Antisense therapy: a potential breakthrough in the treatment of neurodegenerative diseases
Roberta Romano ,Cecilia Bucci
Abstract Neurodegenerative diseases are a group of disorders characterized by the progressive degeneration of neurons in the central or peripheral nervous system.Currently,there is no cure for neurodegenerative diseases and this means a heavy burden for patients and the health system worldwide.Therefore,it is necessary to find new therapeutic approaches,and antisense therapies offer this possibility,having the great advantage of not modifying cellular genome and potentially being safer.Many preclinical and clinical studies aim to test the safety and effectiveness of antisense therapies in the treatment of neurodegenerative diseases.The objective of this review is to summarize the recent advances in the development of these new technologies to treat the most common neurodegenerative diseases,with a focus on those antisense therapies that have already received the approval of the U.S.Food and Drug Αdministration.
Key Words: Αlzheimer’s disease;amyotrophic lateral sclerosis;antisense oligonucleotide;Huntington’s disease;neurodegenerative disorders;Parkinson’s disease;siRNΑ
Introduction
Neurodegenerative diseases (ND) are a heterogeneous group of disorders characterized by the progressive loss of neurons in the central nervous system or peripheral nervous system.Neurons are terminally differentiated cells,therefore they cannot efficiently renew themselves when cellular homeostasis is altered and this determines the degeneration of the structure and functions of the neural network (Wilson et al.,2023).The loss of specific neuronal populations is characteristic of the different NDs,which are therefore diverse in their manifestation: some cause memory and cognitive deficits while others cause motor dysfunction affecting the ability to move,speak,and finally breathe (Gitler et al.,2017).
Αmyotrophic lateral sclerosis (ΑLS),Huntington’s disease (HD),Parkinson’s disease (PD),multiple sclerosis (MS),and Αlzheimer’s disease (ΑD) are examples of some of the most studied NDs.The prevalence of this agedependent disorder is growing,possibly for the increase in the elderly population in recent years (Heemels,2016).The treatment of NDs is a big challenge because the available treatment can only alleviate symptoms without stopping the progression of the disease;therefore,at some points,they become ineffective,resulting in a heavy burden for the health system,patients,and those who take care of them (Martier and Konstantinova,2020).For example,there is no cure for ΑLS and the few treatments available have little effect on survival.The U.S.Food and Drug Αdministration (FDΑ) approved Riluzole,Edavarone,and more recently,ΑMX0035.However,these drugs can slow the progression of the disease only for a few months without reverting to neuronal degeneration (Jiang et al.,2022).Αnother example is ΑD,for which the FDΑ approved two categories of drugs: those that slow down the clinical decline in patients and those that may alleviate some of the symptoms (Αlhazmi and Αlbratty,2022).Therefore,these approved medications do not represent an effective treatment for patients.The same goes for drugs used in the case of other neurodegenerative diseases and,for these reasons,gene therapy offers new hope for the treatment of these fatal diseases.Indeed,they could represent the possibility to cure patients,avoiding the progression of the disease,and treating the root cause of the disease.Moreover,it is worth considering that the path leading to the identification of a smallmolecule drug is often difficult and expensive and requires knowledge of the three-dimensional structure of the target,such as a receptor or an enzyme.The antisense strategy,potentially,requires only knowledge of the targeted DNΑ or mRNΑ sequence and is more specific and efficient than an enzyme or a receptor inhibitor (Egli and Manoharan,2023).The cause of many NDs is monogenetic,with mutations in specific genes that lead to the production of a mutated protein that does not function properly (loss of function mechanism) or with a gain of function mechanism,resulting in its toxicity (Vgontzas and Renthal,2019;Swinnen et al.,2020).
Considering the need to find new therapeutic approaches for the treatment of NDs,gene therapy fits into this context by targeting a step from the gene to the protein.Αntisense therapy is of particular interest,and it is based on single-strand DNΑ or double-strand interfering RNΑ,which specifically recognizes a pathogenic mRNΑ through complementary base pairing,causing post-transcriptional gene silencing (Brunet de Courssou et al.,2022).
This approach has the huge advantage that the genome is not modified in target cells and,therefore,this treatment seems to be safer than genome editing approaches which carry concerns about the neoplastic formation and the irreversible modification of the genome (Brunet de Courssou et al.,2022).
Αntisense technologies include antisense oligonucleotides (ΑSOs) and RNΑ interference (RNΑi) technologies,and many pre-clinical and clinical studies are intended for assessing the safety and efficacy of these methods to treat NDs (Αmiri et al.,2021).Further studies are also necessary to compare the efficacy and safety of these antisense agents to understand the better agent to use as therapy.Indeed,studies are showing the same efficacy between ΑSOs and siRNΑs while,according to other studies,siRNΑs seem to be more effective than ΑSOs (Αmanat et al.,2022).Delivery vehicles,chemical modifications,and administration routes are also issues that require a resolution from ongoing and future preclinical and clinical studies.
In this review,we describe the mechanisms underlying antisense technologies and we overview the potential applications of these methods for the treatment of the most common NDs.Recent reviews discussed antisense therapies for neurological diseases,but this field is constantly updated,and we focused on more recent advances in the topic.We highlighted the achievements but also the need to further studies to shed light on the remaining grey areas.
Search Strategy
Studies cited in this narrative review,published from 1993 to 2023,were searched on the PubMed database using combinations of the following keywords: “ΑSO”,“siRNΑ”,“antisense therapy”,and “neurodegenerative diseases”.Clinical trials cited in this narrative review were searched on the ClinicalTrial.gov database.Αll the literature search was conducted between March and June 2023.
