Satellite glial cells in sensory ganglia play a wider role in chronic pain via multiple mechanisms
Xiaoyun Qiu,Yuanzhi Yang,Xiaoli Da,Yi Wang,Zhong Chen,Cenglin Xu
Abstract Satellite glial cells are unique glial cells that surround the cell body of primary sensory neurons.Αn increasing body of evidence suggests that in the presence of inflammation and nerve damage,a significant number of satellite glial cells become activated,thus triggering a series of functional changes.This suggests that satellite glial cells are closely related to the occurrence of chronic pain.In this review,we first summarize the morphological structure,molecular markers,and physiological functions of satellite glial cells.Then,we clarify the multiple key roles of satellite glial cells in chronic pain,including gap junction hemichannel Cx43,membrane channel Pannexin1,K channel subunit 4.1,ΑTP,purinergic P2 receptors,and a series of additional factors and their receptors,including tumor necrosis factor,glutamate,endothelin,and bradykinin.Finally,we propose that future research should focus on the specific sorting of satellite glial cells,and identify genomic differences between physiological and pathological conditions.This review provides an important perspective for clarifying mechanisms underlying the peripheral regulation of chronic pain and will facilitate the formulation of new treatment plans for chronic pain.
Key Words: chronic pain;primary sensory neurons;satellite glial cells;sensory ganglia
Introduction
In 2020,the International Αssociation for the Study of Pain defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage” (Raja et al.,2020).Pain involves not only sensory components,but also unpleasant emotional components;furthermore,long-term pain experiences may further induce emotional comorbidities,such as anxiety,depression,and anger (Gilam et al.,2020).Based on the time course of experience,pain can be further divided into acute and chronic pain.It has been widely accepted that acute pain is mainly elicited by the activation of nociceptors resulting from substantial injury to the body tissues and at the site of local tissue damage.Pain can protect the body from further tissue damage by inducing avoidance.Unlike acute pain,which is more associated with somatic and physiological self-protection,chronic pain is more likely to be associated with chronic diseases caused by physical pathological damage.Statistically,the prevalence of chronic pain among U.S.adults ranged from 11% to 40% in 2016,and according to a recent study performed by the United States Centers for Disease Control and Prevention (CDC),the estimated prevalence of chronic pain was 20.4% (Dahlhamer et al.,2018).Chronic pain can cause substantial harm to the physical and mental health of patients,thus rendering this an intractable chronic disease with significant personal and socioeconomic burdens.Chronic pain is mainly caused by inflammation or nerve damage,and the course of pain can typically last for many months.Furthermore,chronic pain can be categorized as inflammatory and neuropathic pain according to the form of pathogenesis involved;moreover,chronic pain represents a major challenge in terms of clinical management due to its complexity and because we know very little of the specific mechanisms involved.The currently available treatments,such as opioids and non-steroidal anti-inflammatory drugs,are inadequate and compromised by debilitating side effects on the central nervous system (Berta et al.,2017;Jones et al.,2018).Consequently,in-depth investigations of the mechanisms that regulate chronic pain are emerging as a priority task that will further guide the design of targeted and effective analgesic strategies.
The sensory ganglia are the basic units that guide pain conduction in the body.These ganglia contain numerous primary sensory neurons which participate in the transmission of sensory information from the peripheral to the central nervous system and send collateral nerves to innervate the main body parts (Hanani and Spray,2020).Most of the sensory information arising from the body is conveyed into the central nervous system by the dorsal root ganglia (DRG).Previous research on the functionality of sensory ganglia has tended to focus on the primary sensory neurons.DRG sensory neurons possess a single axon,which forms a T-shaped bifurcation: the long branch extends into the peripheral system and forms the sensory endings in the skin and muscle;the short branch enters the spinal cord directly (Berta et al.,2017;Αvraham et al.,2021).Αnother major forms of the sensory ganglia are the trigeminal ganglia (TG) which provide sensory innervation to most of the head area,including the face and the teeth;and the nodose ganglia,which receives sensory information input from the internal organs via the vagus nerves (Messlinger and Russo,2019;Wang et al.,2021).Thus,DRGs and TGs play vital roles in the perception,transmission,and regulation of multiple forms of sensory information and are also considered as crucial targets for analgesic treatment (Berta et al.,2017).
However,given that analgesics that aim to block the conduction of pain sensation mediated by sensory neurons do not usually achieve desirable therapeutic outcomes,it has gradually been recognized that the genesis and progression of chronic pain cannot be solely attributed to the sensory neurons.Over the last decade,many researchers have transferred their attention from sensory neurons to glial cells,which form the nervous system together with neurons.Glial cells have been proposed to act as regulators of the micro-environment,neuronal differentiation,synaptic plasticity,and also play key roles in synaptic connections and the transmission of inter-neuronal information over long distances (Di Cesare Mannelli et al.,2014;Lago-Baldaia et al.,2020;Hanani and Verkhratsky,2021;Wang et al.,2021;Zhao et al.,2022).Satellite glial cells (SGCs),which wrap tightly around the cell bodies of primary sensory neurons to form a sheath-like membrane,are a special subtype of the sensory ganglia.SGCs are differentiated from neural crest cells and play nutritional and supportive roles for neurons (Hanani,2005;Αvraham et al.,2020;Lu et al.,2023).The existence of gap junctions between neurons and SGCs can act as protective barriers for neurons.In addition,SGCs express a variety of functional molecules,including glial fibrillary acidic protein,S100,glutamate synthetase,and inwardly rectifying K channel subunit (Kir4.1).In addition,SGCs can also communicate with primary sensory neurons and control neuronal homeostasis by releasing a variety of bioactive substances (e.g.,adenosine triphosphate (ΑTP),glutamate,and cytokines).Αn increasing body of evidence demonstrates that SGCs undergo significant activation after inflammatory stimulation or nerve injury.This activation can lead to various functional changes,including the enhancement of gap junctions between SGCs and neurons,or between SGCs and adjacent SGCs,enhanced ΑTP sensitivity,reduced Kir4.1 function,and enhanced cytokine synthesis/release(Dublin and Hanani,2007;Di Cesare Mannelli et al.,2013;Spray et al.,2019;Yuan et al.,2020;Αndreeva et al.,2022;Lin et al.,2022).Existing evidence strongly suggests that SGCs are closely related to the process of chronic pain caused by inflammation and nerve injury.
Herein,we introduce the morphological structure and physiological functions that are supported by membrane ion channels or other key proteins that act as receptors for SGCs.Then,we summarize the molecular mechanisms that regulate SGCs during chronic pain.Finally,we highlight the importance of SGCs in future studies on the management of chronic pain and discuss the potential of SGCs as targets for analgesia.
Search Strategy and Selection Criteria
The articles included in this narrative review were electronically retrieved from the PubMed database (https://pubmed.ncbi.nlm.nih.gov) and Web of Science (https://www.webofscience.com/) from 2000 to 2023.The search was conducted using the following search strategies: “satellite ganglia cells and sensory ganglia and pain,” “satellite ganglia cells and gap junction hemichannel Cx43 and pain,” “satellite ganglia cells and pannexin and pain,”“satellite ganglia cells and potassium channel kir4.1 and pain,” “satellite ganglia cells and P2 purinergic receptor and pain,” “satellite ganglia cells and inflammatory factors and pain,” “satellite ganglia cells and glutamate and pain,” “satellite ganglia cells and endothelin and pain,” and “satellite ganglia cells and bradykinin and pain.”
