一种具有二次谐波发生和适中双折射的紫外透过稀土金属甲酸盐
刘文博 姜春波 吴 超 黄智鹏 张 弛
(同济大学化学科学与工程学院中澳功能分子材料联合研究中心,上海 200092)
0 Introduction
Nonlinear optical (NLO) crystals that can produce coherent light play an important role in modern optics,such as laser printing, lithography, laser guidance, and medical diagnosis[1].In the design of second-order NLO crystals, large second harmonic generation (SHG)response, stability in extreme environments, large bandgap, and high laser damage threshold (LDT) are important requirements that need to be considered in practical applications[2-3].So far, a number of commercialized materials have been developed in the field of second-order NLO optics,such asβ-BaB2O4(β-BBO)[4],LiB3O5(LBO)[5], CsLiB6O10(CLBO)[6], KH2PO4(KDP)[7],AgGaS2(AGS)[8],AgGaSe2(AGSe),and ZnGeP2(ZGP)[9].However, they still have several inherent drawbacks.Exploring and developing new NLO crystals to eliminate these performance defects is still a challenging research direction.
Traditional pure organic oxygen-containing salts have high SHG efficiency, but they are limited in practical applications due to their poor thermal stability.The introduction of inorganic hybrid components into the organic molecular structure is a feasible method that has been proven by recent studies[10-12].According to Chen′s anion group theory[13], anion groups within compounds make a greater contribution to the SHG response.The overall second harmonic coefficient of the material can be calculated by summing the secondorder polarizabilities of the micro components, particularly the anion groups.This provides us with a direction to seek highly efficient second-order NLO materials in terms of structural and group combinations.In addition to the well-known anion group theory in the field of nonlinear optics, considering the coplanar and aligned arrangement of densely packed anionic groups can generate a larger SHG response and sufficient birefringence.Additionally,charge-balanced cations play a role in guiding the formation of configurations when combined with anionic groups.While exploring anionic groups with high first-order hyperpolarizability,we also pay attention to functional cations and their anchoring role in crystal lattice formation[14-16].
Organic - inorganic hybrid perovskites with the general formula ABX4(A=amino, dimethylamine, nitrogen heterocycle, formamid; B=trivalent rare earth cations; X=formate), have received widespread attention in the fields of electric,magnetic,and photonics[17].The anionic structures of these compounds are formed by connecting the rare earth metal ions with the formate ligand to form extended frameworks, where the A site cations are located in the cubic-like cavity of the frameworks.The fully coordinated formate anionic group eliminates terminal oxygen atoms, benefiting to blueshift of the absorption edges[18].The organic cations of A sites interacts with the frameworks through hydrogen bonds and Coulomb forces.These compounds can take advantage of the combination of the organic oxygencontaining formate group with rare earth cations which usually perform variable and asymmetric coordination geometries to form asymmetric coordination[19-22], which are in line with the direction of exploring the synthesis of new noncentrosymmetric (NCS) compounds.Anionic groups withπ-conjugated structures have been extensively developed in the field of NLO optics.Becauseπconjugated cations have similar structural geometry toπ-conjugated anionic groups,π-conjugated cations have received attention in recent study.Theπ-conjugated guanidinium cation (C(NH2)3)+performs a planar structure which is similar to the BO33-, CO32-, and NO3-units[23-27].This structure exhibits a highly anisotropic electron distribution and strong covalent bonding of C—N bonds.It can be forecasted that guanidinecontained NLO crystals may display a large optical anisotropic polarizability and bandgap.Moreover, the H atom of the amino group is not only easy to form hydrogen bonds,promoting crystal growth,but also easily replaced by other planar groups to extend theπconjugated system[26].From the structural perspective,its suitability for use in the second-order NLO optical field can be found.Some studies have shown that introducing the planarπ-conjugated (C(NH2)3)+cation to NLO materials can greatly enhance their SHG efficiencies and birefringences, such as C(NH2)3SO3F (6.2 eV,5.0×KDP, 0.133@1 064 nm)[28].Our group developed aπ-conjugated-cation-based UV NLO phosphate,(C(NH2)3)6(PO4)2·3H2O[29].Unlike existing phosphate systems, (C(NH2)3)6(PO4)2·3H2O has a short UV cutoff edge of 205 nm, an SHG efficiency of approximately 3.8×KDP, and a moderate birefringence (0.078@546 nm).Theoretical calculations show that its optical properties are mainly originated from the synergy effect of theπ-conjugated (C(NH2)3)+cation and the PO43-tetrahedron.The introduction ofπ-conjugated cations such as (C(NH2)3)+provides a paradigm for optimizing the SHG responses and birefringences of NLO materials.