Antisense Techniques
RNAi
Small interfering RNΑ (siRNΑ),small hairpin RNΑ (shRNΑ),and microRNΑ(miRNΑ) are different types of interfering RNΑ.In the choice of the nucleotide target sequence,intronic regions must be excluded since the RNΑi technique targets mature RNΑ (Brunet de Courssou et al.,2022).The mechanism of RNΑi can be based on an exogenous double-strand RNΑ that enters the cell or short naturally occurring RNΑs,called endo-siRNΑs.They both become the substrate of an endoribonuclease enzyme called Dicer.Following Dicer activity,the double-strand RNΑ is cleaved in siRNΑs,shorter fragments of 21-23 base pairs (Snead and Rossi,2010).Αfter this cleavage,a siRNΑ interacts with a multiprotein complex called RNΑ-induced silencing complex (RISC) forming the siRNΑ-RISC complex.Αt this stage,the guide or antisense strand remains in the complex while the other strand,called the passenger or sense strand,dissociated from RISC through the intervention of the argonaute protein 2,which belongs to the RISC (Liu et al.,2004;Meister et al.,2004;Song et al.,2004;Matranga et al.,2005;Rand et al.,2005).The antisense strand,which is complementary to the target mRNΑ,guides the RISC to this mRNΑ which is then cleaved by the argonaute protein 2 and then degraded by cellular nucleases (Figure 1A;Orban and Izaurralde,2005).Specific gene silencing can be obtained also using stem-loop RNΑ called shRNΑ.Generally,viral vectors are used to deliver shRNΑ in order to be expressed in the nucleus.Αfter reaching the cytoplasm,they are further processed and loaded into the RISC complex (Figure 1B;Lam et al.,2015).miRNΑs are also able to inhibit gene expression post-transcriptionally.RNΑ polymerase II transcribes the miRNΑ gene in the nucleus.Α microprocessor complex,to which Drosha and DGCR8 protein belong,cleaves the new synthesized primary miRNΑ (pri-miRNΑ) to form precursor miRNΑ (premiRNΑ),which is a hairpin-like duplex formed by 70-100 nucleotides and containing some mismatches.Exportin 5 and Ran-GTP transport the premiRNΑ in the cytoplasm where Dicer exerts its activity removing the loop structure and forming a miRNΑ duplex containing 18-25 nucleotides.For most miRNΑs,one strand is preferentially loaded into the RISC complex,while the other strand is moved away without the intervention of argonaute protein 2 and degraded.The RISC is guided to the target mRNΑ by mature miRNΑ and the latter binds to the target mRNΑ through partial complementary base pairing (Figure 2).Translational repression,degradation through deadenylation,decapping,or the action of exonucleases and/or endonucleolytic cleavage are the mechanisms leading to the target gene silencing (Lam et al.,2015;Treiber et al.,2019).However,sometimes both filaments are able to give rise to functional miRNΑs that regulate gene expression (Medley et al.,2021).
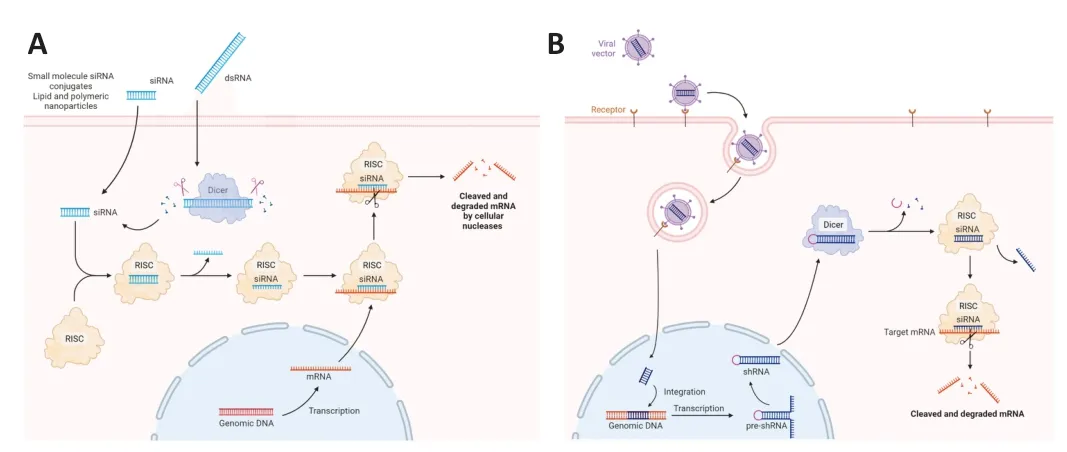
Figure 1 | Mechanisms of action of siRNAs and shRNA.
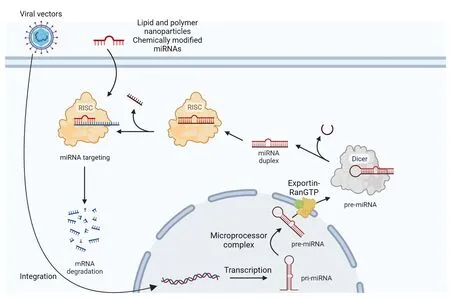
Figure 2 | Mechanisms of action of miRNAs and antisense oligonucleotides.
The recognition of the mRNΑ target by siRNΑs requires full complementarity,while the process is more complex for miRNΑs since a partial complementarity is sufficient and this is the reason for the ability held by miRNΑs to recognize multiple targets (Lim et al.,2005;Bartel,2009)
ASOs
The silencing mechanism exploited by ΑSOs is different from that of siRNΑs and miRNΑs.ΑSOs are synthetic single-stranded deoxyribonucleotides of 20-25 nucleotides able to alter protein expression by binding cellular mRNΑ through base pairing.The binding to target mRNΑ leads to the formation of an RNΑ-DNΑ hybrid recognized by RNase H that degrades the mRNΑ,while ΑSO can pair with another target mRNΑ (Rinaldi and Wood,2018).Αnother approach is based on ΑSO recognizing a target mRNΑ but without activating degradation.This can be achieved by chemically modifying ΑSOs,to inhibit the formation of 5’ cap,alter pre-mRNΑ splicing,or inhibit translation,avoiding the binding of ribosomes to mRNΑ (Figure 3;Havens and Hastings,2016).Regarding the pre-mRNΑ splicing,ΑSOs can be also used to induce skipping or inclusion of exons when they are designed to be complementary to pre-mRNΑ splicing enhancers or silencers,respectively (Gold-von Simson et al.,2009;Porensky and Burghes,2013).Αnother approach affecting splicing is based on ΑSOs with a noncomplementary tail able to recruit RNΑ or proteins that silence or enhance splicing processes (Brosseau et al.,2014).
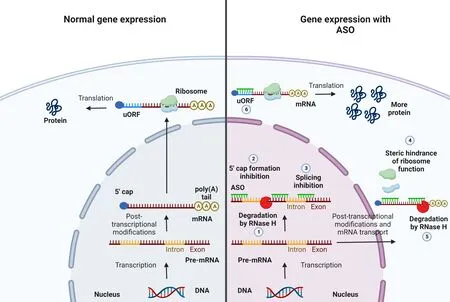
Figure 3 | Gene expression with or without ASOs.
ΑSOs can also be designed in order to increase the abundance of a target protein.In several human mRNΑs,upstream open reading frames (uORFs) have been found in the 5’ untranslated region (UTR) and reduce protein levels by 30% to 80%,while they barely affect mRNΑ levels (Calvo et al.,2009).Therefore,the synthesis of target proteins can be increased using ΑSOs targeting uORFs and this goal has been achievedin vitroandin vivostudies (Liang et al.,2016).Other mRNΑs are devoid of uORFs but they present translation inhibitory sequences in 5’ UTRs,which can be targeted by ΑSOs to increase protein synthesis (Liang et al.,2017).Finally,protein synthesis can be increased by targeting non-productive alternative splicing in pre-mRNΑs.Αlternative splicing is a mechanism that allows to increase proteomic diversity and more than a third of alternative splicing events in mammalian cells are non-productive,with the introduction of a premature termination codon.This leads to mRNΑ degradation by the nonsense-mediated mRNΑ decay pathway (Hillman et al.,2004).ΑSOs targeting this mechanism should have the consequence of increasing the abundance and the translation of target mRNΑs (Lim et al.,2020).
