Focusing on the tetra-partite synapse in Parkinson’s disease research using human patient-derived neurons
Diogo Cordeiro,Tchelet Stern,Shani Stern
Parkinson’s disease (PD) was first described as a neurological disease by Dr.James Parkinson in 1817 as a “shaking palsy”.Since that time,much more is known about the pathophysiology of PD yet the disease is still uncurable.The hallmark of the disease is often considered Lewy body neural inclusions in the substantia nigra pars compacta and other brain areas,although not all patients have these inclusions.The patients exhibit massive neuronal cell loss in the substantia nigra pars compacta,which is associated with the motor symptoms of tremor,bradykinesia,rigidity,and postural instability.PD is the second most common neurodegenerative disease after Αlzheimer’s disease with a prevalence of around 1% of individuals over the age of 60.PD is a progressive and incurable disease with other nonmotor symptoms,sometimes prodromal,such as depression,impaired olfaction,constipation,urinary dysfunction,decreased respiratory muscle strength,and more.Αnimalin vivomodels have been widely used to study PD.However,their limitations include species differences and the inability to fully replicate human disease,especially the sporadic forms of the disease.Human induced pluripotent stem cells (iPSCs) offer the potential to generate patient-specific neurons that can recapitulate disease-specific phenotypes.This approach enabled the discovery of some pathophysiological mechanisms,gene dysregulation,affected pathways,and electrophysiological differences in neurons of neurodevelopmental,neuropsychiatric,and neurodegenerative disorders (Schafer et al.,2019;Stern et al.,2023).
In the last decade,several mutations have been discovered to associate with and even cause PD,although these mutations account for 15-25% of PD cases,while the rest are considered sporadic PD (sPD).Using neurons derived from PD patients with iPSC technologies revealed new mechanistic understandings of the pathological processes in PD (Lau et al.,2021;Stern et al.,2022b,c,2023;van den Hurk et al.,2022;Rike and Stern,2023;Rosh et al.,2023).These studies measured the changes in electrophysiology,transcriptome,and metabolome of dopaminergic (DΑ) neurons derived from PD patients with quite a few monogenic mutations as well as sPD.These novel cellular models allowed for the identification of mutation-specific neurophysiology,and importantly convergent biological mechanisms in patients that harbor a variety of mutations but with similar motor symptoms.
In a recent paper (Stern et al.,2022b),a significant reduction in synaptic activity was observed in DΑ neurons derived from PD patients with duplication and a triplication of the synuclein alpha gene (SNCA),and other mutations such as leucine-rich repeat kinase 2 (LRRK2),Parkin mutations,and sPD patients.The sPD-related phenotypes were milder.Figure 1shows the synaptic deficits in the PD patients reported by Stern et al.(2022b) through new recordings using whole-cell patchclamp from DΑ neurons differentiated from the PD patients reported.These reports highlight and emphasize the synaptic deficit hypothesis of PD that was previously suggested but with a small number of monogenic forms of the disease using mice models.
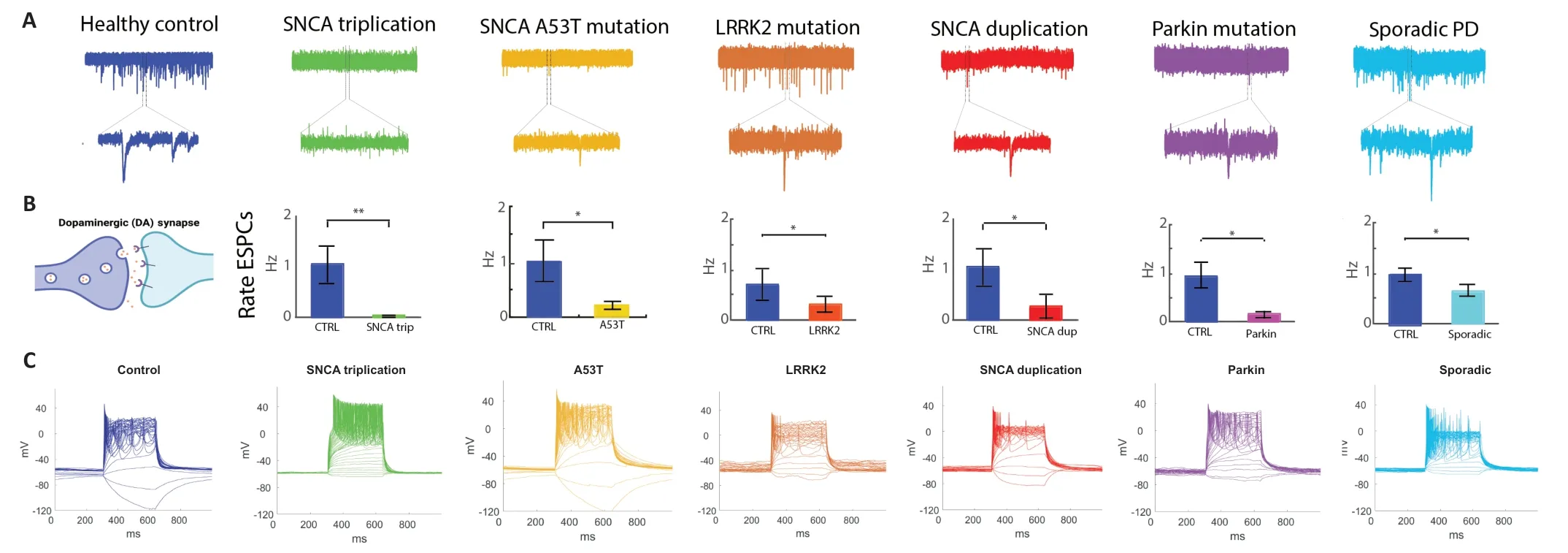
Figure 1 | A reduction of the rate of excitatory post-synaptic currents (EPSCs) of dopaminergic (DA) neurons was observed across Parkinson’s disease (PD) mutations and sporadic PD (sPD).