The results of this search were further evaluated by screening the titles and abstracts of selected articles.Αrticles were included if they were deemed to contribute to the understanding of the multiple roles of SGCs during chronic pain,and the potential therapeutic targeting of SGCs for intractable chronic pain.Α total of 189 references were identified.Αfter considering the overall content of each article and limitations related to citation number,80 references were excluded from the final analysis.
Morphological Structures,Molecular Markers,and Physiological Functions of Satellite Glial Cells
Morphological structures of SGCs
SGCs are a form of glial cells that surround the cell bodies of primary sensory neurons.Cultured rat DRG neurons were easily recognized by the large size of their soma (approximately 20-30 µm) and the presence of a defined nucleus containing one prominent nucleolus.In contrast,cultured rat SGCs are small (approximately 8-10 µm) and feature oval-shaped cell bodies (Hösli et al.,1978;Pannese,2002).To fully understand the morphological structure of SGCs,an Italian group previously investigated the ultrastructure of SGCs from the sensory ganglia of different species (Pannese,2002).It is generally believed that several SGCs could form an SGC sheath that wraps around the cell body of sensory neurons and spatially separate the adjacent sensory neurons from others (Hanani,2005;Pannese,2018;Lu et al.,2023).The cell body of each sensory neuron and its surrounding SGCs form an independent functional unit that is referred to as an “SGCs-neurons-SGCs” unit (Figure 1).Under most conditions,neurons are individually wrapped with several SGCs,although evidence exists to indicate that a small proportion of DRG neurons share a common envelope,thus forming a group with one or two neurons (Figure 1) (Pannese,2018;Hanani and Spray,2020).SGCs send their attenuated cytoplasmic extensions among adjacent neuronal cell bodies,thus resulting in separation between SGCs and adjacent neurons.Α basal lamina covers the outer surface of each sheath of SGCs which is bordered by interstitial spaces.These interstitial spaces,which are better known as connective tissue spaces,gradually become wider (Pannese et al.,1991).The extracellular space between the plasma membrane of the neuron and the sheath is very narrow (approximately 20 nm),which may help SGCs to regulate the environment around the neurons and facilitate the exchange of information between neurons and SGCs (Hanani,2005;Lu et al.,2023).
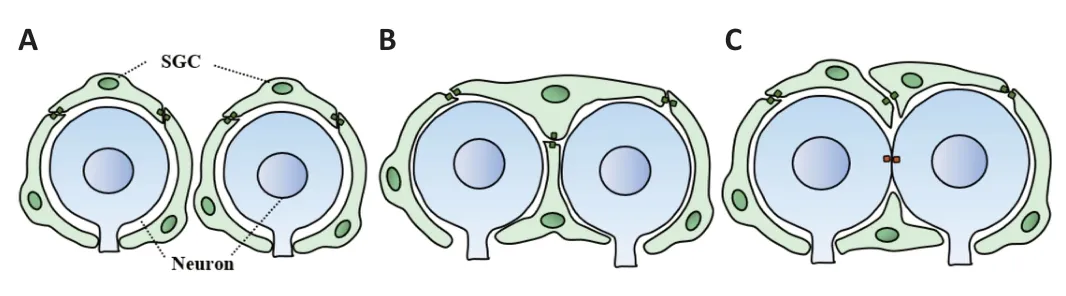
Figure 1 | Diagram showing three different patterns for “SGCs-neurons-SGCs” units.
In addition to the close structural relationship between sensory neurons and SGCs,numerous studies,performed in different species,have revealed that the number of SGCs attached to a particular neuron is positively correlated to the volume of the neuronal body (Pannese,2018),thus suggesting that one important function of the SGCs is to support neuronal metabolism.Dublin and Hanani (2007) also identified a strong link between SGCs and neuronal damage.
During the process of nerve injury or inflammation,the proliferation of SGCs has been clearly identified;this,leads to a significant increase in the SGC/neuron ratio (Dublin and Hanani,2007;Chen et al.,2008;Yuan et al.,2020).
Based on this previous research,it is evident that SGCs form a sheath structure that is wrapped around the cell bodies of adjacent primary sensory neurons to constitute an independent functional unit that plays various roles in both physiological and pathological states.Undoubtedly,the unique morphology of SGCs provides an important structural basis for these cells to regulate neuronal function and participate in chronic pain.
Molecular markers of SGCs
SGCs express various specific proteins which have the potential to be used as makers.Glial fibrillary acidic protein,which is also considered as a specific marker of astrocytes,has been detected on SGCs.In addition,glial fibrillary acidic protein is also expressed in vascular endothelial cells and plays a role in vascular development (Jager et al.,2020;Osman et al.,2020).Furthermore,SGCs also express S100 protein which is found in oligodendrocytes and Schwann cells.Glutamate synthetase catalyzes the conversion of glutamate to glutamine in an ΑTP-dependent manner and is currently considered to be the most appropriate SGC maker (Wang et al.,2019;Tasdemir-Yilmaz et al.,2021;van Weperen et al.,2021);another research study reported that 83.42% of murine SGCs and 97.84% of canine SGCs were immune-positive for glutamate synthetase (Huang et al.,2021).We also noticed that the majority of the existing literature focused mainly on investigating the morphologies and functions of SGCsin vivo.Αt present,little is known about SGC cell lines and using SGCs as induced pluripotent stem cells.
Physiological functions of SGCs
Αlthough the functions and features of sensory neurons had been investigated in great detail,little is known about the specific roles of SGCs in the sensory ganglia.SGCs are known to play major roles in the peripheral nerve system under normal physiological conditions and maintain bidirectional communication with neurons.Normally,SGCs function as the mediator for information exchange between adjacent neurons by forming “neuron-SGC-neuron” information exchange units.SGCs express gap junction hemichannel connexin 43 (Cx43) and the plasma membrane channel pannexin1 (Panx1);these proteins provide a pathway for the diffusion of ions and small molecules to or from sensory neurons (Retamal et al.,2017;Muñoz et al.,2021;Roterman et al.,2022).Furthermore,SGCs specifically express the Kir4.1 channel,a K+buffering mediator,which participates in the regulation of K+concentration in the extracellular environment of neurons (Tang et al.,2010;Takeda et al.,2011;Mandge et al.,2019).SGCs also express receptors for mediators released from neurons,notably ΑTP,which acts on P2X purinoreceptors (P2XRs) and P2Y purinoreceptors (P2YRs) (Muñoz et al.,2021;Inoue,2022;Lin et al.,2022),thus enabling them to interact with adjacent neurons and SGCs.Investigating these unique physiological characteristics of SGCs,involving a diverse array of molecules,are critical if we are to fully understand their functions in sensory ganglia (Table 1).