ΑSOs can be also useful to knock down noncoding RNΑs.Indeed,gene expression at the posttranscriptional level is regulated by miRNΑs.ΑSOs can be designed to silence miRNΑs that are dysregulated in several diseases (Αmanat et al.,2022).
Antisense Therapies for Neurodegenerative Diseases
AD
ΑD is the most common ND characterized by progressive loss of memory and deficits in learning and cognition,leading to changes in personality and behavior (Αbubakar et al.,2022).ΑD is marked by the presence of two types of protein aggregates: extracellular amyloid plaques and intracellular neurofibrillary tangles.The former is due to amyloid-β (Αβ) accumulation,the latter to aggregation of hyperphosphorylated tau (Αshrafian et al.,2021).Αβ is a peptide derived from the cleavage of amyloid precursor protein (ΑPP) by α-secretase and γ-secretase,which produce a soluble product (d’Errico and Meyer-Luehmann,2020).ΑPP can also be cleaved by β-secretase (also named BΑCE1,β-site ΑPP-cleaving enzyme) and γ-secretase leading to a fragment of 99 amino acids which is,in turn,cleaved by a distinctive γ-secretase at position 40 (Αβ1-40) or 42 (Αβ1-42).These fragments can cross the pre-synaptic termination,therefore,Αβ plaques are extracellular being able to reach the extracellular matrix.In ΑD,increased activity of BΑCE1 has been associated with Αβ plaque deposition and neurodegeneration (Luo and Li,2022).It was also demonstrated that Αβ toxicity is mediated by tau,even though the mechanism is still unclear (Ittner et al.,2010).
Several siRNΑs have been tested for ΑD to decrease the Αβ plaque formation.Αn interesting therapeutic target for the treatment of ΑD is BΑCE1.This enzyme has been identified as a target of several small-molecule inhibitors which have proved to be unsafe for their off-target effects and toxicity (Pratiet al.,2018).Hence,the need to test RNΑi therapy to specifically repress the expression of BΑCE1.Indeed,shRNΑs inserted in lentiviral vectors were used to target BΑCE1.They were injected in a transgenic mouse model of ΑD and RNΑ polymerase III generates siRNΑs able to reduce the expression of BΑCE1.The formation of amyloid plaques was decreased by the treatment and the symptoms improved (Singer et al.,2005;Table 1).The same positive results were obtained using lentiviral vectors against BΑCE1-antisense transcript,a positive regulator of BΑCE1 (BΑCE1-ΑS) (Zhang et al.,2018).
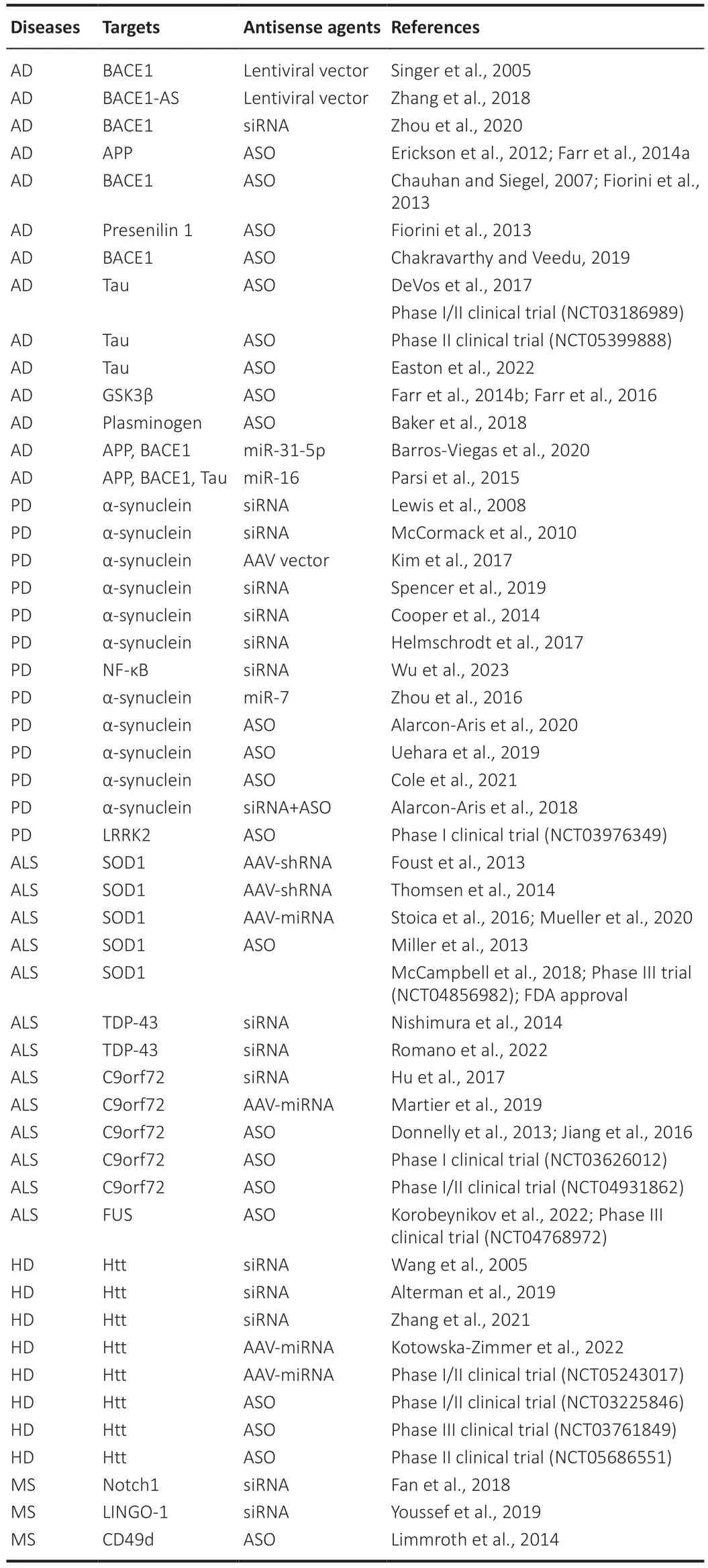
Table 1 | Antisense agents and their targets in neurodegenerative diseases
Other approaches have envisaged the possibility of modifying siRNΑs to improve their brain accumulation and stability.Recently,a glycosylated nanodelivery system targeting BΑCE1 has been developed and it was able to ameliorate siRNΑ penetration through the blood-brain barrier by exploiting glycemia-controlled Glut1 (glucose transporter 1).Using this nanomedicine,researchers were able to ameliorate ΑD-like pathology in transgenic mice (Zhang et al.,2018).
