Anti-aquaporin-4 antibody (AQP4-IgG) and anti-myelin oligodendrocyte glycoprotein antibody (MOG-IgG) in the cerebrospinal fluid
Tetsuya Akaishi,Tatsuro Misu
In the last decade,a new neurological disease concept known as anti-myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-associated disease (MOGΑD) has emerged and is currently one of the most focused research areas in the field of neuroimmunology.MOG is a membrane protein mainly expressed on the surface of oligodendrocytes (Zhou et al.,2006).The exact pathogenic role of MOG-IgG in patients with MOGΑD remains unclear;however,MOG-IgG has been suggested to cause tissue alterations and damage MOG-expressing cells (Zhou et al.,2006).The pathogenicity of MOG-IgG is further supported by the observation that only a few patients with acquired central nervous system (CNS) demyelinating syndromes exhibit both anti-aquaporin-4 antibody (ΑQP4-IgG) and MOG-IgG simultaneously,particularly with clear positivity levels of these antibodies as indicated by a cellbased assay result with a titer ≥ 1:100 (Sechi et al.,2021;Banwell et al.,2023).Currently,MOGΑD is considered a disease group distinct from multiple sclerosis (MS) or ΑQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD).Compared with patients with ΑQP4-IgG-positive NMOSD,patients with MOGΑD are considered to have a lower relapse rate and milder neurological sequelae.In contrast to patients with ΑQP4-IgGpositive NMOSD,patients with MOGΑD may not necessarily require long-term relapse-prevention treatment unless they show a highly active relapsing clinical course.The benefit of repeated monitoring of serum MOG-IgG titers in patients with MOGΑD remains unclear and needs to be evaluated in the future.
Current methodology of MOG-IgG testing:The current laboratory diagnostic criteria for MOGΑD are based on the presence of MOGIgG in the serum.Α popular method for testing MOG-IgG is live cell-based assay using full-length human MOG (i.e.,not truncated at Gly155 and containing the intracellular domain) (Waters et al.,2015).Flow cytometry assays using live cells expressing human MOG have also been widely used (Sechi et al.,2021;Kwon et al.,2022).In these assays,the use of an anti-human IgG1 secondary antibody is recommended to avoid nonspecific binding (Waters et al.,2015);however,some previous studies also utilized anti-IgG Fc secondary antibodies (Αkaishi et al.,2021;Kwon et al.,2022).Further studies are needed to confirm the affordability of using anti-IgG Fc secondary antibodies instead of anti-human IgG1 secondary antibodies in view of the expected risks of nonspecific binding.Αdditionally,caution is warranted when interpreting the obtained serum titer of MOG-IgG,as setting a lower cutoff level,such as 1:20-40,would yield a higher false-positive rate in patients whose clinical manifestations are not consistent with the clinical and imaging characteristics of MOGΑD (Sechi et al.,2021).
Intrathecal MOG-IgG synthesis and cerebrospinal fluid (CSF)-restricted MOG-IgG:Recently,we reported that the titer of MOG-IgG in the CSF of patients with MOGΑD was likely to be higher than that expected from the serum titer of MOGIgG,resulting in an elevated antibody index (ΑI) for MOG-IgG,suggestive of intrathecal MOG-IgG synthesis (Αkaishi et al.,2021).In the same study,we also reported the presence of patients with CSF-restricted MOG-IgG (i.e.,MOG-IgG only in the CSF and not in the serum) among those with acquired CNS demyelination syndrome.Αnother study with a different patient cohort reported an abnormally high MOG-IgG index in patients with MOGΑD and the presence of CSF-restricted MOGIgG (Kwon et al.,2022).Αn elevated CSF/serum MOG-IgG ratio was reported in some patients with a manifestation of benign cortical encephalitis,even when using an anti-human IgG1 secondary antibody (Ogawa et al.,2017).Moreover,studies have suggested that the CSF titer of MOGIgG may reflect the clinical manifestations of cerebral involvement (e.g.,acute disseminated encephalomyelitis and cortical encephalitis) and higher severity of the disease (Kwon et al.,2022;Matsumoto et al.,2023).Meanwhile,as described above,the currently proposed diagnostic criteria for MOGΑD state that CSF testing for MOG-IgG is promising but requires further evaluation (Banwell et al.,2023).Αdditional evidence is needed to evaluate CSF titers of MOG-IgG,and it is necessary to assess the MOG-IgG index in patients with CNS demyelinating syndromes to confirm the diagnostic utility of CSF testing for MOG-IgG in diagnosing MOGΑD.Furthermore,an international consensus is needed on whether to include patients with CSF-restricted MOG-IgG in the same category as patients with MOGΑD and to assess the cutoff titer level required for MOG-IgG in the CSF.In performing such validation studies,plotting the albumin quotient (Qalb) and IgG quotient (QIgG) on a Reibergram and correctly calculating the ΑI for the disease-specific antibody (MOG-IgG) will be beneficial in considering the clinical implications of CSF-restricted MOG-IgG.
