纤维素基磁性水凝胶对左氧氟沙星的吸附










摘要: 针对传统吸附材料吸附容量低、 难以固液分离且易导致二次污染等缺点, "以羧甲基纤维素(CMC)、 聚乙烯醇(PVA)、 丙烯酸(AA)和铁酸锌(ZnFe2O4)为单体, 通过水溶液聚合法, 制备易成型、 毒性低、 吸附容量大且可磁性遥控分离的CMC/PVA/PAA/ZnFe2O4纤维素基磁性水凝胶. 采用扫描电子显微镜(SEM)、 Fourier变换红外光谱(FT-IR)、 X射线衍射(XRD)和振动样品磁强计(VSM)等表征其形态结构并测试其结构性能, 通过吸附动力学和吸附热力学考察其对水中左氧氟沙星(LEV)的吸附性能和吸附机制. 结果表明: 负载ZnFe2O4磁性纳米粒子可增加水凝胶磁性遥控分离能力和吸附能力; 在25 ℃、 pH=5、 吸附4 h时, 水凝胶对LEV的最大吸附量可达405 mg/g, 5次吸附解吸实验后其吸附能力仍可达原吸附能力的84%; 水凝胶对LEV的吸附过程更符合准二级反应动力学和颗粒内扩散模型, 遵循Freundlich等温线模型.
关键词: "羧甲基纤维素; 铁酸锌; 磁性水凝胶; 左氧氟沙星; 吸附
中图分类号: X52""文献标志码: A""文章编号: 1671-5489(2024)06-1499-12
Adsorption of Levofloxacin by Cellulose-Based Magnetic Hydrogels
ZHAO Xinyu1, ZHANG Xinren1, ZHANG Enxu2, SHEN Li3, LIU Wanyi1, OUYANG Yunan1
(1. ""Key Laboratory of Straw Comprehensive Utilization and Black Soil Conservation of Ministry of Education, College of Resource and Environment Science, Jilin Agricultural University, """Changchun 130118, China; "2. Yanji Customs ""Comprehensive Technical Service Center, Yanji 133000, "Jilin Province, China; "3. Jilin Provincial Ecological Environment Monitoring Center, "Changchun 130118, China)
Abstract: ""Aiming at the shortcomings of traditional adsorption materials, such as low adsorption capacity, nbsp;difficulty in solid-liquid separation, "and easy to lead to secondary pollution, "carboxymethyl cellulose (CMC), "polyvinyl alcohol (PVA), "acrylic acid (AA), "and zinc ferrite (ZnFe2O4) were used as monomers to prepare CMC/PVA/PAA/ZnFe2O4 cellulose based magnetic hydrogels that was easy to form, "low toxicity, "large adsorption capacity, "and could be separated by magnetic remote control through aqueous solution polymerization. Their morphological structure and structural performance were characterized by scanning electron microscopy (SEM), "Fourier transform infrared spectroscopy (FT-IR), "X-ray diffraction (XRD), "and vibrating sample magnetometer (VSM). The adsorption kinetics and thermodynamics were used to investigate their adsorption performance on levofloxacin (LEV) in water and adsorption mechanism. The results show that loaded ZnFe2O4 "magnetic nanoparticles can increase the magnetic remote separation ability and adsorption ability of hydrogels. ""The maximum adsorption capacity of the hydrogel for LEV can reach 405 mg/g at 25 ℃, "pH=5 and 4 h of adsorption, "and its adsorption capacity can still reach 84% of the original adsorption capacity after five adsorption and desorption experiments. "The adsorption process of hydrogel for LEV is more in line with the quasi second order reaction kinetics and intraparticle diffusion model, "and follows the Freundlich isotherm model.
Keywords: carboxymethylcellulose; "zinc ferrite; magnetic hydrogel; "levofloxacin; "adsorption
抗生素是一类具有抗细菌、 抗真菌以及致病微生物的活性物质. 左氧氟沙星(LEV)是一种临床普遍使用的第三代氟喹诺酮类(FQs)抗生素, 具有形成“超级细菌”的风险, 可以低浓度存在于环境中且具有活性, 已在多个国家的湖泊、 河流和饮用水中检测出LEV\1"实"验
1.1"试剂与仪器
PVA和AA购于麦克林生化科技(上海)有限公司; 过硫酸铵(APS)购于天津市华东试剂厂; N,N′-亚甲基双丙烯酰胺(MBA)购于罗恩试剂上海易恩化学技术有限公司; CMC购于天津科茂化学试剂有限公司; LEV购于合肥博美责任有限公司; 氯化锌(ZnCl2)、 三氯化铁(FeCl3·6H2O)和甲醇(CH3OH)购于天津鑫铂特化工有限公司; 乙酸(CH3COOH)、 乙二醇(EG)、 乙酸钠(NaAc)和无水乙醇(CH3CH2OH)购于国药集团化学试剂有限公司; 氢氧化钠(NaOH)购于北京化工厂. 以上试剂均为分析纯试剂.
