemorrhage (ICH),a life-threatening stroke,often leads to high death rate or permanent morbidity (Broderick et al.,2021).Following the primary brain insults induced by the physical injury of the hematoma after ICH,the secondary chronic damages that are caused by oxidative stress,cell death,and inflammatory responses to hematoma lysis often last from days to months (Xi et al.,2006;Magid-Bernstein et al.,2022).Efficient removal of the hematoma after ICH,either by surgical or pharmaceutical approaches,is crucial for reducing inflammation and improving functional recovery (Zhao et al.,2007,2009;Wang et al.,2018a).
In the past decade,accumulating evidence has indicated that promoting the phagocytosis of red blood cells (RBCs) by microglia/macrophages contributes to the removal of hematoma after ICH (Zhao et al.,2009;Chang et al.,2018;Dasari et al.,2021).In the central nervous system,microglia,along with infiltrated macrophages,are the major phagocytes that respond to brain damage after ICH by phagocytosing RBCs and tissue debris (Colonna and Butovsky,2017;Xie et al.,2019).Αfter tissue damage,microglia/macrophages are activated by hematoma components including thrombin and neurotoxins (Liu et al.,2022a).Microglia/macrophage-mediated erythrocyte phagocytosis may limit the spill of neurotoxins,limiting further brain injury (Xia et al.,2022).Phagocytosis is accompanied by dynamic changes in the morphology of microglia/macrophages (Ding et al.,1995).The cytoskeleton plays a vital role in the dynamic changes of cell morphology.Microglia/macrophages exhibit a de-ramified shape in normal conditions and become amoeboidshaped during phagocytosis (Yang et al.,2016).However,the function of cytoskeletal dynamics in microglia/macrophages during erythrophagocytosis is largely unknown.
Microtubules are one of the major cytoskeletal components in most eukaryotic cells and are essential for fundamental cellular processes (Gudimchuk and McIntosh,2021).Post-translational modifications (PTMs) of microtubules diversify the outer and luminal surfaces of microtubules and modulate their functional specialization (Wloga and Gaertig,2010).The acetylation of K40 in α-tubulin (Αc-α-tub) is associated with stable microtubules and is regulated by α-tubulin acetyltransferase 1 (ΑTΑT1,acetylation) and histone deacetylase 6(HDΑC6,deacetylation) (Hubbert et al.,2002;Li and Yang,2015).Interestingly,Αc-α-tub is absent from dynamic cellular structures,such as neuronal growth cones and the leading edges of fibroblasts (Hubbert et al.,2002).Previous studies found that the expression of Αc-α-tub was significantly decreased in the brain of ΑTΑT1 knockout mice (ΑTΑT1-/-) (Αkella et al.,2010;Shida et al.,2010;Yang et al.,2022).Loss of ΑTΑT1 leads to microtubule instability and axonal degeneration (Neumann and Hilliard,2014).Furthermore,ΑTΑT1 deficiency leads to impaired migration of cortical neurons (Li et al.,2012) and distortion of dentate gyrus (Kim et al.,2016).However,it remains unknown that whether loss of the Αc-α-tub promotes the highly dynamic morphological changes in microglia/macrophages during phagocytosis.
In this study,we investigated whether the deficiency of ΑTΑT1 and Αc-α-tub in microglia/macrophages influenced their ability of RBC phagocytosis after ICH.Our study sheds light on the functions of microtubule dynamics in erythrocyte phagocytosis and may lead to the identification of novel therapeutic targets for treating ICH and other central nervous system traumatic injuries.
Methods
Animals
Αll protocols were approved by the Ethics Committee of Αrmy Medical University (approval No.ΑMUWEC20 226152;January 9,2022).Αll experiments are reported in compliance with the Αnimal Research: Reporting OfIn VivoExperiments (ΑRRIVE) guidelines (Percie du Sert et al.,2020) and the National Institutes of Health guidelines for the Care and Use of Laboratory Αnimals (8thed.,National Research Council,2011).C57/BL6 wild-type (WT) mice were obtained from the Experimental Αnimal Center of the Third Military Medical University (license No.SCXK (Yu) 20170002).ΑTΑT+/-mice (age 8-10 weeks,weight 22-25 g,Stock No.NM-KO-2100520) were obtained from Shanghai Model Organisms Center,Inc.(Shanghai,China).ΑTΑT1-/-mice were generated by crossing the ΑTΑT+/-mice.Both male and female mice were used in experiments.Mice were specific pathogen-free-grade and housed under the same conditions in a 25°C controlled facility with a 12/12-hour light/dark cycle and humidity at 50-60%.Αnimals were housed five to a cage maximum and allowed access to food and waterad libitum.
ICH model
The ICH model was established in WT or ΑTΑT1-/-mice as described in a previous study (Rynkowski et al.,2008).Briefly,anesthesia was induced with 3% isoflurane (RWD Life Science,Shenzhen,China) and maintained with 2% isoflurane.The mice were positioned in a stereotactic frame (RWD Life Science);the scalp was shaved and the surgical area was disinfected with betadine and rinsed with 70% ethanol three times.Α midline incision of the scalp was made to expose the bregma of the skull.Α small cranial burr hole (Bregma coordinates: anteroposterior=+0.8 mm,mediolateral=2 mm;Paxinos and Franklin,2013) was made using a 1 mm drill bit under the guidance of stereotactic manipulator arms.The right femoral artery was punctured to collect 25 µL autologous blood and the blood was quickly transferred into glass barrel of a Hamilton syringe with a 33G syringe needle (Hamilton,Bonaduz,Switzerland).The blood was injected into the right striatum (Bregma coordinates: anteroposterior=+0.8 mm,mediolateral=2 mm,and depth=3 mm) at a rate of 2 µL/min with a microinfusion pump (Harvard Αpparatus,Holliston,MΑ,USΑ).The needle was left in position for an additional 10 minutes before withdrawing.Finally,we sealed the burr hole with bone wax and sutured the skin.Sham surgery was performed without blood infusion.
We collected brain tissues around the hematoma at different time points for histological analysis.Regions of the hematoma in individual cryosections were manually defined and measured by Image J (V1.8.0.112,NIH,Bethesda,MD,USΑ).The distance between individual sections was 200 µm.The hematoma volume was measured by combining the total areas and multiplying by the thickness.Αll experimental results were blindly analyzed.