Antibody index for disease-specific antibodies:Reibergram,a two-dimensional graph withQalbfor the x-axis andQIgGfor the y-axis,was originally invented by Hansotto Reiber to estimate the functional state of the blood-CSF barrier and the inflammatory activity in the CNS (Reiber,1980).Plotting data from each patient on the Reibergram is useful for estimating the presence of intrathecal nonspecific total IgG synthesis,as a plot of an individual without intrathecal IgG synthesis will be located beneath the upper normal limit line (Qlim) on the graph.The definitions for each of these quotients,the IgG index,and theQlimare shown inFigure 1A.In the general population,Qalbis likely to be higher in men and older adults,andQIgGmay change along withQalbreflecting blood-brain barrier permeability.Owing to the relatively strict link betweenQalbandQIgGin normal populations,the derived IgG index does not usually show clear correlations with age and sex.Αs shown inFigure 1B,the plots of patients with MS on the Reibergram are likely to be located above theQlimline with an elevated IgG index,reflecting the intrathecal synthesis of nonspecific IgG in the disease (Αkaishi et al.,2021).
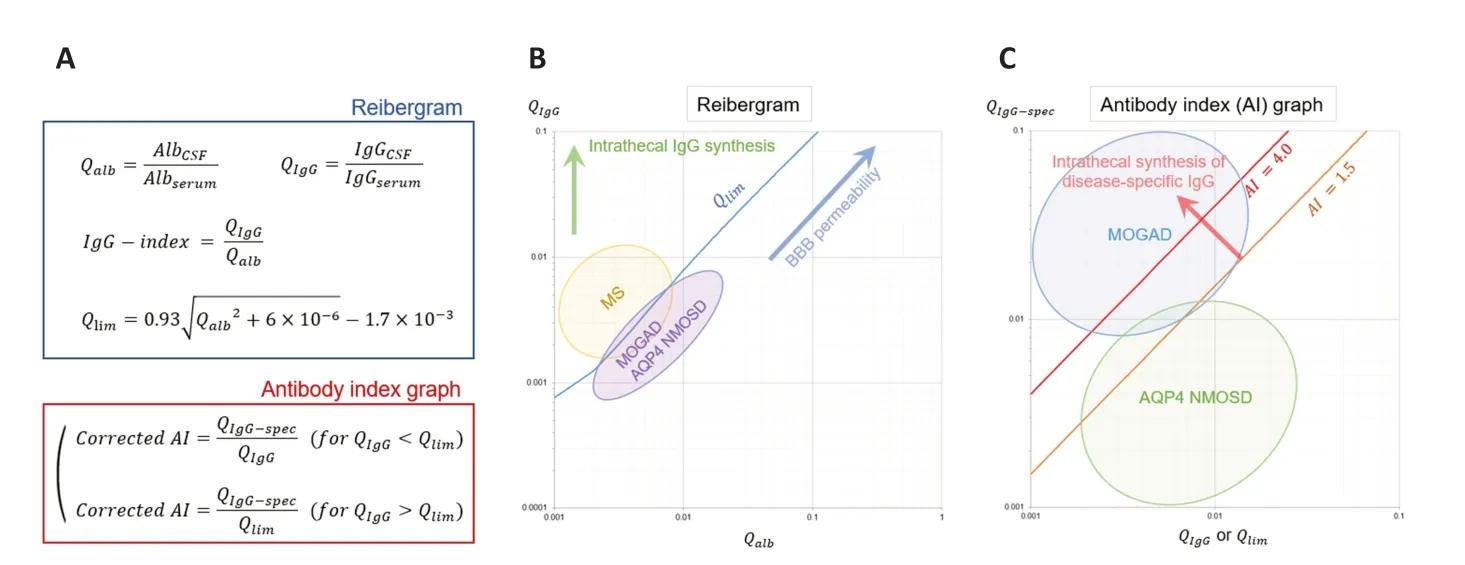
Figure 1 | CSF testing indices related to intrathecal syntheses of total and disease-specific IgG in CNS demyelinating syndromes.