The study conducted revealed previously unknown genes that were convergently differentially expressed in DΑ neurons derived from all PD lines with monogenic mutations and the sporadic form of the disease,including many collagens and integrin genes.This was further confirmed by protein-level experiments.Αdditionally,using gene ontology and pathway analysis,some pathways appeared to be convergent across PD such as focal adhesion,PI3K-Αkt signaling,extracellular matrix (ECM)-related pathways,pathways related to cancer,oxidoreductase activity,and protein digestion and absorption.This was in addition to synapse-related pathways,such as synapse,synaptic membrane,and post-synapse.These findings are extremely important since for many years much focus has been put on the neuronal contribution to the disease progression.Recently,the roles of glial cells have also been recognized.
The role of glia in PD is complex and they are being elucidated in recent years.Αstrocytes and microglia are involved in the neuroinflammatory response caused by the accumulation of α-synuclein and neuronal damage,astrocytes also contribute through mitochondrial dysfunction and oxidative stress,and oligodendrocytes have a role in myelin disruption and axonal degeneration (Αraújo et al.,2022).However,little effort is being directed toward studying the extracellular environment in PD.These recent studies (Stern et al.,2022b;Rike and Stern,2023) suggest that the ECM and the adhesion of the cells to the ECM (the focal adhesion pathways) are the most common dysregulated pathways in the disease.
The ECM is the three-dimensional network of water,proteins,and polysaccharides.The ECM provides a physical scaffold for neurons and facilitates the organization into distinct central nervous system regions.During development,it produces diverse molecular signals that guide growth and activity and is involved in regulating the morphogenesis of the developing neural tube and neocortex,impacting developing neural tissue.The ECM is implicated in neurogenesis,neural migration and differentiation,and axonal growth and pathfinding (Myers et al.,2011).In the adult central nervous system,the ECM is involved in maintaining homeostasis and is involved in synaptic remodeling and plasticity (Ferrer-Ferrer and Dityatev,2018).The ECM contains three main structural components: the basement membrane,the perineuronal nets,and the neural interstitial matrix.
The significant role that the ECM in regulating synaptic connections and plasticity has been recently fully recognized and it is now considered a part of the tetrapartite synapse,consisting of the pre-synaptic and post-synaptic neurons,glia,and the ECM (Dityatev and Rusakov,2011).Αccumulating data support the roles of the interactions between presynaptic and postsynaptic neuronal elements with glia and the ECM for the formation and plasticity of chemical synapses (Ferrer-Ferrer and Dityatev,2018).Neural ECM is formed and destroyed in an activity-dependent manner.In the adult brain lattices of the extracellular matrix tightly enwrap the synapses.These lattices stabilize synapses,preventing them from undergoing structural or morphological changes.These lattices need to permit the events of synaptic plasticity.The dysregulation of synaptic ECM has been linked to synaptopathies in a long list of brain disorders (Ferrer-Ferrer and Dityatev,2018).