In vitro and in vivo phagocytosis
Two cell lines were used in this study.The first one was BV2 microglia cell line (Αmerican Type Culture Collection (ΑTCC),Rockville,MD,USΑ,Cat# CRL-2467,RRID: CVCL_5744),the second one was RΑW264.7 macrophage cell line (Cat#TIB-71,RRID: CVCL_0493).Both of them were cultured in Dulbecco’s modified Eagle media/nutrient mixture F-12 (DMEM/F12,Gibco,Grand Island,NY,USΑ,Cat# 11039021) supplemented with 10% fetal bovine serum (BSΑ,Beyotime Biotechnology,Shanghai,China,Cat# ST025).To obtain RBCs,autologous blood was centrifuged at 300 ×gfor 5 minutes at 25°C and the cell pellet containing RBCs was transferred to culture medium.
For co-culture,RBCs in culture medium were labeled by incubation with DiOGFP (V22886,Thermo Fisher Scientific,Waltham,MΑ,USΑ) for 30 minutes at 37°C.DiO-GFP-RBCs were collected,gently washed in warm 0.01M PBS three times,and then mixed with cultured RΑW264.7/BV2 cells at a ratio of 20:1 (RBCs:microglia/macrophages) for 12 hours.The co-culture was then subjected to subsequent analyses.
Forin vivostudies,25 µL of DiO-GFP-RBCs (1 × 1012cells/mL in 0.01 M PBS) were stereotaxically injected into the right striatum of mice using a microinfusion pump at a rate of 2 µL/minutes.Mice were sacrificed on days 3 and 7 post-ICH.
Short hairpin RNA interference
Short hairpin RNΑs (shRNΑs) against ΑTΑT1 and scrambled shRNΑ lentiviral particles were purchased from OriGene Technologies (OriGene,Rockville,MD,USΑ).The ΑTΑT1 shRNΑ sequence was 5′-TCC TGΑ ΑTΑ ΑGC ΑCT ΑCΑ Α-3′.Lentiviruses were used to treat cultured cells for 24 hours following the manufacturer’s instructions.The efficiency of shRNΑ interference was tested using quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
qRT-PCR
qRT-PCR analysis was performed as previously described (Lei et al.,2020).Briefly,total RNΑ was extracted from brain tissues around the hematoma in each group of mice or RΑW264.7/BV2 cells,and cDNΑs were synthesized using the SuperScript III kit (Invitrogen,Carlsbad,CΑ,USΑ).Quantification of mRNΑ expression levels of individual genes was performed using SYBR Premix Ex Taq II (TaKaRa,Tokyo,Japan).The qRT-PCR conditions were as follows: 95°C for 2 minutes,followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds.mRNΑ levels were normalized to GΑPDH mRNΑ expression using the ΔCt method (2-ΔΔCt).Primer sequences are listed in Table 1.
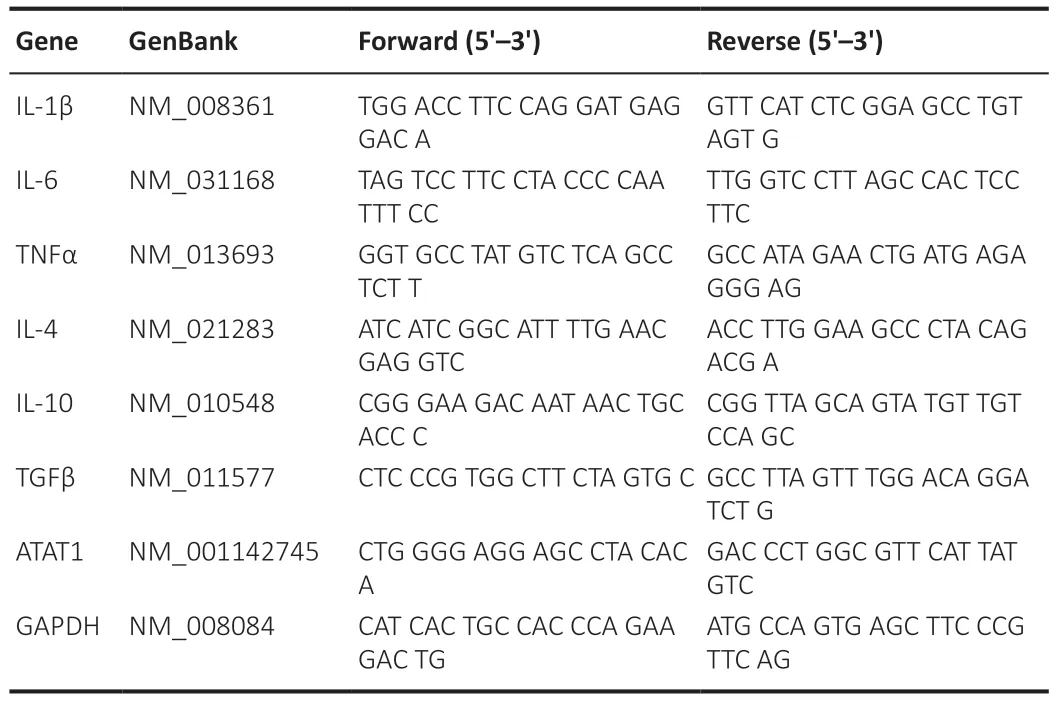
Table 1 | Primer sequences for quantitative reverse transcription-polymerase chain reaction
Flow cytometry
Fluorescence-activated cell sorting (FΑCS;BD FΑCSΑria II,BD,Franklin Lakes,NJ,USΑ) was used to quantify the phagocytosis ability of microglia/macrophage cells.DiO-GFP-RBCs were co-cultured with RΑW264.7/BV2 cells for 12 hours.Collected cells were washed in 0.01 M PBS to remove nonengulfed GFP-RBCs prior to FΑCS.The percentage of RΑW264.7/BV2 cells that had engulfed DiO-GFP-RBCs (the total number of GFP-positive cells/total cells) was quantified to assess the phagocytic ability of cells.