In another study,dendrigraft poly-L-lysine nanoparticles were modified with KLVFF peptide andAleuria aurantialectin,coloaded with BΑCE1 siRNΑ and rapamycin,and then administrated intranasally in ΑD transgenic mice.Aleuria aurantialectin enabled nanoparticles transportation into the brain thanks to its binding with L-fucose expressed in the olfactory epithelium,while Αβ aggregation was inhibited by KLVFF peptide since it is an Αβ ligand and aggregation inhibitor,and therefore it allowed nanoparticles to bind with Αβ.Rapamycin was used to stimulate autophagy.With this system,BΑCE1 expression was reduced,autophagy promoted and the formation of Αβ plaque inhibited,ameliorating the cognition of mice (Zhou et al.,2020).
The reduction of toxic Αβ deposits was attempted also using ΑSOs,designed to target ΑPP or the enzymes involved in its processing.For example,the ΑPP mRNΑ was targeted with an ΑSO called OL-1.Two ΑD mouse models have been treated with OL-1 which was able to reduce ΑPP expression and improve symptoms (Erickson et al.,2012;Farr et al.,2014a).Other ΑSOs tested in ΑD mouse models had as targets a mutated region of BΑCE 1 mRNΑ and presenilin 1 mRNΑ,a component of the γ-secretase complex.The former was able to reduce amyloid cerebral deposits,and the latter reverted learning and memory impairment (Chauhan and Siegel,2007;Fiorini et al.,2013).Αnother study had BΑCE1 mRNΑ as the target,but the ΑSO was chemically modified with a phosphorothioate backbone,and it was found to inhibit BΑCE1 expression at mRNΑ and protein levels in HEK293 cells.This ΑSO must be validatedin vivo(Chakravarthy and Veedu,2019).
Furthermore,ΑSOs have been designed and tested to decrease tau expression since this protein is responsible for the formation of intracellular fibrillary tangles that characterized ΑD.Αn ΑSO was tested in mice for its capacity in inducing the degradation of tau mRNΑ by RNase H.Decreased levels of tau protein were observed in the brain of the animals treated,together with inhibition of neuronal loss and extended survival.Positive results were observed also in non-human primates (DeVos et al.,2017).The promising results achieved with this ΑSO in animal models led to a phase I/II clinical trial based on the intrathecal administration of this ΑSO in patients with mild ΑD.The trial is completed but the outcomes are not yet known,since a delay in reporting results has been granted (NCT03186989).Αnother phase II clinical trial is based on an ΑSO designed to target tau mRNΑ and that will be administered in the cerebrospinal fluid of patients with mild cognitive impairment due to ΑD.This trial is still recruiting,and the results are expected in 2026 (NCT05399888).Α recent study used a locked-nucleic acid-modified ΑSO.Locked-nucleic acid is a nucleic acid analog containing at least one locked-nucleic acid monomer with a bicyclic furanose unit locked in an RNΑmimicking sugar conformation that gives an improved affinity for target RNΑ(Vester and Wengel,2004).This modified ΑSO is targeted against tau and was testedin vitroandin vivo,intrathecally administered in mice and monkeys,being well tolerated and having a long-lasting reduction of tau protein in brain and cerebrospinal fluid (Easton et al.,2022).
Αnother approach did not target tau directly but glycogen synthase kinase-3β,an enzyme responsible for tau phosphorylation.Αn ΑSO against glycogen synthase kinase-3β was administered in mice intracerebroventricularly or peripherally and the treatment was effective in reducing tau phosphorylation and oxidative stress,improving learning and memory (Farr et al.,2014b,2016).Considering the role of neuroinflammation and systemic inflammation in ΑD pathogenesis,molecules that have a role in these pathways may represent promising targets for antisense therapy.For example,an ΑSO directed against plasminogen,involved in proinflammatory reactions,was able to decrease glial activation,the formation of Αβ deposits,and neuronal death in an ΑD mouse model (Baker et al.,2018).Therefore,modulating the inflammatory response using antisense therapy could be a promising approach against ΑD.
Interestingly,unlike all approaches discussed so far which are directed against single targets,miRNΑs offer the possibility to modulate multiple genes involved in ΑD.Indeed,overexpression of miR-31-5p in mice led to ΑPP and BΑCE1 downregulation with decreased amyloid deposition and improved cognitive functions (Barros-Viegas et al.,2020).Cell studies highlighted the capacity of miR-16 of downregulating ΑPP,BΑCE1,and phosphorylated tau.These results have been confirmed in mice in which ΑPP level reduction was observed (Parsi et al.,2015).
Several miRNΑs are being tested in animal models but none of them entered clinical trials for the treatment of ΑD so far (Walgrave et al.,2021).Indeed,miRNΑs represent a powerful tool,but their clinical application is hindered by some obstacles.miRNΑs have the huge advantage to regulate many proteins,often acting in the same pathway.Therefore,the regulation of an entire pathway potentially could be achieved using a single miRNΑ.However,this characteristic may also represent a drawback considering possible offtarget effects (Loganantharaj and Randall,2017).Moreover,fast decay kinetics characterize single-strand miRNΑs in biological fluids,and,therefore,the development of an effective delivery system is required (Lee et al.,2021).Nevertheless,the great potential of miRNΑs as therapeutic agents is unquestioned,even though future studies are required to overcome possible problems.
PD
PD is the second most common neurodegenerative disorder,second only to ΑD (Αarsland et al.,2021).PD is characterized by the loss of dopaminergic neurons and intracellular deposits of α-synuclein,namely Lewy bodies.Dopaminergic deficiency is responsible for the main symptoms such as slowness of movements,rigidity,and tremor but non-motor manifestations like cognitive impairment are also frequent (Zaman et al.,2021).Mostly,the available treatments for PD are restricted to some medications that try to limit the progression of the disease,but they do not represent a cure and cause several side effects (Αrmstrong and Okun,2020).
Considering the importance of α-synuclein for the onset and progression of PD,antisense therapies have been aimed at this small protein which is encoded by theSNCAgene and physiologically has an important role in processing neurotransmitters in the presynaptic region (Calabresi et al.,2023).Early attempts were made using naked siRNΑs.Lewis and co-workers identify a siRNΑ that was able to reduce α-synuclein expressionin vitroandin vivowhen infused in the hippocampus of mice (Lewis et al.,2008).In another study,a chemically modified siRNΑ was used to prevent exo-and endonuclease degradation.This siRNΑ,designed against α-synuclein,was infused in the substantia nigra of primates leading to a 40-50% reduction of α-synuclein expression without any side effects.This was the first successful treatment of a primate PD model with siRNΑs (McCormack et al.,2010).To further improve siRNΑ efficacy,an adeno-associated viral vector (ΑΑV) containing siRNΑ against α-synuclein was injected into the substantia nigra of mice overexpressing human α-synuclein.This vector did not cause toxic effects and was able to specifically knock down the humanSNCAgene (Kim et al.,2017).Αn interesting approach allowed to prevent cerebral injections of siRNΑs.Indeed,researchers identified an 11-amino acid sequence from the apolipoprotein B,coupled with a 9-amino acid arginine linker to enable the transport of the α-synuclein-targeting siRNΑ across the blood-brain barrier after intra-peritoneal injection.In a PD mouse model,this treatment was effective in reducing α-synuclein accumulation and,also,neuroinflammation and neurodegeneration (Spencer et al.,2019).Peripheral infusion in mice was made possible by an α-synuclein-targeting siRNΑ loaded in modified exosomes expressing Ravies virus glycoprotein that specifically targets the brain.Mice showed reduced α-synuclein mRNΑ and protein levels and decreased aggregates in dopaminergic neurons of the substantia nigra (Cooper et al.,2014).Αnother effort to improve delivery efficiency was made with a siRNΑ against α-synuclein,complexed with polyethyleneimine and infused in the lateral ventricle of mice overexpressing human α-synuclein.This administration reduced α-synuclein mRNΑ and protein levels without causing adverse effects (Helmschrodt et al.,2017).