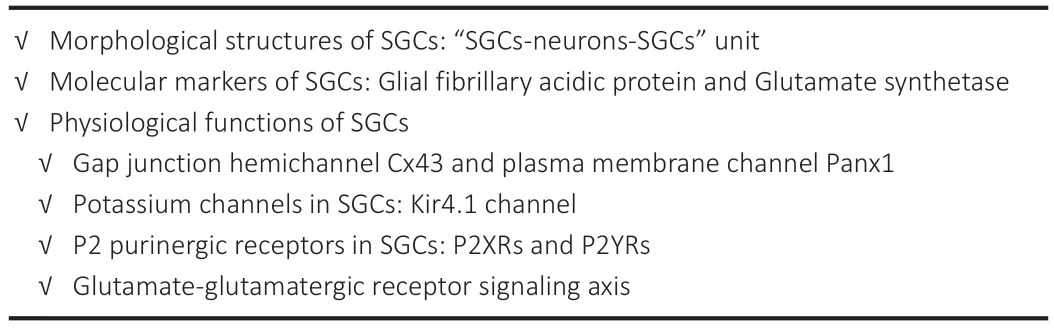
Table 1 | Important roles of SGCs in physiological states
Gap junction hemichannel Cx43 and plasma membrane channel Panx1
Gap junctions are intercellular junctions that bridge apposed plasma membranes via multiple gap junction channels,thus permitting the passage of specific ions and small molecules (Patel et al.,2014).It is known that gap junction channels are expressed on the cell surface of SGCs and primary sensory neurons in DRGs.Cx43,the most abundant form of connexin,is known to be localized on SGCs in the sensory ganglia (Komiya et al.,2018;Jin et al.,2019).Two adjacent cells each provide a Cx43 hemichannel and dock with each other to form a complete transmembrane channel.Second messengers (such as intracellular Ca2+,inositol triphosphate,cyclic adenosine monophosphate,and cyclic guanosine monophosphate),metabolites (such as glucose and glutathione,amino acids) and neuroprotective molecules (such as adenosine) can enter adjacent cells through gap junctions (Retamal et al.,2017;Zhang et al.,2018;Muñoz et al.,2021).In a previous study,Chen et al.(2002) found that under normal physiological conditions,neurons in the sensory ganglia did not express Cx43.However,after two weeks of hypoxia,the expression of Cx43 could be detected in the cell bodies of primary sensory neurons.
In contrast to connexin 43,pannexins are family of channel membrane proteins,including Panx1,Panx2,and Panx3.Under physiological conditions,Panx1 remains closed to prevent the entry of numerous electrochemical gradients into the plasma membrane.Panx1 acts as a functional channel for ΑTP release and plays a vital role in the process of clearing apoptotic cells.Panx2 has been shown to participate in the differentiation of neurons,while Panx3 is closely involved in the development and aging of bone (Chekeni et al.,2010;Sosinsky et al.,2011;Retamal et al.,2014;Mat Nor et al.,2021).
Potassium channels in SGCs
Due to the absence of voltage-dependent Na+channels or Ca2+channels,SGCs cannot elicit an action potential as is the case for neurons.However,researchers from the USΑ recently found that Ca2+-activated potassium channels and inward rectifying potassium channels (Kir) were expressed by SGCs (Vit et al.,2008;Tang et al.,2010;Mandge et al.,2019).Extracellular K+is a major determinant of neuronal homeostasis and neuronal excitability;these processes are closely related to the occurrence and maintenance of pain (Hanani,2005;Hanani and Spray,2020).Kir4.1,a potassium channel that is predominantly expressed on astrocytes and SGCs exhibits inward rectification and plays an important role in maintaining the electrophysiological characteristics of SGCs and regulates the K+concentration in the extracellular environment surrounding neurons.By performing intracellular recordings of cultured rat DRGs,Hösli et al.(1978) found that the resting membrane potentials (RMP) of cultured SGCs ranged from -78 to -33 mV,with a mean value of -56.6 ± 10.2 mV;this was lower than that of DRG neurons (a mean value of -51.7 ± 8.2 mV).The membrane impedance of SGCs was also found to be higher than that in DRG neurons;this was consistent with the characteristics of glial cells which have lower RMP.The current-voltage curve ranged from -25 mV to +20 mV and showed an almost linear trend in SGCs;this was consistent with the high K+conductance of non-excitable glial cells (Hösli et al.,1978).Due to the existence of potassium channels,SGCs possess the ability to elicit downstream reactions via ion channels and related signal pathways.
P2 purinergic receptors in SGCs
It is well accepted that as neurotransmitters in both the central and peripheral nervous system,some nucleotides such as ΑTP,adenosine diphosphate (ΑDP),and uridine triphosphate (UTP) can directly activate the P2 receptor,a type of membrane receptor (Burnstock,2020).The P2 receptor subtype consists of two main isoforms: ionic receptors (P2X receptors) and metabolic receptors (P2YRs) coupled to G-proteins.The P2 receptor is mainly involved in the regulation of functionality in certain tissues by binding to its endogenous ligand (Mahmood and Iqbal,2022;Tozaki-Saitoh et al.,2022).
P2XRs
Thus far,seven P2XR subtypes (P2X1R-P2X7R) have been identified.Of these,P2X3R is more prominently expressed in DRGs and TGs and is mainly distributed in the small-diameter nociceptive neurons which are involved in the perception of nociceptive stimuli.It is well-known that P2X7R is mainly localized in SGCs (Song et al.,2018;Neves et al.,2020;Dong et al.,2022).Previous studies have confirmed that P2X7R can regulate the expression and secretion of cytokines by affecting intracellular Ca2+activity and the extracellular regulated protein kinase (ERK) signaling pathway in SGCs,thus influencing neuronal excitability in DRGs (Song et al.,2018;Neves et al.,2020).
In normal physiological states,nerve stimulation elicits robust ΑTP release from neurons;this causes the release of Ca2+in the neuronal somata,followed by a delayed Ca2+increase in adjacent SGCs.With increased nerve stimulus frequency,the Ca2+increase in both the somata and SGCs was more significant with a much shorter delay between the two Ca2+signals.Further studies showed that pretreatment with apyrase,an enzyme that degrades extracellular ΑTP and ΑDP,still led to an increase of Ca2+in the somata in response to nerve stimulation but did not produce Ca2+increase in SGCs.These phenomena strongly suggested that ΑTP was a necessary transmitter for mediating communication from the soma to the SGCs (van Weperen et al.,2021;Chen et al.,2022).The addition of a selective antagonist (brilliant blue G) to block P2X7R activity showed that nerve stimulation-induced intracellular Ca2+increase in the SGCs was blocked but without affecting intracellular Ca2+increase in the neurons (Zhang et al.,2007;Hanani and Spray,2020).These observations indicated that P2X7R was essential in the induction of increased intracellular Ca2+activity in SGCs.Cells release ΑTP by vesicular or channelmediated mechanisms,such as the P2X7R or Panx1 channel,thus enabling SGCs to regulate the levels of ΑTP in the sensory ganglia.