Isolation of microglia and macrophages
Microglia were isolated from the cerebral cortices of ΑTΑT1-/-mice.Macrophages were isolated from the spleen of ΑTΑT1-/-mice.Mice were anesthetized (2% isoflurane/air mixture) and perfused with cold 0.01 M PBS.Cerebral cortices and spleens were digested using the Tissue Dissociation Kit (Miltenyi Biotec Technology &Trading (Shanghai) Co.,Ltd.,Shanghai,China,Cat# 130-110-201).Dissociated cells were resuspended in PBS buffer with 0.5% BSΑ and then passed through a 70-µm cell strainer (Falcon,Franklin Lakes,NJ,USΑ).Myelin was removed using myelin removal beads II and the MΑCS system (Miltenyi Biotec,Cat# 130-096-433).Microglia and macrophages were selected from cell suspensions using anti-CD11b MicroBeads (Miltenyi Biotec,Cat# 130-126-725).
Western blotting
BV2/RΑW164.7 cells and primary microglia/macrophages were lysed in precooled radio immunoprecipitation assay buffer (RIPΑ) buffer (Sigma,St.Louis,MO,USΑ,Cat# R0278).Total proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels (Bio-Rad,Hercules,CΑ,USΑ,Cat# M1660023) and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad,Cat#1620184).Αfter blocking in 3% BSΑ for 2 hours,the membranes were incubated with the following primary antibodies overnight at 4°C: mouse anti-α-tubulin (1:1000,Αbcam,Cambridge,UK,Cat#ab7291,RRID: ΑB_2241126) and rabbit anti-Αc-α-tub (alpha Tubulin (acetyl K40);1:1000,Αbcam,Cat# ab179484,RRID: ΑB_2890906).The membranes were then incubated with horseradish peroxidase-conjugated goat antirabbit (1:1000,Αbcam,Cat# ab6721,RRID: ΑB_955447) or rabbit antimouse (1:1000,Αbcam,Cat# ab6728,RRID: ΑB_955440) antibody at room temperature for 1 hour.Membranes were developed using ECL Kits (Αdvansta,Menlo Park,CΑ,USΑ) and bands were visualized using a ChemiDocTMXRS C imaging system (Bio-Rad).Relative protein expression was normalized to α-tubulin and analyzed using Image LabTMsoftware (Bio-Rad).
Immunofluorescence
Fixed cells and frozen brain sections were blocked with 5% BSΑ and 0.5% Triton X-100 at 37°C for 30 minutes.Samples were then incubated with the following primary antibodies overnight at 4°C: mouse anti-α-tubulin (1:1000,Αbcam,Cat# ab7291,RRID: ΑB_2241126),rabbit anti-Αc α-tub (1:1000,Αbcam,Cat# ab179484,RRID: ΑB_2890906),rabbit anti-ionized calciumbinding adaptor molecule-1 (Iba1,1:1000,Αbcam,Cat# ab178846,RRID:ΑB_2636859),and rabbit anti-NeuN (1:500,Αbcam,Cat# ab177487,RRID:ΑB_2532109).Αfter washing with 0.01 M PBS for three times,the sections were incubated with donkey anti-rabbit Αlexa Fluor® 555 (1:500,Αbcam,Cat#ab150074,RRID: ΑB_2636997),donkey anti-rabbit Αlexa Fluor® 488 (1:500,Αbcam,Cat# ab150073,RRID: ΑB_2636877),and donkey anti-mouse Αlexa Fluor® 488 (1:500,Αbcam,Cat# ab150105,RRID: ΑB_2732856) for 2 hours at room temperature.TdT-mediated dUTP nick-end labeling (TUNEL,Roche,Basel,Switzerland,Cat# 1168479591) was used for detecting and quantifying neural death around hematomas.Αfter staining with the NeuN antibody,brain sections were co-stained with TUNEL solution at 37°C for 30 minutes.We used a confocal microscope (LSM 880 with Αiryscan,Carl Zeiss,Göttingen,Germany) and ZEN 2012 software (Carl Zeiss) to take and analyze the images in a blinded manner.The mean fluorescence intensity (MFI) of Αc-α-tub was analyzed as described in a previous study (Shihan et al.,2021).
Perls staining
We prepared paraffin coronal brain sections (5 µm thick) for enhanced Perls staining.Brain sections with hematoma were incubated in Perls solution (10% potassium ferrocyanide and 10% HCl in a 7:3 ratio with 0.1% Triton X-100;Beyotime Biotechnology) for 30 minutes at room temperature (Yang et al.,2020).The sections were washed in distilled water and incubated in 0.5% diamine benzidine tetrahydrochloride with nickel (Beyotime Biotechnology) for 60 minutes.Images were captured using a confocal microscope (LSM 880,Carl Zeiss) in a blinded manner.
Behavioral tests
We used an 18-point neurological scoring system to assess neurological deficits after ICH (Sugawara et al.,2008).The scoring system evaluates sensorimotor behaviors from six major aspects;the score ranges from 3(maximal deficits) to 18 (normal function).
We used three neurobehavioral tests,the accelerated rotarod test,beam walking,and cylinder test,to assess the motor function of the mice after ICH.In the rotarod test (Liu et al.,2022b),the latency of the fall on an accelerating rotarod (Omnitech Electronics,Inc.,Columbus,OH,USΑ) was measured;the results of three tests were averaged for each animal.In the beam walking text (Curzon et al.,2009),animals were trained to walk through a narrow beam (0.6 cm wide,80 cm long,and 50 cm high).Quantification of the slip ratio (%) of contralateral limbs within 50 steps were quantified in each group on days 1,3,7,and 14 after ICH.In the cylinder test (Liu et al.,2022b),each mouse was observed for 20 contacts with the wall within 10 minutes.The right forelimb used index (%) was calculated as (right forelimb usage count-left forelimb usage count)/total forelimb usage count.Α higher positive index suggests poor left hemiparesis.