SA31000型比表面积/孔径分布和孔隙分析(BET)仪(美国Bechman Coulter公司); XL-30扫描电子显微镜(SEM, 美国FEI 公司); IRAffinity-1S型Fourier变换红外光谱(FT-IR)仪(日本岛津公司); XRD-7000型X射线衍射(XRD)仪(日本津岛公司); 7307型振动样品磁强计(VSM, "美国LakeShore公司); TGA-DSC1型热重分析(TGA)仪(美国Mettler Toledo公司); "Nano ZS90型Zetasizer纳米粒度电位(DLS)仪(英国Malvern Panalytical公司).
1.2"纤维素基磁性水凝胶的制备
ZnFe2O4磁性纳米粒子的制备: "将适量FeCl3·6H2O、 ZnCl2和NaAc溶于350 mL EG溶液中, 磁力搅拌上述混合溶液60 min, 将其转移至聚四氟乙烯反应釜中, 密封后于220 ℃恒温保持6 h. 反应结束后, 待反应釜冷却至室温, 将混合物进行强磁分离收集沉淀物, 用CH3CH2OH溶液和去离子水反复洗涤沉淀物, 经充分干燥后得到ZnFe2O4磁性纳米粒子.
CMC/PVA/PAA水凝胶的制备: "准确称取1.0 g CMC, 于100 mL去离子水中完全溶解; 准确称取10.0 g AA于50 mL去离子水中, 用NaOH调节AA溶液中和度至70%; "准确称取2.0 g PVA于20 mL去离子水中, 于90 ℃恒温搅拌至完全溶解; 将引发剂APS和交联剂MBA加入上述三者共混溶液中, 以超声脱气, 于60 ℃氮气保护条件下搅拌至共混溶液开始出现凝胶状态为止, 于75 ℃恒温水浴反应12 h, 用CH3OH溶液和去离子水将反应产物洗涤浸泡, 得到纯净CMC/PVA/PAA水凝胶.
CMC/PVA/PAA/ZnFe2O4水凝胶的制备: 以上述相同溶液质量比加入CMC,AA,PVA后, 在共混体系中加入0.8 g ZnFe2O4, 通过超声使混合液分散均匀, 按上述相同步骤得到CMC/PVA/PAA/ZnFe2O4水凝胶.