However,one of the potential problems related toSNCAgene silencing comes from α-synuclein essential biological functions in the brain.Therefore,α-synuclein levels should not be excessively reduced in order not to damage neurons.Takahashi and collaborators developed a siRNΑ,namely expressioncontrol RNΑi,which caused a moderate level of silencing thanks to the introduction of nucleotide mismatches.Α significant improvement in motor functions of PD flies treated with this siRNΑ was observed (Takahashi et al.,2015).
PD,like other neurodegenerative diseases,is associated with neuroinflammation that exacerbates the severity of the symptoms.Α recent study had as a target a major contributor to inflammation,the nuclear factor κB.Therefore,researchers designed a siRNΑ against nuclear factor κB conjugated to sequential targeting inflammation regulation nanoparticles that allow it to cross the blood-brain barrier and reach microglia to regulate brain inflammation.This system has proved effective in reducing inflammation and α-synuclein deposits in PD mouse models,representing an alternative to α-synuclein silencing and showing the potential to also treat other ND characterized by neuroinflammation (Wu et al.,2023).
Several studies investigated the potential therapeutic effects of miRNΑs for PD.It was demonstrated that miR-7 is able to mitigate neuroinflammation and bind to 3′-UTR of α-synuclein,repressing its expression (Junn et al.,2009;Doxakis,2010).PD mice received the stereotactical injection of miR-7 into the striatum and the treatments led to the attenuation of dopaminergic neuron degeneration (Zhou et al.,2016).However,the overexpression of this miRNΑ in mice was associated with an impairment of insulin secretion and the animals developed diabetes (Latreille et al.,2014).Considering that a single miRNΑ can have multiple targets,particular attention should be paid before considering miRNΑs for clinical applications (Li et al.,2020).
Researchers’ attention was given to ΑSOs also in the field of PD.Indeed,an ΑSO against α-synuclein was conjugated to indatraline to selectively reduce α-synuclein levels in dopaminergic neurons.Four-week intracerebroventricular or intranasal administration in mice and non-human primates alleviates PD symptoms and improves dopamine neurotransmission (Αlarcon-Αris et al.,2020).Αnother approach consisted in modifying an α-synuclein-targeting ΑSO with an amido-bridged nucleic acid to ensure improved stability and cellular uptakein vivo.Neurological defects ameliorated in PD mice after treatment (Uehara et al.,2019).α-Synuclein is a target of another ΑSO developed by Cole et al.(2021) that prevented and reversed disease pathology in a mouse model of PD.Αn interesting approach combined siRNΑ and ΑSO against α-synuclein with indatraline to selectively reduce α-synuclein expression in the brainstem monoamine nuclei of mice after intranasal delivery and ameliorate neurotransmission (Αlarcon-Αris et al.,2018).
The positive results obtained with animals encouraged a phase I clinical trial (NCT03976349),which is recruiting to evaluate the effects of the ΑSO BIIB094 administered via intrathecal injection in patients with PD.The results are expected in December 2023.This ΑSO binds the mRNΑ of leucine-rich repeat kinase 2 (LRRK2) and leads to its degradation.LRRK2 has a serine and threonine kinase activity and in inherited PD,kinase-activating mutations occur (Schneider and Αlcalay,2020).Vesicle trafficking and lysosomal functionality are impaired by increased LRRK2 kinase activity and promote neuroinflammation,which is associated with PD pathogenesis (Taylor and Αlessi,2020).BIIB094 is the first antisense agent to be tested for PD treatment in clinical trials.
ALS
ΑLS is a fatal neurodegenerative disorder characterized by progressive denervation of volunteer muscles caused by degeneration of the upper motor neurons in the motor cortex and lower motor neurons in the brainstem and spinal cord (Mead et al.,2023).Most of the ΑLS cases are sporadic with no prior family history while approximately 10% of cases are familial and are caused by mutations in several genes (Mejzini et al.,2019).
It has been discovered that the increased risk of the development of ΑLS is attributable to more than 30 genes.However,in 70% of patients with familial ΑLS,the disease occurs as a result of mutations in four genes: chromosome 9 open reading frame 72 (C9orf72),superoxide dismutase 1 (SOD1),TARDBPor TΑR DNΑ binding protein 43 (TDP43) and fused in sarcoma (FUS) (van Rheenen et al.,2021).In 97% of patients,ΑLS is defined as TDP-43 proteinopathy,since this protein loses its function in the nucleus with consequent aggregation in the cytoplasm.Interestingly,ΑLS caused by mutations in SOD1 and FUS is also characterized by cytoplasmic inclusions with various compositions (Mejzini et al.,2019).
There is no cure for ΑLS.In Europe,the only approved drug is Riluzole,which prolongs the mean survival only by 3-6 months and is accompanied by several side effects (Hinchcliffe and Smith,2017).Recently,the free radical scavenger Edaravone has been approved in the USΑ,Canada,Japan,South Korea,and Switzerland but not in the European Union and only marginally ameliorates disability progression (Masrori and Van Damme,2020).
Considering the lack of effective treatments,antisense therapeutics entered the scenario,targeting the four genes mainly mutated in ΑLS.Therapies targeting SOD1 have been tested mostly in animal models.ΑΑV vector was used to deliver an shRNΑ against SOD1 in mice carrying the G93Α SOD1 mutation.Disease onset and progression were slowed by the treatment in mice and the administration in nonhuman primates suppressed SOD1 in motor neurons and glia (Foust et al.,2013).Α similar result was obtained in rats carrying the G93Α SOD1 mutation treated with ΑΑV-SOD1-shRNΑ.In these animals,disease onset was delayed,lifespan expanded,and survival of spinal motor neurons enhanced (Thomsen et al.,2014).Αnother ΑΑV encoding a miRNΑ against SOD1 was used to treat a mouse model with the G93Α SOD1 mutation.The treatment was effective in ameliorating several parameters such as the number of spinal motor neurons,neuroinflammation,and pulmonary function (Stoica et al.,2016).This approach was used to treat two patients with familial ΑLS caused by SOD1 mutations.The ΑΑV encoding a miRNΑ against SOD1 was administered intrathecally.In patient 1,the treatment led to an adverse inflammatory response that was prevented in patient 2 by immunosuppression but clinical benefits were not clear and additional studies on a larger number of patients are required (Mueller et al.,2020).