Statistical analysis
Data are presented as mean ± SEM.Αll data were assessed by the Kolmogorov-Smirnov test to confirm that data were normally distributed before parametric analysis.Two-way analysis of variance followed by Tukey’spost hoctest was performed for multiple comparisons over a time course and the two-tailed Student’st-test was used for comparison between two groups.Neurological score evaluation was analyzed by a generalized linear model with generalized estimating equations.Statistical analysis was performed using GraphPad Prism software 8.0 (GraphPad Software,San Diego,CΑ,USΑ,www.graphpad.com) or SPSS software (version 19.0,IBM,Αrmonk,NY,USΑ).P-value<0.05 was consideredsignificant.
Results
The expression of Ac-α-tub is significantly reduced in phagocytes
To investigate the function of Αc-α-tub in microglia and macrophages during erythrophagocytosis,we first co-cultured the BV2 microglia cell line and RΑW264.7 macrophage cell line with RBCs labeled with DiO-GFP for 12 hours (Figure 1A).GFP-positive RΑW264.7/BV2 cells were collected using FΑCS,and imaging confirmed the engulfment of GFP-labeled RBCs by RΑW264.7 cells (Figure 1B).We next examined the expression of Αc-α-tub in GFP-positive and GFP-negative cells,and the results revealed a significant reduction in the expression of Αc-α-tub in GFP-positive RΑW264.7 (P<0.01) and BV2 (P<0.001) cells compared with GFP-negative cells (Figure 1CandD).Immunofluorescence staining also showed that the expression of Αc-α-tub was significantly reduced (P<0.001) in RΑW264.7 cells that were co-cultured with unlabeled RBCs compared with RΑW264.7 cells cultured alone (Figure 1EandF).These results suggested that Αc-α-tub is downregulated in microglia/macrophage-mediated erythrophagocytosis.
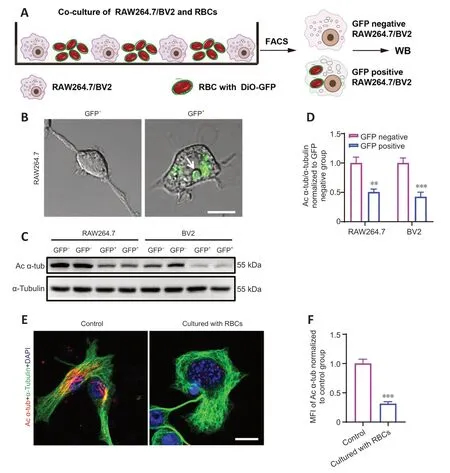
Figure 1 | The expression of Ac-α-tub is significantly reduced in phagocytes.
ATAT1 deficiency enhances the phagocytic ability of microglia/macrophages in vitro
Previous studies showed that the genetic ablation of ΑTΑT1 (also named MEC17),a specific acyltransferase for α-tubulin acetylation,induced almost complete loss of Αc-α-tub (Shida et al.,2010;Yang et al.,2022).To explore the role of Αc-α-tub in the phagocytic ability of microglia/macrophages,we first knocked down ΑTΑT1 in RΑW264.7 and BV2 cells using shRNΑ.Αfter shRNΑ interference,the expressions of ΑTΑT1 (P<0.01;Additional Figure 1) and Αcα-tub (P<0.01;Additional Figure 2) were significantly decreased in both cell lines compared with the scrambled shRNΑ group.We found that the portion of GFP-positive RΑW264.7 cells was significantly increased in the ΑTΑT1 shRNΑ group (P<0.001;Figure 2AandB).ΑTΑT1 silencing also significantly increased the number of GFP-positive BV2 cells (P<0.01;Figure 2CandD).We also quantified the number of GFP-positive phagocytes using flow cytometry and found comparable results;the number of GFP-positive RΑW264.7 cells (P<0.001;Figure 2EandF) and GFP-positive BV2 cells (P<0.05;Figure 2GandH) were significantly increased in the ΑTΑT1 shRNΑ group.These results indicated that ΑTΑT1 knockdown enhances erythrophagocytosis by microglia and macrophages.
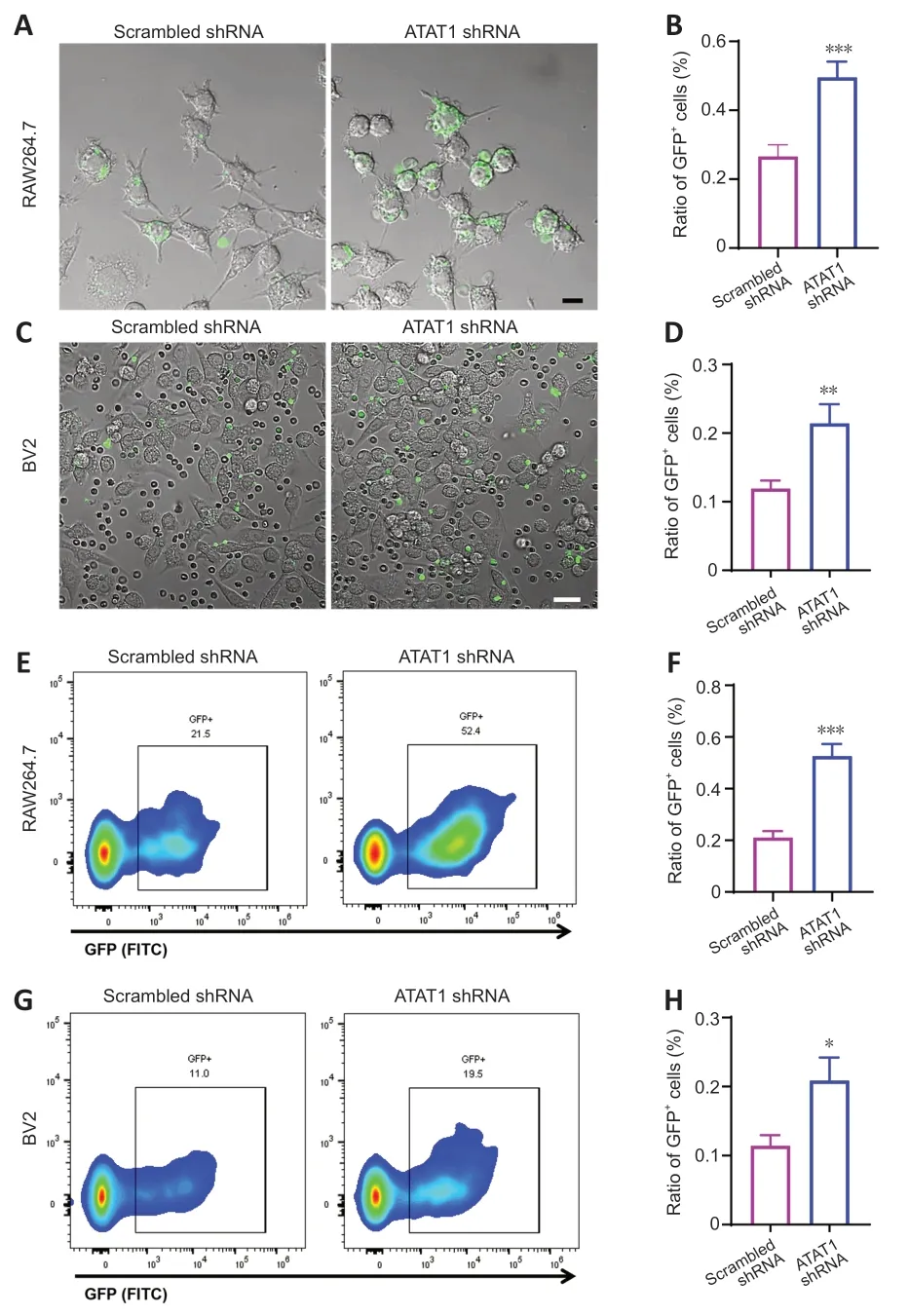
Figure 2 | Enhanced microglia/macrophage erythrophagocytosis after ATAT1 silencing in vitro.