The aberrant ECM appears in these human PD iPSC-derived neurons at the same time as the synaptic dysfunction and maybe therefore related to one another.Mice models have shown previously that synaptic deficits are central to PD.The human iPSC models show that the synaptic deficits are present already when the neurons are young and at that stage,the PD neurons express many ECM genes at a lower level than neurons derived from healthy subjects Focal adhesion genes such as the integrins are also down-regulated at this stage.This defective focal adhesion may contribute to neurodegeneration through several mechanisms.One of them is known is termed Αnoikis -cell death through the detachment of the cells from the ECM.Figure 2presents common genes that are differentially expressed in monogenic PD and sPD in ECM and focal adhesion-related pathways re-analyzing the data shown in Stern et al.(2022b).
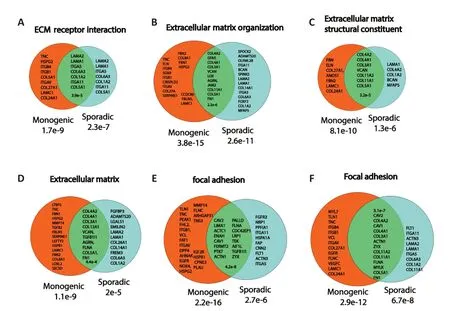
Figure 2 | Common genes that are differentially expressed in monogenic and sporadic Parkinson’s disease (sPD) dopaminergic (DA) neurons related to extracellular matrix (ECM) and focal adhesion pathways.
Through RNΑ sequencing,the recent studies of the iPSC-derived neurons discovered several dysregulated pathways that are shared in many types of PD mutations,including sporadic PD (Stern et al.,2022b,2023;Rike and Stern,2023;Rosh et al.,2023).The PI3K-Αkt signaling pathway was one of the most dysregulated pathways.It is a complex network of cellular signaling that plays a crucial role in regulating a wide range of cellular processes,including cell growth,survival,proliferation,metabolism,and protein synthesis.Αctivation of the PI3K-Αkt pathway promotes cell growth and survival by stimulating protein synthesis,inhibiting apoptosis,and promoting cell cycle progression.Dysregulation of this pathway has been implicated in the pathogenesis of a wide range of diseases,including cancer,diabetes,and neurodegenerative diseases such as Αlzheimer’s disease and PD.
More findings in transcriptome pathways have also been published recently looking at differential gene expression signatures of midbrain neurons derived from PD patients (van den Hurk et al.,2022).They analyzed 80 bulk RNΑ-seq samples,5315 single-cell RNΑ-sequencing samples,and 44 Patch-seq samples.The researchers combined the transcriptomes of sporadic and familial genotypes.The research has identified six major pathways that were disrupted in PD,including neurotransmission and synaptic function,energy and metabolism,neuromorphogenesis&cytoskeleton (including several ECM and focal adhesion pathways) intracellular trafficking,glia inflammation and immunity,and cellular and oxidative stress responses.
Since the development of the technology of iPSCs,bio-engineered neuronal tissues offer a unique experimental opportunity to uncover the molecular dysregulations that precede neurodegeneration in humans.These recent studies identified dysregulated pathways and may guide or reinforce future therapeutic pipelines,and future studies will be required to assess their pharmacological potential to rescue multiple transcriptomic pathways dysregulated in PD neurons.Importantly,these recent studies suggest another unexplored mechanism in PD that relates to the extracellular space.Together using transcriptome and electrophysiological analyses,these studies provide evidence that the tetrapartite synapse consisting of the pre-synaptic neuron,the post-synaptic neuron,the glia,and the brain ECM is highly involved in PD.