Based on the above research, considering the diversity of metal ions and organic ligands coordination combinations in the ABX4family, when screening for potential candidates from the ICSD database, an NCS material (C(NH2)3) [Er(HCOO)4] captured our attention[30].So far, there are no NLO optical properties reported for it.We found that (C(NH2)3)[Er(HCOO)4]exhibited a large optical bandgap of 4.76 eV, a sufficient birefringence (0.066@546 nm) and a phase-matching SHG response of 0.20×KDP.Furthermore, theoretical calculations were performed to unveil the relationship between the NCS structure and optical properties.
1 Experimental
1.1 Reagents
Guanidine carbonate ((C(NH2)3)2CO3, 99.00%,Xiya Reagent), formic acid (HCOOH 98.00%, Sinopharm Chemical Reagent), erbium nitrate pentahydrate(Er(NO3)3·5H2O, 99.00%, Xiya Reagent), methanol(CH3OH, Analytical grade, Tansole Chemical Reagent)andN,N-dimethylformamide (DMF, Analytical grade,Xiya Reagent) were commercially available and used as received without further purification.
1.2 Synthesis of(C(NH2)3)[Er(HCOO)4]
Er(NO3)3·5H2O (0.50 mmol), HCOOH (10 mmol),and (C(NH2)3)2CO3(1.0 mmol) were dissolved in mixed solvents of methanol and DMF (2∶1,V/V, 30 mL).The solution was sealed and left undisturbed.Block pink crystals were obtained overnight.The crystals were harvested, washed with methanol, and air-dried.The yield was approximately 60%(based on Er).
1.3 Single crystal X-ray diffraction
Single-crystal X-ray diffraction data collection of(C(NH2)3)[Er(HCOO)4] was carried out on a Bruker D8 VENTURE CMOS X-ray diffractometer using graphitemonochromatic MoKαradiation (λ=0.710 73 nm) at room temperature.APEX Ⅱsoftware was applied to collect and reduce data.Semi-empirical absorption corrections based on equivalent reflections were applied for both data sets using the APEX Ⅱprogram.The structure was solved by direct methods and refined onF2by full-matrix least-squares methods using the SHELXTL-97 software package.All hydrogen atoms were placed in calculated positions and refined with a riding model.All non-hydrogen atoms were refined anisotropically.Both structural data sets were also checked for possible missing symmetry using the program PLATON, and none were found.For (C(NH2)3)[Er(HCOO)4], in a range of 2.71°<θ<27.09°, a total of 6 535 reflections were collected and 2 357 were independent withRint=0.018 9,R1=0.012 9, andwR2=0.031 7.The detailed crystallographic data and structural refinement parameters of the compound are summarized in Table S1 (Supporting information).Selected bond distances (nm) and angles (°) are given in Table S2, while atomic coordinates and equivalent isotropic displacement parameters are given in Table S3.
1.4 Powder X-ray diffraction
Powder X-ray diffraction (PXRD) was used to confirm the phase purity of (C(NH2)3)[Er(HCOO)4].The PXRD data of each sample were carried out on a Bruker D8 X-ray diffractometer equipped with CuKαradiation(λ=0.154 18 nm)in a 2θ range of 5°-70°with a step size of 0.02°.The operating current was 40 kV and the operating voltage was 40 mA.