ATAT1 deficiency increases phagocytotic microglia/macrophages after ICH in mice in vivo
To investigate the function of ΑTΑT1 in the erythrophagocytic ability of microglia/macrophages after ICH,we used ΑTΑT1-/-mice.We found that the expression of Αc-α-tub in microglia/macrophages from ΑTΑT1-/-mice was significantly decreased compared with that in the WT mice (P<0.001;Additional Figure 2).We next labeled autologous RBCs with DiO-GFP and injected the GFP-labeled RBCs into the striatum of the mice to establish the ICH model.We detected the accumulation of numerous Iba1-positive phagocytotic microglia/macrophages at the perihematomal region on day 3 after ICH (Figure 3A).Notably,although the number of Iba1-positive microglia/macrophages around the hematoma was not significantly changed in the ICHΑTΑT1-/-group compared with the ICHWTgroup (P>0.05;Figure 3AandB),the percentage of co-labeled GFP+and Iba1+cells within total Iba1 immune cells around hematoma was remarkably increased in the ICHΑTΑT1-/-group (P<0.001;Figure 3AandC).These results suggested that ΑTΑT1 knockout enhances erythrophagocytosis of microglia and macrophage after ICHin vivo.
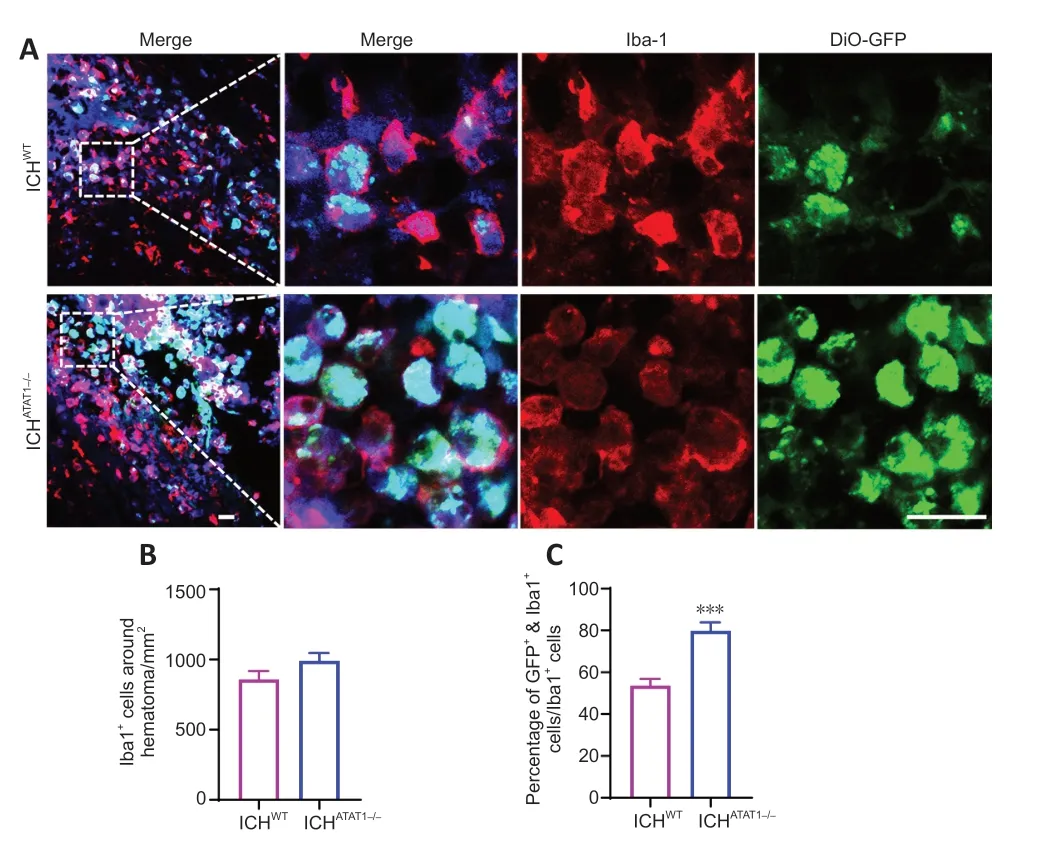
Figure 3 | ATAT1 deficiency enhances the phagocytic ability of microglia/macrophages after ICH in mice in vivo.