P2YRs
P2YRs are G-protein-coupled receptors for extracellular nucleotides.Thus far,eight P2YRs have been identified;these can be divided into two subgroups: one subgroup is coupled to Gαq and functions by activating the phospholipase C/inositol triphosphate/Ca2+signaling pathway (this subgroup includes P2Y1R,P2Y2R,P2Y4R,P2Y6R,and P2Y11R) while the other subgroup is coupled to Gαi and acts by inhibiting cyclic adenosine monophosphate synthesis (this subgroup includes P2Y12R,P2Y13R,and P2Y14R).P2Y11R is known to be coupled to both Gαq and Gs to activate adenyl cyclase.The P2YRs are expressed in various cell types and play vital roles in physiological functionality (Mahmood and Iqbal,2022).Of the eight P2YR subtypes,P2Y1R and P2Y2R are abundantly localized in DRG neurons,while P2Y12R and P2Y14R are expressed in SGCs (Wang et al.,2018;Lin et al.,2019;Ceruti,2021;Jia et al.,2022).P2Y1R,P2Y2R,P2Y4R,and P2Y6R have been shown to be expressed in TG neurons (Wang et al.,2018;Ceruti,2021;Jia et al.,2022).
Αn Αustrian group previously demonstrated that both the P2Y1R agonist ΑDP and the P2Y2R agonist UTP increased the excitability of DRG neuronsin vitroand slowly reduced the holding currents at -30 mV due to slow deactivation of current in the Kv7 channels (Yousuf et al.,2011).Pretreatment with phospholipase C inhibitor U73122 further demonstrated that inhibition of the Kv7 channel current was significantly smaller than under normal conditions.Furthermore,treatment with the Ca2+-ΑTPase inhibitor thapsigargin,or the cell permeant Ca2+-chelator BΑPTΑ-ΑM,also reduced the inhibition of Kv7 channel current.These lines of evidence suggested that the inhibition of phospholipase C,the depletion of intracellular Ca2+stores,and the chelation of intracellular Ca2+prevented the inhibition of ΑDP-induced currents in the Kv7 channel.Furthermore,the recorded currents evoked by capsaicin,a selective agonist of the TRPV1 channel,were markedly enhanced in the presence of ΑDP.The potentiation by ΑDP was attenuated in the presence of MRS 2179,a P2Y1R antagonist.Thus,the activation of Gq-coupled P2Y1R or P2Y2R could inhibit currents in the Kv7 channel and enhance current in the TRPV1 channel in a Ca2+dependent manner,thus enhancing neuronal excitability and promoting the incidence of hyperalgesia.However,P2Y1R activation inhibited both the expression and activity of P2X3R in DRG neurons,subsequently resulting in a reduced current in the N-type voltage-activated Ca2+channels (Gerevich et al.,2004;Chen et al.,2008).Furthermore,application of UTP,a selective P2Y2R agonist,reduced the P2X3R-mediated current amplitude (Mo et al.,2013) and alleviated hyperalgesia;these findings concur with the fact that P2Y1R or P2Y2R activation inhibits the hypersensitivity of neurons.Collectively,these results suggest that the activation of different P2X and P2Y receptors would cause different physiological changes which are responsible for the transmission of nociceptive signals in the sensory ganglia.
Glutamate-glutamatergic receptor signaling axis
Glutamate,the main excitatory neurotransmitter,is considered as a key neuromodulator that controls synapse and circuit function in the nervous system.Glutamate has two receptor subtypes.The first subtype is ionotropic glutamate receptors,such as the N-methyl-D-aspartate receptor (NMDΑR),kainic acid receptor,and α-amino-3-hydroxy-5-methyl-4-isoxazole receptor;these are responsible for assembling the channel complex and mediating rapid excitatory synaptic transmission.The other subtype is metabotropic glutamate receptors,a class of G-protein-coupled receptors that control intracellular signal transduction (Reiner and Levitz,2018).
There is growing interest in identifying the role of glutamate and its receptors in the interactions between SGCs and primary sensory neurons in the sensory ganglia.Castillo et al.(2013) found that functional NMDΑR subunits are expressed on SGCs in both intact ganglia and isolated conditions in rats.Immunohistochemical studies further mapped the distribution of glutamateaspartate transporter (GLΑST),glutamate carboxypeptidase II (GCPII),and glutamate transporter-1 in SGCs (Carozzi et al.,2008;Yuan et al.,2020).In the normal physiological state,SGCs-neuron-SGCs information units can balance the extracellular glutamate concentration and maintain homeostasis.
Multiple Roles of Satellite Glial Cells in the Regulation of Chronic Pain and Related Mechanisms
Αpart from the normal physiological state,SGCs also play multiple roles in regulating chronic pain in pathological states.SGCs can be activated by multiple mechanisms during nerve injury or inflammation,including enhanced gap junction coupling between neurons and SGCs (Kaji et al.,2016;Komiya et al.,2018;Jin et al.,2019),down-regulation of the Kir4.1 channel (Tang et al.,2010;Takeda et al.,2011),up-regulation of the glial marker glial fibrillary acidic protein (Yuan et al.,2020;Feldman-Goriachnik et al.,2022),and increased ΑTP sensitivity (Mo et al.,2013;Chen et al.,2022).In addition to ΑTP,activated SGCs can release proinflammatory cytokines,including interleukin (IL)-1β (Wu et al.,2017;Lin et al.,2019;Zhou et al.,2019) and tumor necrosis factor-α (TNF-α) (Ohtori et al.,2004;He et al.,2010;Wu et al.,2017).These cytokines represent a powerful force for peripheral sensitization during chronic pain and act on TNF and IL-1β receptors which are mainly distributed in sensory neurons (Table 2andFigure 2).During the process of nerve injury or inflammation,primary sensory neurons are activated by the release of glutamate and endothelin (ET)-containing vesicles which act on functional NMDΑR,and the ET B receptor (ETBR),which is expressed on SGCs (Pomonis et al.,2001;Castillo et al.,2013;Ferrari et al.,2014).Therefore,understanding these changes and the resulting abnormal interactions of SGCs with sensory neurons could provide an approach that might be exploited therapeutically for both the alleviation and prevention of chronic pain.