1.5 UV-Vis diffuse reflectance spectrum
Optical diffuse-reflectance spectrum was collected on a Cary 5000 over a spectral range of 200-800 nm at room temperature and a BaSO4plate was used as a 100% reflectance standard.The diffuse reflectance spectral data were converted to the function of reflectance using the Kubelka-Munk function[31].
1.6 Second-order NLO measurements
The SHG intensities of(C(NH2)3)[Er(HCOO)4]were measured according to the modified method of Kurtz and Perry[32].A Q-switched Nd:YAG laser with 1064 nm radiation was employed for the visible SHG study.Because the SHG efficiency is related to the particle size, the polycrystalline samples of (C(NH2)3)[Er(HCOO)4] were ground and sieved into several particle size ranges (0-26, 26-50, 50-74, 74-105, 105-150,150-200, and 200-280 µm).Crystalline KDP with the same particle size ranges was used as references.
1.7 Birefringence measurements
The birefringence of crystalline (C(NH2)3)[Er(HCOO)4] was assessed with a polarizing microscope (ZEISS Axio Scope.A1) equipped with a Berek compensator.The wavelength of the light source was 546 nm.The birefringence was calculated according to Eq.1:
where ΔRdenotes the optical path difference,Nerepresents the maximum value of the refractive index,Norepresents the minimum value of the refractive index,Δnrepresents the birefringence, andδis the thickness of the crystal.The positive and negative rotation of compensation affords the relative retardation.
1.8 Theoretical calculations
First-principlescalculationson(C(NH2)3)[Er(HCOO)4] were performed using the CASTEP package, a total energy package based on pseudopotential density functional theory (DFT).The correlationexchange terms in the Hamiltonian were described by the functional developed by Perdew, Burke, and Ernzerhof in the generalized gradient approximation form.Optimized norm-conserving pseudopotentials in the Kleinman-Bylander form were adopted to model the effective interaction between the valence electrons and atom cores, which allows the choice of a relatively small plane-wave basis set without compromising the computational accuracy.A kinetic energy cutoff of 850 eV and dense Monkhorst-Packk-point meshes spanning less than 1.5×10-5nm3in the Brillouin zone was chosen.