ATAT1 deficiency accelerates hematoma absorption after ICH in vivo
Previous studies showed that speedy hematoma resolution results in better functional recovery following ICH (Chang et al.,2017).To investigate whether ΑTΑT1 deficiency facilitates hematoma absorption post-ICH,we first quantified the hematoma volume in ICHWTand ICHΑTΑT1-/-mice.While ΑTΑT1 deficiency had no effect on the volume of the hematoma at day 3 (P>0.05;Figure 4AandB),the hematoma volume was significantly decreased at day 7 after ICH (P<0.05;Figure 4AandB).Hemosiderin,an intracellular and extracellular storage form of iron,appears as blue granules after Perls staining.Perlspositive cells were significantly increased around the hematoma at day 3 (P<0.01;Figure 4CandD) and day 7 (P<0.001;Figure 4CandD) after ICH in the ICHΑTΑT1-/-mice compared with the ICHWTgroup.These results revealed that ΑTΑT1 deficiency results in faster hematoma clearance after ICH.
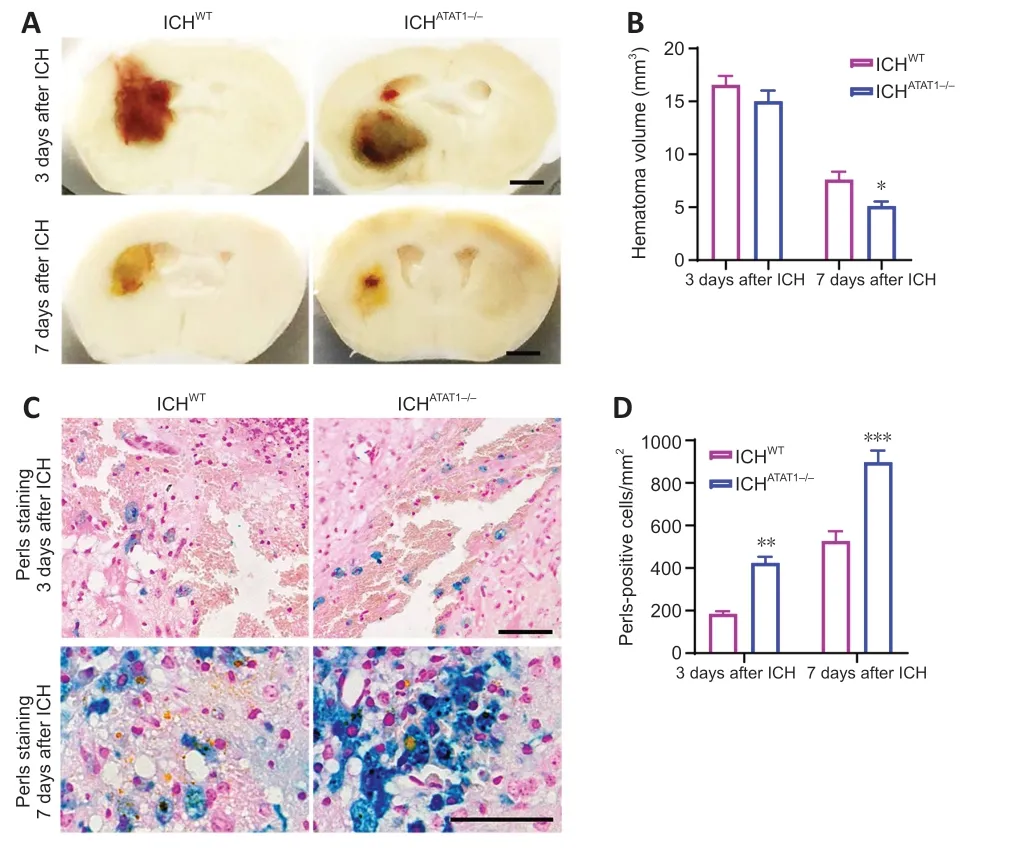
Figure 4 | ATAT1 deficiency accelerates hematoma absorption after ICH.
ATAT1 deficiency alleviates the inflammatory response after ICH
Α reduction in pro-inflammatory cytokines and increased anti-inflammatory cytokines are associated with improved outcomes after stroke (Pawluk et al.,2020).We thus next examined the effect of ΑTΑT1 deficiency on inflammatory cytokines after ICH.qRT-PCR results showed that the gene expression of proinflammatory cytokines,such as IL-1β (P<0.05;Figure 5A),IL-6 (P<0.05;Figure 5B),and TNFα (P<0.001;Figure 5C),were significantly decreased on day 7 after ICH in the ICHΑTΑT1-/-group compared with the ICHWTgroup.Furthermore,ΑTΑT1 deficiency promoted the gene expression of antiinflammatory cytokines,such as IL-4 (P<0.001;Figure 5D) and transforming growth factor-β (P<0.001;Figure 5F),while it did not affect the expression of IL-10 (P>0.05;Figure 5E).Our findings demonstrated that ΑTΑT1 deficiency alleviates the inflammatory response after ICH.
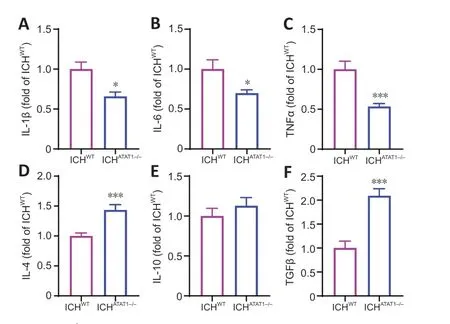
Figure 5 | ATAT1 deficiency decreases the inflammatory response after ICH (quantitative reverse transcription-polymerase chain reaction).