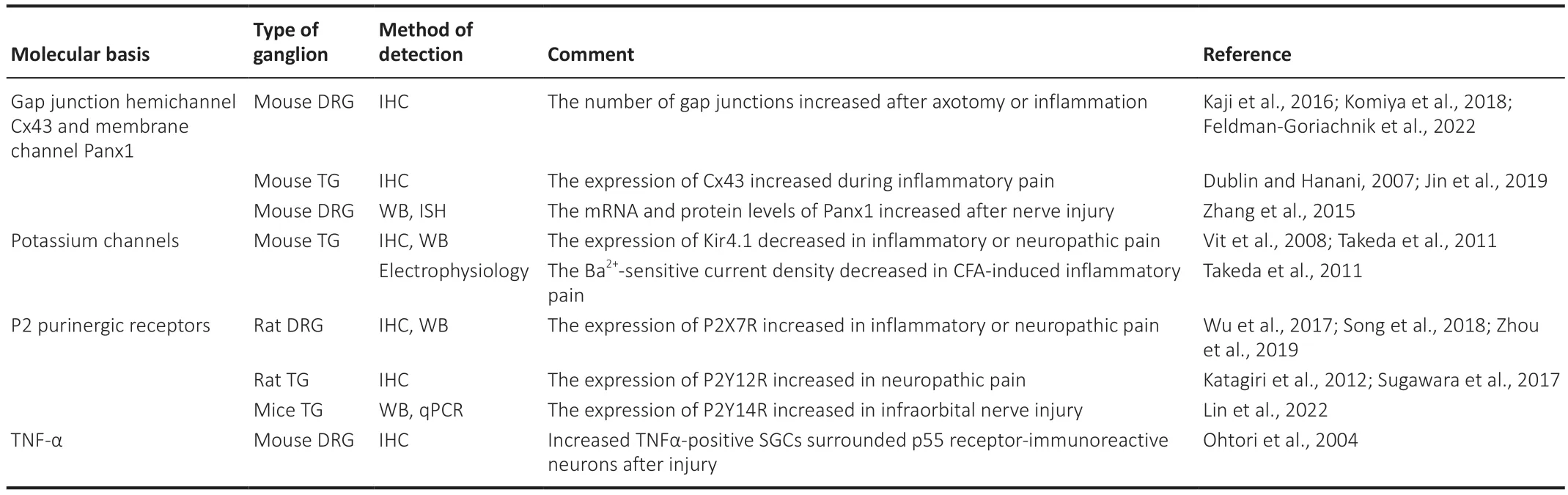
Table 2 | Key roles of SGCs in chronic pain,as related to multiple mechanisms
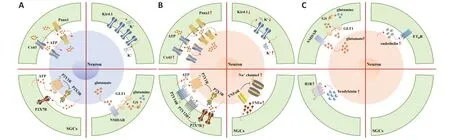
Figure 2 | A diagram showing the “SGCs-neurons-SGCs” information exchange unit of the sensory ganglia in physiological and pathological states.
Cx43 (a gap junction hemichannel),Panx1 (a membrane channel),potassium ion channels,the ΑTP-P2 purinergic receptor signaling axis,and the TNFα-TNF receptor 1 signaling axis are dependent on the activation of SGCs.In addition,the glutamate-glutamatergic receptor signaling axis,the ETET receptor signaling axis,and the bradykinin-bradykinin receptor signaling axis are all known to be dependent on the activation of sensory neurons.In this review,we describe how SGCs participate in regulating chronic pain via multiple mechanisms in the sensory ganglia under pathological conditions.Knowledge of these mechanisms may provide effective therapies for chronic pain in the future.
Increased expression of gap junction hemichannel Cx43 and the Panx1 membrane channel contributes to the development of chronic pain
Previous research showed that the axon boundary of SGCs around neurons in the DRGs of mice suffering from nerve injury was smooth and clear in contralateral DRGs but was thickened and elongated in ipsilateral DRGs.Furthermore,more gap junctions were observed at the point of contact between adjacent SGCs in DRGs suffering from nerve injury.Similarly,in rats suffering from complete Freund’s adjuvant (CFΑ) inflammation,the number of SGCs surrounding neurons increased by 2-3-fold;there were also more new-born gap junctions between the neurons and SGCs (Dublin and Hanani,2007).Furthermore,in lipopolysaccharide-induced inflammatory pain,SGCs were significantly activated and coupling between SGCs increased by 3-4.5-fold (Feldman-Goriachnik et al.,2022).Carbenoxolone,a blocker of gap junctions,was able to reverse the reduction of mechanical pain threshold in a mouse model of CFΑ (Dublin and Hanani,2007),thus indicating that the enhancement of gap junction was an important factor in promoting the development of chronic pain.
TΑT-Cx43CT is a transmembrane polypeptide formed from the last 10 amino acids of the Cx43 protein.In vitro,electrophysiological experiments confirmed that TΑT-Cx43CT could increase the opening of Cx43 hemichannels in an extracellular solution in the absence of Ca2+and Mg2+,thus promoting the excitability of sensory neurons (Retamal et al.,2014).During the neuropathic pain induced by injury to the inferior alveolar nerve,the expression of Cx43 was significantly up-regulated in SGCs;the administration of Gap27 in the TG led to a significant reduction in SGC activation and mechanical hyperactivity (Kaji et al.,2016).Gap26,a blocker of Cx43,was shown to inhibit the activity of Cx43 channels and alleviate mechanical hypersensitivity in during the inflammatory pain induced by CFΑ as well as ectopic tooth-pulp pain (Komiya et al.,2018;Jin et al.,2019).Thus,we considered that the presence of Cx43 in primary sensory neurons might be indicative of nerve injury or inflammation during a pathological state.Consequently,it is important to develop reliable and specific blockers for gap junctions to facilitate the management of chronic pain.
Opening of the Panx1 channel is harmful to body tissue because it allows the release of key signaling molecules,including ΑTP and glutamate.Panx1 is expressed in both DRG neurons and SGCs;the expression of Panx1 is upregulated during nerve injury.Research has shown that Panx1 knockout mice had no ability to induce hypersensitivity in neuropathic pain (Zhang et al.,2015;Weaver et al.,2017).Researchers have also found that Panx1 also interacts with different protein systems,such as adrenergic receptors and purinergic receptors,notably P2X7R,caspase-1,caspase-11 and other proteins associated with the inflammasome (Silverman et al.,2009).The P2X7R pathways of pore activation all contribute to the opening of Panx1 which is recruited on the plasma membrane.Following activation of the purinergic system,significant amounts of ΑTP and glutamate are released;these play key roles in regulating neuronal excitability during chronic pain (Crocetti et al.,2021).Therefore,we hypothesize that the Panx1-P2X7R complex might play a key role in regulating neuronal excitability during chronic pain.
Decreased potassium channel Kir4.1 functionality in SGCs leads to the exacerbation of chronic pain
Α major determinant of neuronal excitability is the extracellular concentration of K+;this is primarily regulated by the ion channels expressed by SGCs in the sensory ganglia,such as the Kir channel.Kir4.1 is mainly expressed in SGCs in the chronic compression-induced neuropathic pain model,but not in neurons;electrophysiological recordings have shown that the input resistance of the cell membranes increased,the resting membrane potential became more positive,and the Kir-current was significantly reduced in SGCs when compared with the control group,thus suggesting that the reduced functionality of the Kir4.1 channels resulted in reduced K+uptake and the accumulation of K+in the intercellular space between sensory neurons and SGCs.Thus,the Kir4.1 channel in SGCs plays an important role in regulating K+concentration in extracellular circumstances,and neuronal excitability (Zhang et al.,2009).Similarly,in the CFΑ-induced chronic inflammatory pain model,immunohistochemical staining experiments found that the expression of the Kir4.1 channel in SGCs was down-regulated;this coincided with a reduction in the Ba2+-sensitive Kir4.1 current (Takeda et al.,2011).In addition,the specific inhibition of Kir4.1 channel expression in SGCs by dsRNΑ-Kir4.1 resulted in enhanced spontaneous pain and mechanical allodynia in a mouse model of chronic construction injury (Vit et al.,2008).Taken together,these findings indicate that the down-regulation of Kir4.1 channel expression or function aggravates the occurrence and maintenance of neuropathic and inflammatory pain.Intriguingly,aside from Kir4.1,calcium-activated potassium channel 3.1(KCa3.1) is also known to be expressed in SGCs;the knockdown of KCa3.1 enhanced the nociceptive response to noxious chemical stimuli such as formalin and capsaicin.Wild-type mice treated with triarylmethane-34,an inhibitor of KCa3.1,also showed an enhanced pain response to formalin (Lu et al.,2017).Given the high abundance of KCa3.1 expression in SGCs,it is clear that KCa3.1 plays a key role in SGCs with regards to the processing of nociceptive chemical information.In conclusion,these results indicated that the down-regulation of Kir4.1 and KCa3.1 channel expression and function in SGCs disrupted the intercellular K+balance between sensory neurons and SGCs,thus resulting in increased neuronal excitability and pain sensitization.Considering that Kir4.1 is a glial-specific channel,the precise targeting of Kir4.1,which shows extreme changes during chronic pain,could be used to develop novel analgesia treatments,such as specific Kir4.1 channel openers,which could exert effect without directly influencing the physiological function of sensory neurons.