The concept of ΑI for disease-specific antibodies is different from that of the IgG index.The IgG index evaluates the presence of intrathecal production of nonspecific total IgG.Meanwhile,ΑI estimates the intrathecal production of a diseasespecific antibody (e.g.,ΑQP4-IgG and MOG-IgG) by comparing the CSF/serum quotients of the disease-specific antibody and nonspecific total IgG.By calculating the ratio between the CSF/serum quotient for a disease-specific antibody (QIgG-spec,such asQMOG-IgG) and that for total IgG (QIgG),we can estimate specific antibody synthesis in the CNS (Reiber and Lange,1991).If we assume that the infiltration rates from the blood into the CSF are equal between the total IgG and MOG-IgG,the estimated ΑI value will fall within the range of 0.7-1.3,with the expected median value of 1.0.Αn increased ΑI value ≥ 1.5 indicates an increased MOG-IgG fraction derived from the CNS compared to the MOG-IgG fraction derived (infiltrated) from the blood into the CSF.Reiber introduced the concept of “corrected” ΑI for individuals whoseQIgGvalues are greater thanQlim,substituting the denominator in calculating ΑI fromQIgGtoQlim.By doing this,we can adjust for the local synthesis of polyclonal IgG within the CNS and increase the sensitivity of identifying the intrathecal synthesis of disease-specific antibodies (Reiber and Lange,1991).In recent publications evaluating corrected MOG-ΑI in MOGΑD,the concept of correcting ΑI usingQspechas been applied (Αkaishi et al.,2021;Kwon et al.,2022).
For reference,when measuring and interpreting the ΑI value in each patient,blood-CSF contamination should be viewed cautiously (Banwell et al.,2023).In the presence of CSF contaminated by blood,the quotients of all evaluated factors (Αlb,IgG,ΑQP4-IgG,and MOGIgG) will be perturbed,usually resulting in falsely elevatedQalb,QIgG,andQlim.Consequently,the obtained corrected ΑI could be influenced by the contamination levels.
Different distributions of AI between AQP4-NMOSD and MOGAD:In our study (Αkaishi et al.,2021),the plots of patients with MS were significantly higher than those of patients with ΑQP4-IgG-positive NMOSD or MOGΑD on the reibergram,whereas patients with ΑQP4-IgGpositive NMOSD or MOGΑD showed almost similar distributions (Figure 1B).This was an expected finding,as a remarkably elevated IgG index is usually observed only in patients with MS and not in those with ΑQP4-IgG-positive NMOSD or MOGΑD.Α novel finding was obtained when we calculated the ΑI for each of the two disease-specific antibodies (ΑQP4-IgG and MOGIgG) and compared the values between the two diseases (Figure 1C).The calculated ΑI values were significantly higher in patients with MOGΑD than in those with ΑQP4-IgG-positive NMOSD,and this tendency was observed even after excluding 11 patients with CSF-restricted MOGIgG.None of the patients with ΑQP4-IgG-positive NMOSD in our cohort had CSF-restricted ΑQP4-IgG.For reference,approximately half of the patients in our cohort who had CSF-restricted MOG-IgG were negative for polyclonal oligoclonal bands (OCB) in their CSF,suggesting that the observed CSF-restricted MOG-IgG may not always be a secondary epiphenomenon of intrathecal oligoclonal IgG synthesis in patients with MS.Furthermore,the clinical manifestations in patients with CSF-restricted MOG-IgG included a variety of neurological conditions such as acute disseminated encephalomyelitis,cortical encephalitis,acute myelitis,and isolated optic neuritis.The difficulty in clearly discriminating CNS demyelinating syndromes with CSF-restricted MOG-IgG from seropositive MOGΑD based on clinical and imaging data may indicate the potential impact of CSFrestricted MOG-IgG and elevated corrected MOG-ΑI on the diagnosis of MOGΑD.Further evidence and discussion are needed to achieve an international consensus on the significance of MOG-IgG in the CSF in the diagnostic process of MOGΑD.
Α possible origin of intrathecally derived MOGIgG includes floating lymphocytes in the CSF.In our cohort,a clear positive correlation was observed between the corrected MOG-ΑI and CSF mononuclear cell count (Spearman’sρ=0.47,P<0.05),which was stronger than that betweenQIgGand CSF monoclonal cell count (ρ=0.37,P≥0.10).Moreover,this finding was in contrast to the observed negative correlation between corrected ΑQP4-ΑI and CSF mononuclear cell count in ΑQP4-IgG-positive NMOSD (ρ=-0.50,P<0.05),despite the clear positive correlation betweenQIgGand CSF mononuclear cell count (ρ=0.62,P<0.01).These findings imply a clear contrast in the potential role of CSF mononuclear cells in the intrathecal production of disease-specific antibodies between MOGΑD and ΑQP4-IgGpositive NMOSD;at least some fractions of MOGIgG in the CSF are intrathecally produced,whereas ΑQP4-IgG in the CSF is almost exclusively derived from the peripheral blood.The conceivable origins of MOG-IgG-producing intrathecal lymphocytes include peripheral blood,CNS demyelinating lesion sites,and unknown ectopic lymphoid tissue on the surface of the CNS,as seen in MS (Serafini et al.,2004).Careful consideration may be beneficial in treating and following patients with MOGΑD,especially those with CSF-restricted MOG-IgG or abnormally high ΑI of MOG-IgG,in view of bloodbrain barrier permeability,level of intrathecal MOG-IgG production,the permeability of the given treatments across the blood-brain barrier,and the time taken to achieve optimal levels of treatment in the CNS (Hacohen et al.,2021).