2 Results and discussion
2.1 Powder SHG response
(C(NH2)3)[Er(HCOO)4] crystallizes in NCS chiral space groupP212121, and therefore, the powder SHG property was measured using the Kurtz-Perry method under a 1 064 nm laser source.As shown in Fig.1, the NLO measurement results indicated that the SHG intensity of (C(NH2)3)[Er(HCOO)4] in the sizes of 105-150µm was approximately 0.20 times that of KDP (Fig.1a).The SHG intensity signal gradually increased and finally tended to be constant with the increase of particle size,which indicates that (C(NH2)3)[Er(HCOO)4] is phasematchable at 1 064 nm(Fig.1b).
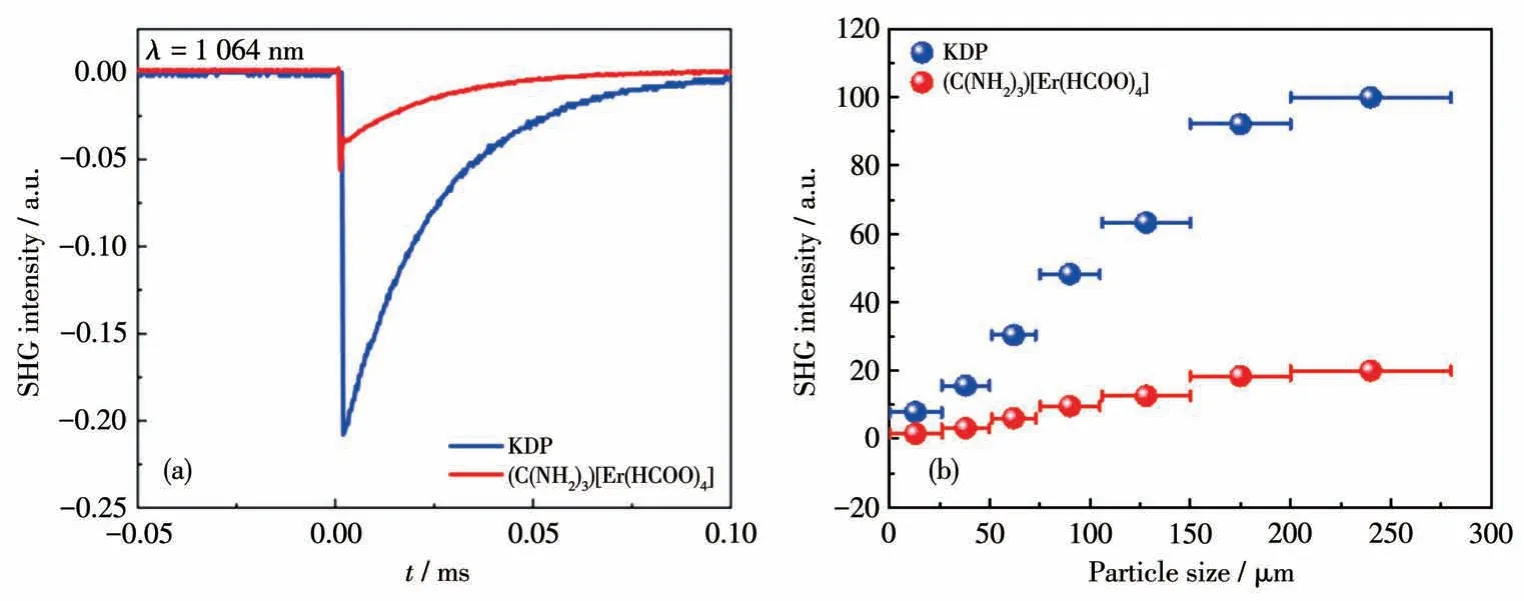
Fig.1 (a)Oscilloscope traces of the SHG signals(105-150µm)for(C(NH2)3)[Er(HCOO)4]with KDP sample as a reference;(b)Plots of measured SHG intensity versus particle sizes of(C(NH2)3)[Er(HCOO)4]under 1 064 nm laser radiation with KDP sample as a reference
2.2 UV-Vis diffuse reflectance spectrum
The test result of the UV-Vis diffuse reflectance of the powder samples in a range of 200-800 nm is shown in Fig.2.The absorption (K/S) data were calculated by the Kubelka-Munk functionF(R)=(1-R)2/(2R)=K/S, withR,K, andSrepresenting the reflectance,absorption, and scattering, respectively.The absorption spectrum showed that the compound (C(NH2)3)[Er(HCOO)4] had an optical bandgap of 4.76 eV (Fig.2,Inset), corresponding to the UV cutoff edge of 260 nm.The result indicates that (C(NH2)3)[Er(HCOO)4] is a potential UV NLO crystal.The peaks within the range of 400-800 nm are consistent with the pink nature of the title compound.

Fig.2 Optical properties of(C(NH2)3)[Er(HCOO)4]
2.3 Birefringence measurement
The birefringence of (C(NH2)3)[Er(HCOO)4] was measured by a Zeiss Axio A1 polarizing microscope,and the test result shows that the compound achieved complete extinction.The optical path difference of(C(NH2)3)[Er(HCOO)4] with a thickness of 70.447 µm was measured to be 4.65 µm (Fig.3), corresponding to a measured birefringence of 0.066@546 nm.The birefringence was comparable to other guanidinium-based NLO materials,such as[C(NH2)2NHNO2](C(NH2)3)(NO3)2(0.071@550 nm),(C(NH2)3)3PO4·2H2O(0.055@546 nm).