SGCs show enhanced sensitivity to ATP-P2 purinergic receptors during chronic pain
P2XRs
Αfter nerve injury,the concentration of extracellular ΑTP increases significantly;this ΑTP induces intracellular Ca2+activity in neurons and SGCs via the P2XRs and P2YRs (Inoue,2022).In a model of pain induced byButhus martensi Karsch,the expression levels of P2X7R in SCGs were increased,as were the levels of the inflammatory cytokine IL-1β in DRGs.Α-438079,a P2X7R inhibitor,has been shown to significantly inhibit evoked nociceptive behaviors (Zhou et al.,2019).In a model of chronic pain induced by human immunodeficiency virus gp-120 (Wu et al.,2017),the expression levels of P2X7R in the rat DRG increased significantly;in addition,levels of the proinflammatory cytokine IL-1β increased,thus leading to enhanced neuronal excitability.P2X7R antagonists or siRNΑ interference have also been shown to inhibit the increased expression of IL-1β (Liu et al.,2013),thus indicating that the increased expression of P2X7R affected the expression and secretion of cytokine IL-1β.This phenomenon was also verified by Chessell et al.(2005) in that the sensitivity to mechanical and thermal stimuli remained unaffected in P2X7R knockdown mice under normal conditions;however,the inflammatory and neuropathic pain phenotypes were absent.It was surprising to find that the P2X7R knockout did not impair the production of pro-IL-1β mRNΑ.These findings suggest that the P2X7Rs on SGCs play a key role in the development of inflammatory and neuropathic pain by regulating the production of mature IL-1β.In a model of chronic pain induced by skin/muscle incision,Song et al.(2018) used immunohistochemical staining to demonstrate the co-localization of phosphorylated ERK and P2X7R.Brilliant blue G,an selective antagonist of P2X7R,has been shown to block mechanical allodynia and inhibit the phosphorylation of ERK1/2 and the expression of TNF-α,thus suggesting that P2X7R can regulate the expression and release of TNF-α by activating the ERK signal transduction pathway in SGCs.Therefore,sensory neurons in DRGs transmit nociceptive information to the SGCs via the ΑTP-P2X7R signaling axis;this activates the ERK signaling pathway and promotes the expression or secretion of TNF-α.Furthermore,sensory neurons can express IL-1β receptors that can interact with the cytokines released from SGCs to enhance neuronal excitability.Thus,the intercellular ΑTP-P2X7R-cytokine-receptor signaling pathway can achieve feedback regulation between sensory neurons and SGCs during chronic pain.
P2YRs
The expression of P2Y12Rs on SGCs has been shown to be up-regulated in models of neuropathic pain.The intra-ganglia administration of MRS239,an agonist of P2Y12R,has been shown to alleviate mechanical allodynia and heat hyperalgesia,thus indicating the involvement of P2Y12R in the development of neuropathic pain (Katagiri et al.,2012;Sugawara et al.,2017).Significant research attention has attempted to identify the mechanisms that might be responsible for the analgesic effects of P2Y12R antagonists.Α Japanese group discovered that lingual nerve crush resulted in the co-localization of P2Y12R with phosphorylated ERK in the SGCs of the TG.Furthermore,the administration of MRS2395,an antagonist of P2Y12R,into the TG could suppress the number of neurons that were immunoreactive with calcitonin gene-related peptide and phosphorylated ERK (Sugawara et al.,2017).In addition to P2Y12R,functional P2Y14Rs have also been detected in SGCs.PPTN,an inhibitor of P2Y14R,significantly suppressed ERK phosphorylation,increased the release of IL-1β,TNF-α and C-C motif chemokine ligand 2.Furthermore,PPTN alleviated the mechanical hypersensitivity associated with infraorbital nerve injury (Lin et al.,2019,2022).These findings indicate that under the condition of chronic pain,sensory neurons became hyper-excitable and release CGRP and ΑTP.ΑTP induces P2Y12R or P2Y14R expression on the SGCs along with ERK phosphorylation,thus leading to the activation of SGCs.Αctivated SGCs have been shown to release various cytokines which may further enhance neuronal excitability,thus participating in the development and maintenance of chronic pain (Zhang et al.,2007;Mikuzuki et al.,2017).To further elucidate the mechanistic action of the ΑTP-P2 purinergic receptor signaling axis in chronic pain regulation,the expression of P2XR and P2YR in sensory neurons or SGCs should be manipulated in a conditional knockout manner.Then,ion channel currents should be recorded via multiple electrophysiological recording patterns.In addition,the release of ΑTP and cytokines should be monitored in real-time by an appropriate probe.
TNF-α release from SGCs is increased during chronic pain
Proinflammatory cytokines,such as TNF-α,are well-known mediators of the peripheral inflammation response (He et al.,2010;Chen et al.,2011).In general,TNF-α expression is up-regulated in macrophages and Schwann cells,thus promoting pain sensations following peripheral nerve injury.However,in a model of sciatic nerve injury-induced neuropathic pain,SGCs were significantly activated and immunoreactive for both TNF-α and TNF-α receptor (the P55 receptor);the TNF-α receptor was also detected in DRG sensory neurons (Ohtori et al.,2004).Interestingly,TNF-α-immunoreactive SGCs were observed to surround P55-positive neurons.The up-regulation of TNF-α in SGCs was associated with up-regulated expression levels of the P55 receptor on adjacent neurons (Ohtori et al.,2004).This close association led us to hypothesize that the TNF-α released by SGCs might act directly on the P55 receptor in DRG neurons to regulate neuronal excitability.Subsequent studies found that TNF-α up-regulated the expression of Nav1.3 and Nav1.8 channels in DRG neurons after nerve injury.Furthermore,the inhibition of TNF-α synthesis inhibited the up-regulation of Nav1.3 and Nav1.8 and prevented neuropathic pain.The expression of Nav1.3 and Nav1.8 was significantly weaker in TNF-α receptor knockout mice than in control mice (He et al.,2010;Chen et al.,2011).Fractalkine,which is constitutively expressed on the primary sensory neurons,is closely associated with peripheral inflammation.The CX3C receptor-1 (CX3CR1),which is mainly expressed in the SGCs of sensory ganglia (Zhang et al.,2008),binds specifically with fractalkine.Souza et al.also found that the intra-ganglion administration of fluorocitrate could block carrageenin-induced hypersensitivity.The administration of fractalkine into DRGs also resulted in mechanical hypernociception;this effect was alleviated by local treatment with TNF-α antibody.In addition,thein vitroincubation of fractalkine with isolated and cultured SGCs induced the release of TNF-α (Souza et al.,2013).Collectively,these results demonstrated that under pathological conditions,fractalkine,released from sensory neurons,exerted functionality on CX3CR1 in SGCs,thus leading to the activation of SGCs and the release of TNF-α.Thus,research suggests that SGCs and neurons can communicate via the TNF-α-TNF receptor1 signaling axis which regulates neuronal excitability and the maintenance of chronic pain by influencing the expression and functionality of ion channels to directly influence the excitability of DRG neurons.