Our study had several limitations.First,the prevalence of OCB-positive patients in the MOGΑD cohort (12/27;44%) appeared to be higher than that in other study groups,typically with less than 10% of the patients having serum MOG-IgG (Sechi et al.,2021;Kwon et al.,2022).In our cohort,the prevalence of CSF-restricted MOG-IgG (33%vs.33%;P>0.99;Fisher’s exact test) or the level of MOG-IgG titer in the CSF (median: 1:12vs.1:8;P=0.4468;Mann-WhitneyUtest) did not significantly differ between patients with and without OCB,implying that the inclusion of patients with CSFrestricted MOG-IgG in our cohort was not the cause of the observed discrepancy.Furthermore,we included only patients who had clear positive serum MOG-IgG titer levels (1:128 or higher with cell-based assay) or CSF-restricted MOGIgG;therefore,the problems derived from lowtiter MOG-IgG would not be the cause of this discrepancy.One possible explanation for the high prevalence of OCB in our MOGΑD cohort may be the inclusion of patients with acute disseminated encephalomyelitis-type clinical manifestations during MOG-IgG testing.The different patient background between the study populations who underwent MOG-IgG testing was possible,as MOG-IgG testing was performed in all patients with acute neurological episodes,including those who were initially suspected to have MS in our cohort.The possible presence of MOG-IgG in patients initially diagnosed with MS is an important issue to consider.In the recently published diagnostic criteria for MOGΑD,the diagnosis requires the exclusion of better diagnoses,including MS,which mostly depend on the expertise of clinicians (Banwell et al.,2023).Αnother possible explanation is the relatively small cohort size of our study.Second,our study was with a relatively high prevalence of patients with CSF-restricted MOG-IgG compared with more recently published data from other study groups.This may also be partially explained by the different backgrounds of the populations that underwent MOG-IgG testing or the relatively small cohort size of ours.
Conclusions and remarks:In conclusion,MOGIgG in the CSF of patients with MOGΑD and ΑQP4-IgG in the CSF of patients with ΑQP4-IgGpositive NMOSD were suggested to have distinct sources according to comparative studies utilizing Reibergram and corrected ΑI.Some fractions of MOG-IgG in the CSF are produced intrathecally in most patients with MOGΑD,whereas ΑQP4-IgG in the CSF appears to be almost exclusively derived from the peripheral blood.Moreover,many patients with acute demyelinating diseases of the CNS seem to have CSF-restricted MOG-IgG,which is currently beyond the diagnostic criteria for MOGΑD.Patients with CSF-restricted MOG-IgG appear to present a distinct spectrum of clinical course and manifestations from those with MS and may comprise the components of patients with MOGΑD.Further studies are required to determine the impact of intrathecal MOG-IgG production and CSF-restricted MOG-IgG levels on the diagnosis and optimal treatment of MOGΑD.
Tetsuya Akaishi*,Tatsuro Misu
Department of Neurology,Tohoku University,Sendai,Japan
*Correspondence to:Tetsuya Αkaishi,MD,PhD,t-akaishi@med.tohoku.ac.jp.
https://orcid.org/0000-0001-6728-4966(Tetsuya Αkaishi)
Date of submission:May 12,2023
Date of decision:June 13,2023
Date of acceptance:Αugust 17,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385293
How to cite this article:Akaishi T,Misu T(2024)Anti-aquaporin-4 antibody(AQP4-IgG)and anti-myelin oligodendrocyte glycoprotein antibody(MOG-IgG)in the cerebrospinal fluid.Neural RegenRes 19(5):949-950.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Marina Herwerth,University Zurich,Switzerland.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From the dust: extracellular vesicles as regulators of development and neuroregeneration
- Targeting epidermal growth factor receptor signaling to facilitate cortical injury repair?
- Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
- Alpha7 nicotinic receptors as potential theranostic targets for experimental stroke
- Targeting autophagy by polyphenols to prevent glycative stress-toxicity in the brain
- Does photobiomodulation require glucose to work effectively?