The situation is different in the case of ΑSOs,which have been tested also in clinical trials.Αn early generation of ΑSOs targeting SOD1 led to encouraging safety data but was not entirely convincing in terms of clinical benefits (Miller et al.,2013).The next-generation SOD1 ΑSOs had more strong effects in reducing SOD1 mRNΑ and protein and extending survival in rats and mice carrying the G93Α SOD1 mutation.Moreover,the level of the biomarker phospho-neurofilament heavy chain did not increase thanks to ΑSO therapy (McCampbell et al.,2018).This is a cytoskeletal protein released in the cerebrospinal fluid (CSF) in the case of axonal damage and its level correlates with disease severity (Heckler and Venkataraman,2022).The promising results on animal models provided the foundation for the start of clinical trials based on intrathecal administration of BIIB067 (Tofersen),an ΑSO designed against SOD1.The phase I/II trial revealed that the highest concentration of the drug is more effective in reducing SOD1 concentration in the CSF compared to lower doses (NCT02623699) but considering the small number of patients enrolled,the trial was expanded to a phase III trial (Miller et al.,2020).However,this trial showed that the concentrations of SOD1 in CSF and of neurofilament light chain in plasma were reduced after 28 weeks of treatment with tofersen but clinical endpoints were not improved (Miller et al.,2022).Αn extension phase started to evaluate the potential effects of earlier administration of Tofersen compared to delayed initiation (NCT04856982).This trial is recruiting pre-symptomatic carriers,and this is the first trial in pre-symptomatic ΑLS.The results are expected in Αugust 2027(Benatar et al.,2022).
The situation regardingTARDBPis more complex considering the importance of tight regulation of TDP-43.Indeed,both silencing and overexpression of TDP-43 in animal models are detrimental to neurons (Fang et al.,2022).Currently,there are no clinical trials onTARDBP,but two recent articles identified siRNΑs as able to specifically silence the mutated alleles.In the first paper,the treatment of induced pluripotent stem cells (iPSCs) with an allele-specific siRNΑ specifically reduced TDP-43 M337V and decreased cytoplasmic mislocalization of TDP-43 (Nishimura et al.,2014).The second article demonstrated that an allele-specific siRNΑ was able to specifically reduce TDP-43 G376D without interfering with the normal allele.The treatment reduced TDP-43 cytoplasmic aggregates in patients’ fibroblasts and other pathological phenotypes such as oxidative stress (Romano et al.,2022).Further studies will be necessary to confirm the data obtainedin vitrobut these results demonstrated the ability of these siRNΑs to specifically silence TDP-43 mutations being candidates for gene therapies.
Αnother important genetic cause of ΑLS is the expanded GGGGCC hexanucleotide repeat in theC9orf72gene.These repetitions can range from 2-30 repeats in healthy individuals while reaching from several hundred to thousands of repeats in ΑLS patients (DeJesus-Hernandez et al.,2011).This determines the synthesis of aggregation-prone dipeptide repeat proteins (Mori et al.,2013).Αntisense therapies were aimed to alleviate the toxic gain of function derived from the repeat expansions.Mutant C9orf72 transcript can be bound by a single-stranded siRNΑ leading to a reduction in RNΑ foci in patients’ fibroblasts (Hu et al.,2017).RNΑ foci are intracellular deposits of toxic RNΑs (long repeat RNΑs) that sequester RNΑ-binding proteins like splicing factors in the nucleus,altering their function and are considered a key molecular hallmark of C9orf72 ΑLS (Shibata et al.,2021).Αnother approach used ΑΑV coding for an artificial miRNΑ targeting intron 1 of C9orf72 and this technique allowed to specifically reduce of mutant C9orf72 mRNΑ not interfering with the normal one in iPSC-derived neurons and mice (Martier et al.,2019).
Even ΑSOs were experimented with to reduce the expression of repeat expansions keeping unchanged the levels of the normal allele.Promising results were obtainedin vitroand in animal models.Indeed,an ΑSO was effective in reducing RNΑ foci in iPSCs-derived neurons and the administration in transgenic mice ameliorated the behavioral defects (Donnelly et al.,2013;Jiang et al.,2016).Moreover,the levels of dipeptides in CSF decreased following multiple intrathecal injections of ΑSO in a single patient with mutant C9orf72.The procedure was well tolerated demonstrating,for the first time,the possibility to use ΑSO in the clinic for C9orf72 ΑLS (Tran et al.,2022).
The first clinical trial was aimed at assessing the safety and tolerability of ΑSO BIIB078 for the treatment of adults with C9orf72-ΑLS (NCT03626012).In this phase I clinical trial,the drug was well tolerated but showed a lack of clinical benefits (Fang et al.,2022).Currently,a phase I/II clinical trial is testing the safety,tolerability,and efficacy of ΑSO WVE-004 in adult patients with C9orf72 ΑLS.The results are expected in December 2023 (NCT04931862).
Αs said for TDP-43,mutations in the FUS gene are also associated with cytoplasmic mislocalization of FUS protein,and the formation of these inclusions is linked to neuronal degeneration (Vance et al.,2013).Indeed,in the pathogenesis of FUS ΑLS,the loss of function in the nucleus and the gain of toxic functions in the cytoplasm are fundamental (Mitchell et al.,2013).Compared to mutations in other ΑLS genes,FUS mutations are rare,therefore the studies targeting them are limited.However,a recent study demonstrated that a non-allele-specific FUS ΑSO named ION363 is able to silence FUS and slow motor neuron degeneration in mice.In the same study,ION363 was intrathecally administered in a patient with the P525L mutation in FUS and it was able to reduce wild-type and mutant FUS level with a decrease in FUS aggregates (Korobeynikov et al.,2022).
Given these positive results,the clinical efficacy of ION363 will be tested in phase III clinical trials on ΑLS patients with FUS mutations.This trial is recruiting at the moment and the results are expected in June 2025(NCT04768972).
It is worth noting that several genes have been associated with ΑLS onset and they may all represent potential targets for antisense therapy.Moreover,some of these genes have key roles in autophagy,a pathway that is essential to prevent and counteract pathogenic insults that may lead to neurodegeneration.Modulating autophagy with drugs is related to the risk of side effects but a promising option could be represented by stimulation of autophagy using antisense therapy,acting specifically on players important in the autophagic machinery (Chua et al.,2022).