Fig.3 (a)Positive and(b)negative rotations of(C(NH2)3)[Er(HCOO)4]under the compensator;(c)Thickness of(C(NH2)3)[Er(HCOO)4]
2.4 Relationship between crystal structure and optical properties
Because the crystal structure was already well described by Liu et al.[30], the key structural features are briefly reviewed in this section.Each Er3+ion is coordinated by eight oxygen atoms from six formate groups, forming a [ErO8] polyhedron with Er—O bond lengths ranging from 0.226 2(2) to 0.241 4(3) nm(Fig.4a).Each [ErO8] building block is corner-shared six [ErO8] units by sixμ2-HCOO-groups to form a perovskite-like [Er(HCOO)4] framework[31].In the rhombohedral framework cavity, the (C(NH2)3)+cations act as charge balance cations located in the cavity of the[Er(HCOO)4]framework(Fig.4b and 4c).

Fig.4 (a)(C(NH2]3)+group and[ErO8]polyhedron in(C(NH2)3)[Er(HCOO)4];(b)Structure of(C(NH2)3)[Er(HCOO)4]viewed along the a-axis;(c)Monolayer of(C(NH2)3)[Er(HCOO)4]
The strength of the SHG effect serves as a crucial criterion for evaluating NLO materials.When analyzing the SHG effect of (C(NH2)3)[Er(HCOO)4], we can examine the arrangements of its structural units.According to Chen′s anion group theory, the SHG effect of(C(NH2)3) [Er(HCOO)4] is largely determined by the anions.As for [ErO8] units, they appear in parallel states but with different directions, resulting in a cancellation of polar orientations (Fig.5a).When examining the arrangement of the (C(NH2)3)+groups within the crystal structure, their polar orientations cancel each other out (Fig.5b).The Er3+ions exhibit absorption in the visible region.These factors are not beneficial to generate a strong SHG effect.
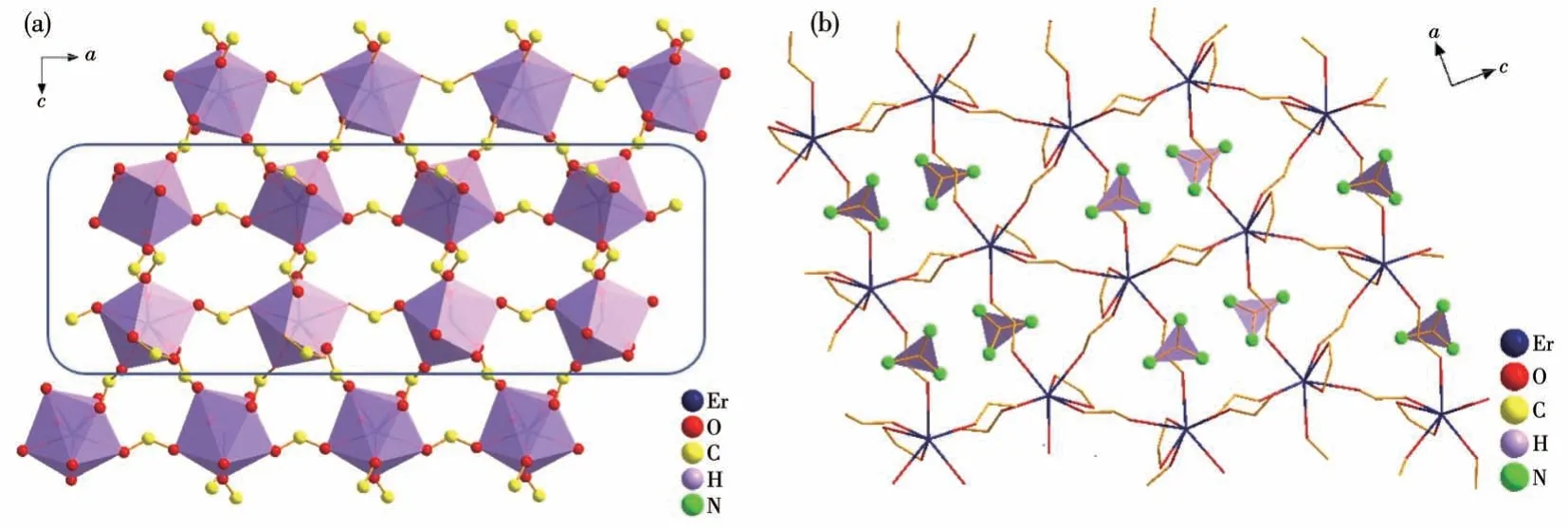
Fig.5 (a)Arrangement of[ErO8]polyhedra(purple polyhedra)in(C(NH2)3)[Er(HCOO)4];(b)Arrangement of guanidinium groups(purple triangles)in(C(NH2)3)[Er(HCOO)4],with the Er—O bonds connections depicted using wireframes
The birefringence is another important characteristic of NLO optical material,and(C(NH2)3)[Er(HCOO)4]exhibited a moderate birefringence of 0.066.This birefringence can also be explained through an analysis of the crystal structure arrangement.Regarding its arrangement of [ErO8] units or the orientation of guanidinium within the framework, it is evident that they are highly parallel and orderly (Fig.5a).The [ErO8] octahedra, with their terminal oxygen atoms provided by formate, are controlled by the formate, which influences the distortion direction of the polyhedra.The distortion directions of the polyhedral throughout the crystal structure are parallel and exhibit cumulative strength.This can account for the substantial birefringence of 0.066 observed in(C(NH2)3)[Er(HCOO)4].