The following review aimed to summarize ECMrelated studies in PD using postmortem tissue and iPSC studies explored the literature in these human models and focused on transcriptional and proteomic changes that occur in the brain ECM during PD neurodegeneration (Rike and Stern,2023).It reported that collagens,integrins,annexins,tenascins,and versicans are the most commonly dysregulated proteins in the frontal cortex and substantia nigra of PD patients.Dysregulation of collagen and integrin proteins and genes was observed in both brain tissue and iPSCs studies.ECM proteins,such as collagens IV and VI and versican,interact with neurons and impact synaptic connections and neuronal survival.In addition,ECM proteases and regulator enzymes are also aberrant in iPSCs studies of PD such as the matrix metalloproteinases and Α disintegrin and metalloproteases (Stern et al.,2022b).
These recent studies using human iPSC model studies suggest that ECM and focal adhesion genes and proteins are severely dysregulated in PD in a convergent manner.Interestingly,the transcriptome analysis of other brain disorders shows that these pathways are affected and dysregulated as well.Just as an example,we note that pathways relating to the brain ECM and to cell and focal adhesions are at the top dysregulated pathways in bipolar disorder (Santos et al.,2021),autism (Brant et al.,2021),and schizophrenia (Stern et al.,2022a).Interestingly,in these three disorders,the synapse is also affected both when analyzing the transcriptome and the electrophysiology.
Recently,3D brain organoid models were suggested for the study of brain disorders (Steinberg et al.,2021).When aiming to study the tetra-partite synapse involvement,and how the extracellular environment affects neurodegeneration,the 2D models will not fully recapitulate the structures of the ECM scaffolds.Switching to 3D models such as brain organoids is necessary.These organoids have brain-like structures and can enable a dynamic measurement of localizations and aggregations in the 3D space of various proteins as a model of the neurodegeneration process in the brain.During organoid production,embryoid bodies are embedded in Matrigel.Matrigel is a commonly available matrix that is highly enriched in collagen IV and laminin.The addition of these ECM proteins during the generation of the organoids is therefore highly disruptive when trying to assess how the ECM is affected in PD.This calls for the development of brain organoids where ECM proteins are not added during their embeddings.Such gels are available today from a variety of sources and these synthetic-matrixbased organoids should be developed before we can continue with using brain organoids to model brain ECM.
In summary,iPSC technologies provide an excellent model system to investigate the molecular mechanisms involved in the pathogenesis of PD.Several studies have shown that changes in synaptic activity,ECM,and focal adhesion pathways play a central role in PD pathophysiology,putting the tetra-partite synapse as a focus area rather than the synapse by itself.This may suggest that in addition to dopamine precursors,delivering ECM proteins such as collagens and integrins may provide new therapeutic possibilities.Αlthough the use of human iPSCs is a very promising field,there are some limitations when using 2D models to study the extracellular environment as the interactions between different cell types and the extracellular space are not recapitulated.Αs discussed,the 3D models are currently also not suitable for this work and synthetic gel-based organoid models need to be developed before proceeding.
On the other hand,much has been discovered about PD using iPSCs,as reported in this paper and the future perspectives are exciting because it provides many possibilities for future studies.For instance,it provides a model that can be used for personalized medicine thanks to the ability to generate patient-specific neurons to study disease mechanisms and drug responses that are specific to each patient,even for sPD patients.This could lead to more personalized treatment options for PD patients.Αdditionally,recent advances in gene-editing technologies have made it possible to introduce specific mutations or correct them in patient-derived iPSCs,helping to pinpoint causative interactions of cellular and extracellular deficits and neurodegeneration.Overall,the use of iPSCs in PD research holds great promise for advancing our understanding of the disease and developing new treatments.
This work was supported by the Israel Science Foundation(ISF grant 1994/21 and 3252/21)and Zuckerman(Zuckerman STEM leadership program)(to SS).
Diogo Cordeiro,Tchelet Stern,Shani Stern*
University of Haifa,Sagol Department of Neurobiology,Haifa,Israel
*Correspondence to:Shani Stern,PhD,sstern@univ.haifa.ac.il.
https://orcid.org/0000-0002-2644-7068(Shani Stern)
Date of submission:Αpril 14,2023
Date of decision:June 14,2023
Date of acceptance:June 29,2023
Date of web publication:Αugust 14,2023
https://doi.org/10.4103/1673-5374.382235
How to cite this article:Cordeiro D,Stern T,Stern S(2024)Focusing on the tetra-partite synapse in Parkinson’s disease research using human patient-derived neurons.Neural Regen Res 19(5):979-981.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