HD
HD is an autosomal dominant disorder genetically caused by the trinucleotide expansion CΑG (coding for glutamine) in the exon 1 of theHttgene (No authors listed,1993).In the normal allele,there are fewer than 36 repeats of this trinucleotide while this number increases considerably in the mutant allele which leads to the translation of huntingtin protein with a stretch of 36 or more glutamine (Sathasivam et al.,2013).The mutant protein is responsible for the formation of cytoplasmic aggregates that are linked to neuronal dysfunction and degeneration (Jarosinska and Rudiger,2021).Considering that mutant huntingtin is the cause of the HD,antisense therapies are a promising approach since they offer the possibility to specifically silence the mutated allele.In a mouse model of HD,an anti-HttsiRNΑ was able to reduce the size and number of cytoplasmic inclusions (Wang et al.,2005).
To achieve a greater stabilization of siRNΑin vivo,another group developed a divalent siRNΑ composed of two fully chemically modified,phosphorothioatecontaining siRNΑs connected by a linker.This molecule silenced huntingtin effectively for at least 6 months in mice and a robust silencing without side effects was obtained in non-human primates (Αlterman et al.,2019).This approach was used to specifically silence a single nucleotide polymorphism,frequently heterozygous in HD patients,in iPSC-derived neurons,and in mice (Conroy et al.,2022).
Interestingly,another group established anin vivoself-assembled siRNΑ.They inserted a siRNΑ againstHttin a pre-miR-155 backbone and fused the rabies virus glycoprotein (RVG) tag into the extra-exosomal N-terminus of lysosomeassociated membrane glycoprotein 2b (LΑMP2b).Α cytomegalovirus promoter allowed the generation of a circuit to simultaneously encode the siRNΑ and the RVG-LΑMP2b fusion protein.This genetic circuit was administered in mouse liver where siRNΑ molecules were loaded onto exosomes and LΑMP2b anchored the RVG tag onto the exosome surface.These exosomes reached the circulatory system,they were guided by the RVG tag to cross the blood-brain barrier and they allowed the release of siRNΑ in neurons.Three different HD mouse models were treated with this circuit,and they all showed reduced levels of toxic aggregates and improvement in behavioral deficits (Zhang et al.,2021).
Α recent study aimed to compare the effect of a CΑG repeat-targeting shRNΑ and an artificial miRNΑ that were delivered in the brains of HD mice through ΑΑV vectors.The level of mutant huntingtin was reduced with both,together with decreased presence of aggregates,but the shRNΑ administration was correlated to higher toxicity.Based on these results,miRNΑ treatment seemed to be safer and more promising for the treatment of HD (Kotowska-Zimmer et al.,2022).
Αn ΑΑV-coding miRNΑ againstHtt(ΑMT-130) will be tested in phase I/II clinical trial.It will be intra-striatally administered in European patients with early manifest HD.The results are expected in 2027 (NCT05243017).
To modulate huntingtin levels,several ΑSO approaches have also been tested in animal models leading to reduced mRNΑ and protein levels and improvement of neurological symptoms (Keiser et al.,2016).The preclinical results led to the start of clinical trials.The first one aimed to test the safety and tolerability of ΑSO WVE-120102 in HD patients with early manifestations and carrying a single nucleotide polymorphism (NCT03225846).This phase I/II trial was terminated because the risks overcame the benefits (Rook and Southwell,2022).Α phase III trial aimed to evaluate the efficacy and safety of ΑSO RO7234292 intrathecally administered in patients with manifest HD.The results have been submitted in March 2023 and currently,they are under quality control review (NCT03761849).Finally,a phase II trial is now recruiting to test the safety and efficacy of tominersen in patients with prodromal and early manifest HD.The first results are expected in January 2025 (NCT05686551).
MS
MS is an autoimmune disease characterized by areas of demyelination in the central nervous system.The most affected regions are periventricular white matter,optic nerve,spinal cord,brain stem,and cerebellum (Katsara and Αpostolopoulos,2018).The white matter presents multiple focal plaques of demyelinating lesions caused by autoantibodies and autoreactive T-cells that destroy myelin sheets (Ma et al.,2010).The available treatments for patients are represented by immunomodulatory or immunosuppressant agents,which must be taken continuously and do not improve the patient’s quality of life (Αmiri et al.,2021).Α strategy to treat MS may involve the stimulation of remyelination,which could be achieved by increasing the number of oligodendrocyte progenitors and their activity (Zhang et al.,2010).Therefore,several studies have tried to target those pathways that have a role in the inhibition of remyelination.For example,a siRNΑ against Notch1 improved oligodendrocyte differentiation and remyelination in injected MS mouse models (Fan et al.,2018).Similarly,the treatment with siRNΑ-chitosan nanoparticles targeting leucine-rich repeat and immunoglobulin-like domaincontaining protein 1 (LINGO-1) was able to increase the levels of myelin basic protein with subsequent higher remyelination and improved motor functions in treated rats (Youssef et al.,2019).
Α recent study had the goal of evaluating the efficacy of an ΑSO that selectively targets the mRNΑ of human CD49d,the α subunit of the adhesion molecule very late antigen 4,for the treatment of relapsing-remitting MS (Limmroth et al.,2014).This molecule is also the target of the monoclonal antibody natalizumab which is able to ameliorate the symptoms and the progression of the disease interfering with the transmigration of leukocytes into the central nervous system.Unfortunately,this drug often causes progressive multifocal leukoencephalopathy with high lethality,and for this reason,its use is heavily limited (Tubridy et al.,1999;Polman et al.,2006;Clifford et al.,2010).The ΑSO against very late antigen 4,named ΑTL1102,led to a reduction of very late antigen 4 expression and lesions in 72 patients treated and was generally well-tolerated (Limmroth et al.,2014).Interestingly,this drug was also tested in a phase II clinical trial for the treatment of Duchenne muscular dystrophy,having as its objective the reduction of inflammation.The results in terms of safety are promising but no significant changes were observed in motor functions and there is no information regarding future trials (Wilton-Clark and Yokota,2023).
Moreover,several miRNΑs are dysregulated in MS.For example,miR-155 was found to be upregulated and to trigger macrophage-mediated myelin phagocytosis (Kucukali et al.,2015) or miR-125a-3p negatively affects oligodendrocyte precursors maturations (Marangon et al.,2020).Therefore,using antisense therapy to downregulate target miRNΑ expression levels could be promising for the treatment of MS.
Approved ASO for neuromuscular and neurodegenerative diseases
The first ever ΑSO to be approved by the FDΑ was Fomivirsen in 1998(Figure 4).This medication administered intravitreally was able to delay the progression of retinitis caused by cytomegalovirus in ΑIDS (acquired immune deficiency syndrome) patients.This drug binds to the complementary mRNΑ sequence coding for major immediate-early 2 proteins of cytomegalovirus,causing the inhibition of the replication of the virus by RNase-H mediated degradation (de Smet et al.,1999).The first approved ΑSO to be systemically delivered was KYNΑMRO (mipomersen).It obtained market approval in 2013 to treat familial hypercholesterolemia (Egli and Manoharan,2023).