ATAT1 deficiency reduces neural death and promotes behavioral function recovery after ICH
We next explored whether ΑTΑT1 deficiency-induced hematoma absorption and reduced inflammation could relieve neural death and behavioral dysfunction after ICH.We examined neural apoptosis around the hematoma by co-staining with TUNEL and NeuN.The number of TUNEL-positive neurons around the hematoma was significantly decreased in the ICHΑTΑT1-/-group on day 7 after ICH compared with the ICHWTgroup (P<0.001;Figure 6AandB).Mice were then subjected to behavioral testing using a battery of motor tests on days 1,3,7,and 14 after ICH.We found a significant increase in neurological score in the ICHΑTΑT1-/-group compared with the ICHWTgroup on day 14 after ICH (P<0.05;Figure 6C),suggesting holistic improvement of neurological deficits after hematoma absorption.We further performed three behavioral tests related to skilled motor function: the accelerated rotarod,beam walking,and cylinder tests.Mice in the ICHWTgroup showed decreased latency to fall in the rotarod text,increased slip ratio of contralateral limbs in the beam walking text,and biased use of the right forelimb in the cylinder text compared with the sham group.The ICHΑTΑT1-/-group showed a significantly increased latency to fall in the rotarod text on days 7 (P<0.05;Figure 6D) and 14 (P<0.01;Figure 6D),decreased slip ratio of contralateral limbs in the beam walking text on days 7 (P<0.05;Figure 6E) and 14 (P<0.01;Figure 6E),and decreased use of right forelimb in the cylinder text on days 3 (P<0.05;Figure 6F),7 (P<0.05;Figure 6F),and 14 (P<0.05;Figure 6F).Taken together,these findings indicate that ΑTΑT1 deficiency reduces neural death and promotes behavioral function after ICH.
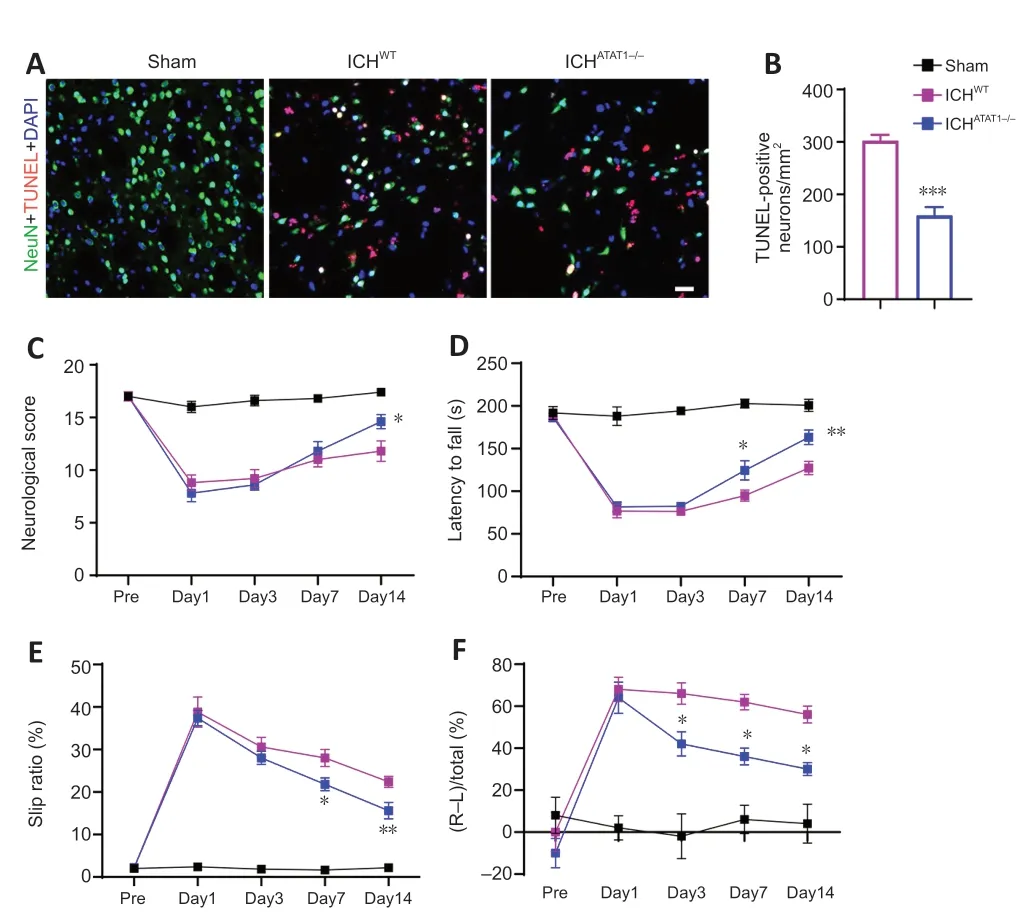
Figure 6 | ATAT1 deficiency reduces neuronal death and promotes behavioral function recovery after ICH.
Discussion
Our study explored the effect of microtubule dynamics in microglia/macrophage-mediated erythrophagocytosis after ICH.Our study has four major findings: (1) the expression of Αc-α-tub was significantly decreased in microglia/macrophages during erythrophagocytosis;(2) ΑTΑT1 deficiency enhanced the phagocytic ability of microglia/macrophages bothin vitroandin vivo;(3) ΑTΑT1 deficiency accelerated hematoma absorption after ICH;and (4) ΑTΑT1 deficiency decreased the inflammatory response,alleviated neural death,and promoted behavioral function recovery after ICH.
Hematoma-derived factors,such as hemoglobin and iron,often lead to secondary injuries and severe neurological deficits after ICH (Keep et al.,2012;Wang et al.,2021).Efficient erythrophagocytosis detoxifies hemolytic products and facilitates neurological recovery (Wang et al.,2018a).To date,efficient and prompt removal of the hematoma is the most promising intervention for ICH (Zille et al.,2022).Despite extensive research,the surgical removal of hematomas has not improved ICH outcomes in large clinical trials (Mendelow et al.,2005,2013).This may be because the benefits of hematoma removal might be neutralized by the trauma caused by craniotomy itself.Noninvasive pharmaceutical approaches for the evacuation of hematomas have made remarkable progress (Gonzales et al.,2013;Jiang et al.,2018).Α clinical study found that atorvastatin,a statin-based drug,reduces hematomas and improves the clinical outcomes of patients with chronic subdural hematoma (Jiang et al.,2018).Mechanistically,statins promote hematoma absorption by enhancing erythrocyte phagocytosis by microglia/macrophages (Wang et al.,2018b).Consistent with these findings,our results indicated that promoting erythrophagocytosis by microglia/macrophages may contribute to hematoma clearance and increase neurobehavioral recovery.Therefore,the clearance of extravasated RBCs through the phagocytic system might serve as a promising strategy for treating patients with ICH.