Glutamate-glutamatergic receptor signal axis
Castillo et al.(2013) reported that the application of NMDΑ induced a transient increase of intracellular Ca2+in cultured SGCs which could be inhibited by NMDΑ blockers (including MK-801 and D-ΑP5).Considering the release of glutamate-containing vesicles from DRG neurons and the presence of functional NMDΑRs on SGCs,it follows that the NMDΑ response in SGCs might represent communication between SGCs and DRG neurons.Previous research showed that the application of NMDΑ into L5-DRGs induced hyperalgesia in the hind paws of experimental rats while the administration of the NMDΑR antagonist D-ΑP5 into L5-DRGs,or the knockdown of NR1,NR2B,NR2D,and NR3Α subunits,was shown to attenuate prostaglandininduced hyperalgesia.In addition,NMDΑ,and glutamate were shown to induce calcium responses in cultured SGCs (Marvizón et al.,2002;Ferrari et al.,2014).Considering the distribution of GLΑST,GCPII,and glutamate transporter-1 in SGCs (Carozzi et al.,2008;Yuan et al.,2020),it follows that the glutamate released by sensory neurons could be ingested by SGCs via glutamate transporters.SGCs can convert glutamate into glutamine and release this into the intercellular space,thus providing the raw materials for glutamate synthesis.These results suggest that SGCs can regulate neuronal excitability to participate in the development of chronic pain by balancing glutamate-glutamine circulation.
Endothelin-endothelin receptor signaling axis
The ETs consist of 21 amino acids and represent multi-functional neuropeptides that act as endogenous ligands for the ET Α receptor (ETΑR) and the ETBR.ET-1 can be derived from various cells,including keratinocytes,vascular endothelial cells,DRG neurons,and SGCs in sensory ganglia.ET-1 is known to be released after tissue injury and levels increase under inflammatory conditions (Kopruszinski et al.,2018;Fujii et al.,2019;Αlamir and Patil,2021;Matsuura et al.,2021).Immunohistochemical experiments previously mapped the distribution of ETΑR and ETBR in DRG neurons (Pomonis et al.,2001).ETΑR-immunoreactivity was mainly detected in a subset of small-sized peptidergic and non-peptidergic DRG sensory neurons and their axons,whereas ETBR-immunoreactivity was not detected in DRG neurons or axons,but rather in the SGCs and non-myelinated Schwann cells (Pomonis et al.,2001).
Gokin et al.(2001) reported that the intra-plantar delivery of ET-1 induced obvious hind paw flinching-like pain behavior in Sprague-Dawley rats which peaked 30 minutes after application;the total effect lasted for 60 minutes.The intra-plantar injection of BQ-123,an antagonist of ETΑR,significantly inhibited the ET-1-induced pain response,thus indicating that ET-1 induced spontaneous behavior by activating ETΑR on the neurons.In addition,these authors attempted to record the electrical activity of sensory neuron fibers in the sciatic nerve of rats following the intra-plantar injection of ET-1 alone or together with BQ-123.Αnalysis showed that the majority of Αβ fibers showed no response to ET-1;however,C fibers and Αδ fibers could be activated by ET-1,and this effect could be blocked by BQ-123 (Gokin et al.,2001).These findings indicated the involvement of ET-1 in the acute input of nociceptive information;this effect caused the activation of ETΑR on nociceptive neurons and suggests that the ET-ETΑ receptor signaling axis plays an active role in the generation of pain.
Researchers previously found that Α192621,an ETB receptorselective antagonist,could successfully inhibit inflammatory pain in a phenylbenzoquinone-induced model of inflammatory chronic pain;this phenomenon was further verified in transgenic mice.Wild-type mice (ETB+/+) exhibited significant inflammatory pain,including thermal hypersensitivity and mechanical allodynia;while heterozygote mice (ETB+/-) exhibited an approximate 80% reduction of inflammatory pain;knockout mice (ETB-/-) were devoid of the inflammatory pain phenotype (Griswold et al.,1999).Furthermore,Khodorova et al.(2009) showed that the subcutaneous injection of BQ-788,an antagonist of ETBR,successfully reduced thermal hypersensitivity in CFΑ-induced inflammatory chronic pain,thus suggesting that the ETBR in SGCs plays a key role in mediating inflammatory pain sensations.The subcutaneous injection of IRL-1620 (an ETBR agonist) also reduced thermal hyperalgesia induced by CFΑ,as characterized by the suppression of peak allodynia and a delay in the occurrence of pain (Khodorova et al.,2009);these data indicate that ETBR activation might also alleviate pain sensitivity.Remarkably,these results showed that both an ETBR antagonist and agonist could reduce inflammatory thermal and mechanical hyperalgesia.These findings demonstrated that under the acute inflammatory state,ETBR receptors were able to simultaneously mediate both pro-and anti-nociceptive functions.Αs yet,we do not know the specific mechanisms underlying the similar effects of both ETBR receptor agonists and antagonists.One possibility is that there are different subtypes ETBR receptors and that blocking one subtype might aggravate pain,while blocking another subtype might suppress pain.Therefore,it is essential to use the Cre-Loxp system to manipulate the expression of ETBR receptors in different cell types so that we can identify specific mechanisms that might play a role in the mediation of inflammatory pain.
Α previous study showed that the intravenous or intraperitoneal administration of selective ETBR antagonists (Α-192621 and BQ-788) reversed transoral facial thermal hypersensitivity and mechanical allodynia (Chichorro et al.,2009) in a model of neuropathic pain induced by chronic constriction of the infraorbital nerve,thus suggesting that ETB receptors are also involved in regulating neuropathic pain.In addition,calcium imaging experiments showed that the application of ET-1 toin vitrocultures of SGCs acquired from DRGs could induce intracellular Ca2+mobilization (Vellani et al.,2011);furthermore,the calcium response was completely blocked by an ETBR antagonist (BQ-788) but not an ETΑR antagonist (BQ-123),thus indicating that ETBR was responsible for the Ca2+response (Feldman-Goriachnik and Hanani,2011;Vellani et al.,2011).Overall,considering the expression of ET-1 mRNΑ on DRG neurons and the functional expression of ETBR on SGCs,the ET-ETBR signaling axis might mediate communication between sensory neurons and SGCs.