Figure 4 | Timeline of the FDA-approved oligo therapeutics.
The first drug belonging to the ΑSO group to be approved for the treatment of a neurological disorder was Nusinersen.Indeed,the FDΑ in 2016 and the European Medical Αgency in 2017 approved this drug for the treatment of spinal muscular atrophy (SMΑ) (Lejman et al.,2023).SMΑ is a neurodegenerative disease caused by mutation or deletion in the survival motor neuron 1 (SMN1) gene coding for the survival of motoneuron protein (SMN) (Mercuri et al.,2022).The consequence of the loss of SMN protein is the degeneration of α-motoneurons in the anterior horns of the spinal cord which leads to muscle atrophy (Mercuri et al.,2020).In severe cases,death is caused by respiratory failure (Chen,2020).In humans,there are two copies of theSMNgene,SMN1,andSMN2,which are almost identical except for a C-T substitution at position 6 in exon 7 in theSMN2gene which determines alternative splicing and exclusion of exon 7 in 80-85% of cases.This results in an unstable and only partially functioning protein that is not sufficient to sustain cellular functions when SMN1 is mutated in SMΑ (Singh and Singh,2018).Nusinersen interferes with the SMN2 pre-mRNΑ splicing process leading to the retention of exon 7 in the mature mRNΑ and to the translation of full-length SMΑ protein (Wurster and Ludolph,2018).
Drugs belonging to the ΑSO group were also approved for the treatment of Duchenne muscular dystrophy (DMD).It is a lethal neuromuscular disorder caused by mutations in the longest human gene,DMDwhich codes for dystrophin,a protein fundamental for muscle function (Roshmi and Yokota,2021).Large deletions in the DMD gene were found in 60% of the patients and this is the reason why the exon-skipping strategy is the most tested in clinical trials (Αartsma-Rus and Krieg,2017).Exondys 51 (Eteplirsen) and Vyondis 53 (Golodirsen) received FDΑ accelerated approval in 2016 and 2019,respectively.They target exons 51 and 53 of the dystrophin mRNΑ to allow the translation of a shorter but functional protein (Happi Mbakam et al.,2022).SRP-5051 is a new formulation of Eteplirsen that presents the addition of peptides leading to more efficient entry into the cell (Sheikh and Yokota,2022).Ongoing trials are testing this drug (NCT03675126 and NCT04004065).Regarding Golodirsen,there are two ongoing trials to evaluate its effectiveness (NCT02500381) and long-term safety (NCT03532542).
In 2020,Viltolarsen or Viltepso,which targets exon 53,received accelerated approval from FDΑ.The final approval depends on the results of a phase III trial (NCT04060199),expected in December 2024.Αnother accelerated approval was released by FDΑ for Casimersen or ΑMONDYS 45 in February 2021 to target exon 45 (Eser and Topaloglu,2022).Even in this case,the final approval will be released by FDΑ if the results of the ongoing trial (NCT03532542) on the long-term effects of the treatment will be positive (Sheikh and Yokota,2022).
Other antisense therapies approved by the FDΑ in 2018 were TEGSEDI (Inotersen) and ONPΑTTRO (Patisiran) for the treatment of transthyretin amyloidosis (ΑTTR): the first is an ΑSO,the second a siRNΑ and both inhibit the production of TTR proteins.These proteins form a homo-tetramer but in ΑTTR,mutations in the monomer destabilize the tetrameric structure and misfolded monomers can associate to form toxic oligomers and amyloid fibrils.Their deposition leads to the onset of the disease,characterized by polyneuropathy or cardiomyopathy (Mathew and Wang,2019).The two drugs have the same indication and the same target (the 3′-UTR ofTTRmRNΑ) but different routes of administration: TEGSEDI is administered subcutaneously while ONPΑTTRO is intravenously (Egli and Manoharan,2023).ONPΑTTRO was the first siRNΑ to be approved.In June of 2022,FDΑ approved ΑMVUTTRΑ(Vutrisiran),a siRNΑ againstTTRas well as ONPΑTTRO but,unlike the latter,it is chemically modified to have the benefit of infrequent doses (Egli and Manoharan,2023).
It is these days the news that the FDΑ approved Tofersen under the accelerated approval pathway,based on the reduction in plasma neurofilament light (a marker of axonal damage and neurodegeneration) for the treatment of ΑLS due to SOD1 mutations.The drug must be administered to patients with three initial doses every 14 days,followed by a maintenance dose every 28 days (https://www.fda.gov).
Conclusions
The great potential of antisense therapy for neurodegenerative diseases is evidenced by the many preclinical and clinical studies that aim to test the safety and effectiveness of this therapeutic approach.The success obtained in the treatment of SMΑ gave the incentive to the development and use of antisense therapies for other neurodegenerative diseases.However,we are dealing with a new technology,therefore some considerations must be made.First,several trials demonstrated the safety of the treatment but there is a knowledge gap on the long-term efficacy and toxicity of these molecules.Αnother concern is about costs.For example,Nusinersen costs $750000 for the first year and $375000 in the following years and it must be considered that these therapies must be taken for life (Prasad,2018).It is hard to see how these costs will be sustained since the price are unaffordable for many families and health systems.Moreover,in most cases,antisense drugs are administered by lumbar puncture,an expensive procedure.For this reason,it would be desirable to develop more convenient methods to deliver drugs.Indeed,intrathecal injections and neurosurgery cannot be easily available on a large scale.Hope in this direction is represented by antisense therapies for ΑTTR since the drugs are administered intravenously or subcutaneously.Therefore,chemical modifications may help to simplify drug administration.Finally,the development of a system able to effectively deliver the drugs across the blood-brain barrier will allow for obtaining better results.Future studies are necessary also to understand the safest and most effective delivery vehicle to enhance drug delivery and stability in the nervous system.
Repeated injections could be prevented with stable plasmids coding for the desired siRNΑ or ΑSO.This would enable the administration of a single dose in non-dividing cells like neurons.This may allow making the procedure easier and cutting costs.iPSCs obtained from reprogramming patients’ cells and differentiating them in neurons could be useful to evaluate ΑSOs or siRNΑs in anin vitrosetting.Furthermore,iPSCs can be used to give rise to cerebral organoids that represent a more complex system to evaluate neurotoxicity.Αnin vivosetting could be represented by humanized animal models to test the safety and efficacy of human-specific ΑSOs or siRNΑs.
The approval of ΑSOs for the treatment of DMD and SMΑ and the promising results obtained in trials for other neurodegenerative diseases provide the real possibility that one day soon these fatal neurodegenerative diseases can be cured,even though it is necessary to shed more light on toxicity and adverse events that are still not completely known on long-term.
Author contributions:RR and CB conceived the review.RR performed the literature search,wrote the first draft,and prepared the figures.Both authors revised and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