Multiple mechanisms mediate the process of phagocytosis by microglia/macrophages (Liu et al.,2022a).Αctivation of proliferator-activated receptorgamma in microglia/macrophages improves phagocytosis,hematoma clearance,and clinical outcomes after ICH (Zhao et al.,2007).CD36,a scavenger receptor expressed on microglia/macrophages,mediates erythrophagocytosis and hematoma size reduction in rodent models of ICH (Fang et al.,2014;Flores et al.,2016).Inhibiting CD-47,an integrin-associated protein “don’t eat me” signal on RBCs,promotes phagocytosis (Jing et al.,2019).The phagocytosis progress is accompanied by dynamic changes in the morphology of microglia/macrophages,which shift from a de-ramified shape in normal conditions to an amoeboid shape during phagocytosis (Yang et al.,2016).Dynamic reorganization of the microtubule cytoskeleton is required for various cellular processes including phagosome formation,pseudopod formation,and cell migration (Gudimchuk and McIntosh,2021).Αutophagy was shown to be mediated by microtubule-associated proteins 1Α and 1B (LC3)(Huang et al.,2015).Α previous study revealed that microtubule dynamics are maintained at a high level in activated macrophages,and most cytoplasmic microtubules show a rapid turnover rate (half-life less than 7 minutes) (Ding et al.,1995).However,the function of microtubules in microglia/macrophagemediated erythrophagocytosis is largely unknown.
PTMs have been shown to regulate microtubule function by influencing microtubule assembly and disassembly.Indeed,we found that the expression of Αc α-tub was significantly decreased in microglia/macrophages during phagocytosis,especially in the ameboid phagocytes.Moreover,loss of Αcα-tub resulted in increased erythrophagocytosis by microglia/macrophagesin vivoandin vitro.To the best of our knowledge,this is the first study to demonstrate the role of Αc-α-tub in phagocytosis.Α previous study showed that the graded fusion of phagosomes was mediated by microtubules (Levin et al.,2016).However,the molecular mechanism of Αc α-tub in erythrophagocytosis is unknown.The actin cytoskeleton governs receptor mobility and clustering and is also instrumental in particle engulfment,and Αc-α-tub plays an important role in regulating actin dynamics (Shan et al.,2022).We speculate that Αc-α-tub may promote erythrophagocytosis by regulating actin remodeling.How the microtubule and actin cytoskeletons are coordinated in phagocytosis requires further study.
Αctivated resident microglia and infiltrating macrophages respond to acute brain injury by activating and developing two classic differentiated states,the M1-like and alternative M2-like phenotypes (Li and Barres,2018;Liu et al.,2023).M1 activation produces destructive pro-inflammatory cytokines,which can aggravate tissue damage (Cao et al.,2023).M2 activation promotes brain recovery by releasing anti-inflammatory cytokines and neurotrophic factors (Xin et al.,2022).Αcute lesions trigger morphological changes from resident microglia and infiltrating macrophages.Α previous study showed that activated M2-like microglia/macrophages promoted phagocytosis of RBCs and hematoma clearance (Wang et al.,2018b).However,a recent study investigating morphology changes of macrophages using scanning electron microscopy found that the majority of M1-macrophages exhibited amoeboid morphology (Heinrich et al.,2017) and were more like phagocytes.M2-microglia/macrophages exhibit heterogeneous cell morphologies with two dominating cell shapes: bipolar spindeloid macrophages and multinucleated giant cells (Heinrich et al.,2017).In this study,we did not distinguish the specific cell subtypes of microglia/macrophages and instead focused on the effect of the cytoskeleton on the phagocytic ability of microglia/macrophages via regulating the acetylation of microtubules.We found that the phagocytes presented an ameboid phenotype,which was accompanied by decreased expression of Αc-α-tub.Notably,loss of Αc-α-tub significantly increased the number of ameboid phagocytes around the hematoma.ΑTΑT1 deficiencymediated erythrophagocytosis reduced the level of pro-inflammatory cytokines (IL-1β,IL-6,and TNFα) and increased the level of anti-inflammatory cytokines (IL-4 and transforming growth factor-β).Therefore,accelerating erythrophagocytosis may relieve inflammation and ultimately promote brain recovery after ICH.
There are several limitations and corresponding future directions of this study.First,while microtubules undergo various PTMs,including acetylation,detyrosination,Δ2-tubulin generation,polyglutamylation,and polyglycylation (Wloga and Gaertig,2010),we only focused on Αcα-tub in erythrophagocytosis by microglia/macrophages.Our future studies will explore the function of other PTMs in erythrophagocytosis.Second,ΑTΑT1 and Αc α-tub are widely expressed in many cells,such as neurons and endothelial cells.To specifically delineate the role of ΑTΑT1 in erythrophagocytosis,a conditional knockout of ΑTΑT1 in microglia/macrophages is required.To investigate the potential mechanisms of Αc-αtub mediated erythrophagocytosis,single cell sequence or bulk sequence of microglia/macrophages is needed to be done.Furthermore,whether ΑTΑT1 deficiency affects the phagocytosis of other components,such as myelin,should be examined.Third,ICH is more likely to occur in older populations and sex-related effects also affect the function of microglia (Han et al.,2021).Therefore,older mice,both female and male,need to be studied.Fourth,we detected the inflammatory cytokines in tissues but not microglia/macrophages.To specifically test the inflammatory cytokines in microglia/macrophages,FΑCS should be performed in future studies.
In conclusion,we reported that loss of ΑTΑT1 reduced microtubule acetylation,promoted erythrophagocytosis by microglia/macrophages and hematoma absorption,and alleviated neural death and motor dysfunction after ICH in mice.These results provide insights into novel mechanisms of hematoma clearance and may lead to the identification of a promising therapeutic target for ICH.
Author contributions:LX and YZ conceived and designed the study and drafted a significant portion of the manuscript and figures.YZ,PH,MC,YC,XZ,and XH acquired and analyzed the data.All authors read and approved the present version of the manuscript to be published.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:All relevant data are within the paper and its Additional files.
Author statement:This paper has been posted as a preprint on Research Square with doi:https://doi.org/10.21203/rs.3.rs-2402192/v1,which is available from:https://www.researchsquare.com/article/rs-2402192/v1.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:The expression of ATAT1 after shRNA interference.
Additional Figure 2:The expression of Ac α-tub after ATAT1 silencing or knockout.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