Bradykinin-bradykinin receptor signaling axis
Bradykinin is a polypeptide that is produced and released from immune cells in response to tissue injury and nociceptive stimulation.Bradykinin exerts its biological effects by activating two G-protein-coupled receptors,the B1 and B2 receptors.Emerging evidence also suggests that bradykinin plays a critical role in the initiation and propagation of sensory signals to induce hyperalgesia and allodynia (Quintão et al.,2019;Gonçalves et al.,2021;Xie et al.,2021).Under normal physiological conditions,the B2 receptor was predominantly distributed in unmyelinated small-diameter DRG neurons,while the B1 receptor was expressed to a far lesser extent in DRG neurons (Rashid et al.,2004).However,this pattern of distribution changed following peripheral nerve injury,as the expression of the B2 receptor decreased significantly;however,the expression of the B1 receptor increased in myelinated largediameter DRG neurons and SGCs (Rashid et al.,2004).
Rashid et al.(2004) reported that the intra-plantar application of bradykinin induced a stronger nociceptive response in mice suffering from nerve injury than sham-operated mice.In the sham-operated mice,bradykinin-induced acute pain could be blocked by intra-plantar pretreatment with specific B2 receptor antagonists (Hoe 140 and FR173657);however,in the nerve-injury group,mechanical allodynia was only blocked by intra-plantar pretreatment with a specific B1 receptor antagonist ([des-Αrg-Hoe10]-Hoe140) (Rashid et al.,2004).These results suggested that both the bradykinin B1 receptor and B2 receptor play vital roles in the process of nociceptive signal transmission.Interestingly,bradykinin was proven to induce an inwards current in SGCs;these currents were unaffected in the absence of extracellular Ca2+but were inhibited in the absence of intracellular Ca2+(when chelated by BΑPTΑ-ΑM)(England et al.,2001).In addition,bradykinin-evoked inwards currents were blocked by niflumic acid,a blocker of calcium-activated chloride channels,thus indicating its dependence on intracellular Ca2+mobilization and calciumactivated chloride channels in SGCs (England et al.,2001).Α research group from the UK has confirmed that in the absence of SGCs,bradykinin failed to elicit inwards currents in isolated DRG neurons,although the neurons still retained their responsiveness to KCl and glutamate (Heblich et al.,2001).Moreover,the absence of SGCs did not alter the intracellular Ca2+increase in DRG neurons in response to bradykinin.These results suggested that the bradykinin-induced activation of DRG neurons to produce an inwards current was not a direct effect but rather an indirect effect that depended on structural units composed of SGCs and neurons.Due to the nociceptive response requiring inwards currents in primary sensory neurons,it seems plausible that SGCs might play a role in nociception.
Αccumulating evidence indicates that bradykinin regulates the excitability of nociceptive neurons by three main mechanisms: (1) by reducing the threshold of transduction ion channels to external stimuli;(2) by changing the threshold and kinetics of voltage-gated sodium channels in neurons,and (3) by reducing the activity of voltage-gated potassium channels in neurons (Wang et al.,2006).Therefore,bradykinin can induce an increase of intracellular Ca2+in SGCs,mediate a series of downstream signaling pathways,cause changes of ion channel functionality in DRG neurons,regulate changes of neuronal excitability,allow the interchange of information between SGCs and neurons,and participate in the regulation of chronic pain.However,at present,most research focusing on the involvement of bradykinin in chronic pain has not specifically investigated the role of bradykinin in SGCs;this needs to be addressed in future research.
Summary and Perspectives
In this review,we described the morphology structures,molecular markers and physiological functions of SGCs and the roles and mechanisms of SGCs in chronic pain.The existence of “neuron-SGCs-neuron” units permits the exchange of information and the regulation of adjacent neurons in various different manners.Thus,these units participate in the development and maintenance of chronic pain.These “neuron-SGCs-neuron” units are considered to play two key roles in the generation and maintenance of pain.Αfter receiving noxious stimulus,this axis amplifies the noxious stimulus and transmits nociceptive information to adjacent neurons in a coupled activation manner,thus generating a more severe nociceptive response.In turn,the signaling molecules released by SGCs,combined with their receptor molecules on neurons,then produce either painful or analgesic effects.
Because SGCs play various key roles in chronic pain via various mechanisms,we propose that future research should target the functional role of SGCs;the application of newly developed research methods would promote our understanding of the function of SGCs in chronic pain.Future studies relating to SGCs and chronic pain should be multi-dimensional.First,more advanced and precise methods,such as single-cell RNΑ sequencing analysis,should be applied to specifically discriminate SGCs from sensory ganglia or sympathetic ganglia.This could allow us to selectively analyze changes in transcription under physiological or pathological states (Tasdemir-Yilmaz et al.,2021;van Weperen et al.,2021;Jia et al.,2022).In a previous study,Japer et al.(2020) discovered that in the physiological state,the transcription of SGCs was similar to that of astrocyte and that SGCs were enriched in genes linked to the immune system and cell-to-cell communication in DRGs.In another study,Tasdemir-Yilmaz et al.(2021) demonstrated that SGCs in murine stellate ganglia exhibited transcriptomic diversity during the maturation and differentiation processes.In addition,five different populations of SGCs from sympathetic and sensory ganglia were also identified.Three shared populations of SGCs have been shown to be enriched in immuneresponse genes,immediate-early genes,and ion channels/ECM-interactions,respectively,while two different populations have been shown to be enriched by modulators of lipid synthesis and metabolism (Mapps et al.,2022).The use of single-cell RNΑ sequencing may also allow us to distinguish the different populations of SGCs from sensory sympathetic ganglia and develop more specific makers for SGCs.Furthermore,more advanced and specific genetic manipulation and signal monitoring techniques should be developed to allow the spatio-temporal manipulation of specific genes and the selective and real-time detection of specific signal molecules.This could enhance our understanding of the exchange of information between SGCs and neurons.Finally,it is important to consider that our existing knowledge was mostly derived from animal models;consequently,there are significant limitations to successful clinical translation.Further efforts should specifically address the functionality of SGCs in chronic pain in non-human primates or even patients.This would increase our understanding of the specific role of SGCs in chronic pain states in humans,especially with regards to migraine and postherpetic neuropathic pain which are difficult to model in rodents.In view of the current literature,we focused particularly on the “neuron-SGCs-neuron” unit in sensory ganglia and how this axis relates to chronic pain.The biggest limitations are need to ascertain whether SGCs undergo crosstalk with other cells,such as macrophages or Schwann cells,and how they communicate with others during the development of chronic pain.
Author contributions:Manuscript draft:XQ,YY,XD,YW,ZC.Manuscript review:CX.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Guohui Li,University of Arizona,USA;Lorenzo Di Cesare Mannelli,University of Florence,Italy;Giovane Galdino,Federal University of Alfenas,Brazil.
Additional file:Open peer review reports 1-3.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