2.5 Theoretical calculations
To further understand the structure-function relationship of (C(NH2)3)[Er(HCOO)4], theoretical calculations using the CASTEP program based on DFT were performed.The band structure diagram in Fig.6a shows that (C(NH2)3) [Er(HCOO)4] had a direct bandgap of 4.84 eV, which was slightly larger than the experimental value of 4.76 eV.The discrepancy can be attributed to the omission of excitonic effects in the calculation.The density of states (DOS) of the title compound is depicted in Fig.6b.The electronic transitions among the states near the Fermi level play a dominant role in the optical properties.The CBs (conduction bands)located between -10 and 0 eV are mainly occupied by Er4f, N2p, C2p, O2p, and H1sorbitals, with a smaller contribution from O2sorbital.The VBs (valance bands)between 0 and 10 eV are mainly composed of N2p,C2p, O2p, and H1sorbitals, as well as a little of Er4dorbital.Overall, this demonstrates that the bandgap and optical properties of (C(NH2)3) [Er(HCOO)4] are dominated byπ-conjugated (C(NH2)3)+cations, the[ErO8]polyhedron,and HCOO-groups.
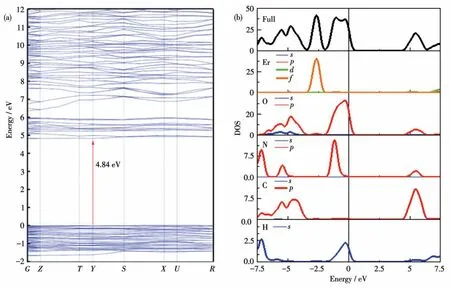
Fig.6 (a)Band structure of(C(NH2)3)[Er(HCOO)4];(b)Total DOS and partial DOS diagrams of(C(NH2)3)[Er(HCOO)4]
3 Conclusions
In summary, we successfully synthesized an NLO active rare earth metal formate, (C(NH2)3)[Er(HCOO)4].The optical study indicates that (C(NH2)3)[Er(HCOO)4]possessed a large optical bandgap of 4.76 eV.NLO measurements show that (C(NH2)3)[Er(HCOO)4] exhibited moderate and phase-matchable SHG intensity of 0.20 times that of KDP.In addition to its wide bandgap and SHG response, (C(NH2)3) [Er(HCOO)4] exhibited sufficient birefringence (0.066@546 nm).Theoretical calculation results reveal that the SHG response and birefringence of (C(NH2)3)[Er(HCOO)4] mainly originate from the cooperation ofπ-conjugated (C(NH2)3)+cations, the [ErO8] polyhedra, and HCOO-groups.These properties make (C(NH2)3)[Er(HCOO)4] a potential UV NLO material.
Declaration of competing interest:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information is available at http://www.wjhxxb.cn