将CMC/PVA/PAA水凝胶和CMC/PVA/PAA/ZnFe2O4水凝胶于65 ℃恒温干燥至恒质量, 研磨过筛, 得到固体粉末, 以备后续表征及吸附实验使用. 其中SEM表征所用样品为-40 ℃冷冻干燥制备所得样品.
1.3"吸附实验
准确称取0.01 g干凝胶粉末于50 mL LEV溶液中, 160 r/min恒温振荡至吸附平衡, 用紫外分光光度计在290 nm处测试吸附反应后LEV溶液的吸光度, 并计算水凝胶对LEV的吸附量(Qe)和去除率(R)[9]
1.4"循环吸附实验
使用强磁铁收集吸附LEV后的CMC/PVA/PAA/ZnFe2O4水凝胶, 并用V(CH3OH)∶V(CH3COOH)=9∶1的溶液和去离子水对其进行洗脱、 干燥, 进行反复5次吸附解吸实验, 考察水凝胶的循环使用性能.
2"结果与讨论
2.1"材料的表征
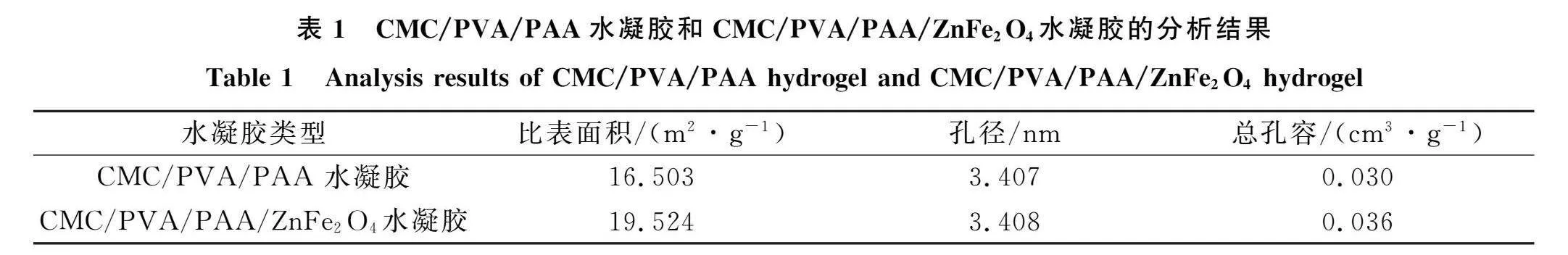
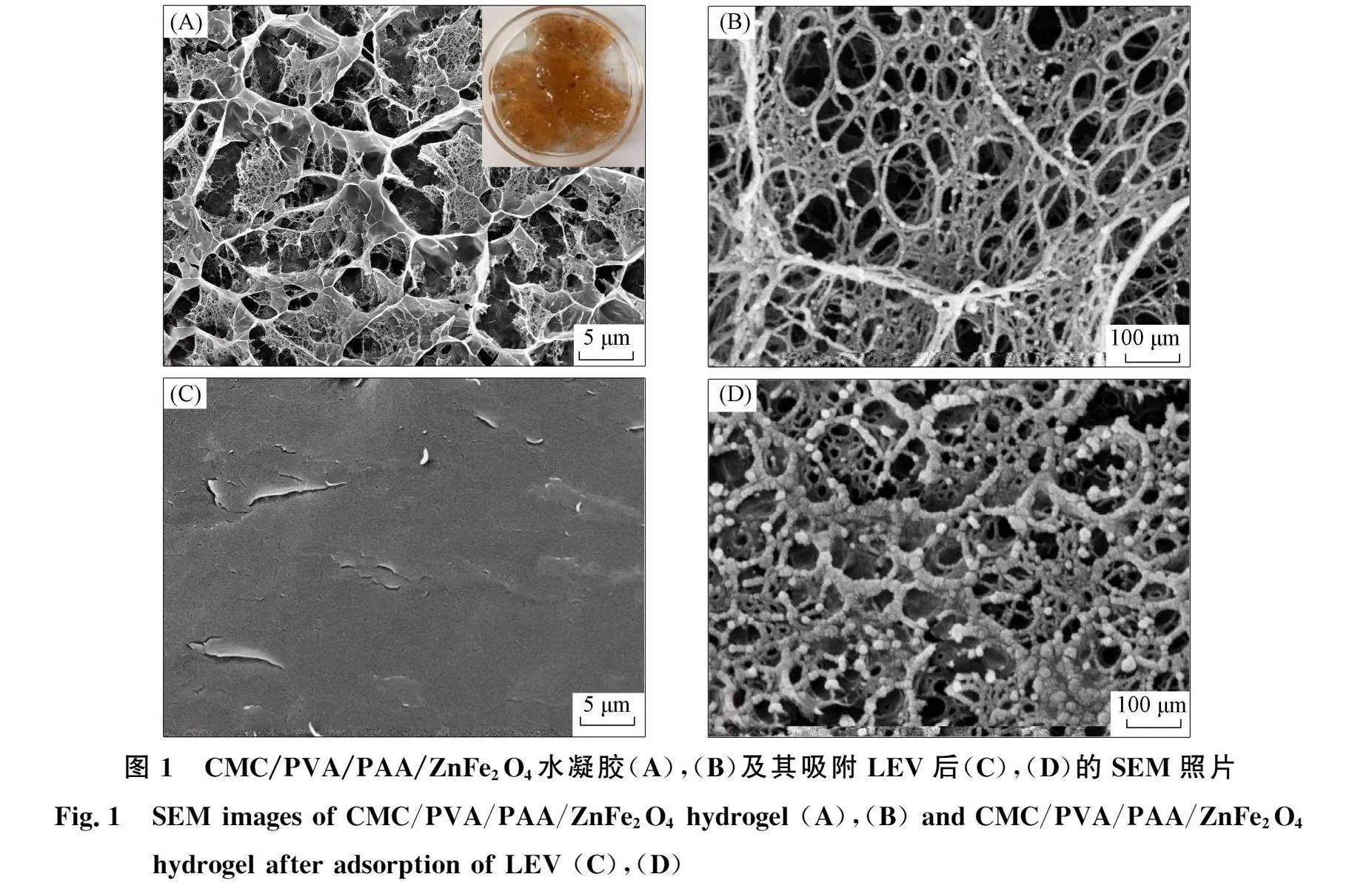
吸附LEV前后的CMC/PVA/PAA/ZnFe2O4水凝胶SEM表征结果如图1所示. 由图1(A)可见, 水凝胶内部可见明显清晰丝状网格结构, 其结构疏松多孔, 孔洞均匀密集, 该结构是聚合过程中CMC,PVA和AA剧烈离子交联所致, 提高了水凝胶对LEV的吸附性能[10]由图I(B)可见,水凝胶网格结构中均匀分布大量凸起的球形颗粒状物质,表明ZnFe,04磁性纳米粒子已成功负载于所制备水凝胶内部网络.CMC/PVA/PAA水凝胶和CMC/PVA/PAA/ZnFe,()。水凝胶的分析结果列于表l.由表1可见,包埋于CMC/PVA/PAA水凝胶内部的ZnFe2 04磁性纳米粒子与粒子之间形成了更致密的网状交联结构,使负载ZnFe2 04磁性纳米粒子后的水凝胶的比表面积、孔径和总孔容均有增加.
由图1(C),(D)可见, "吸附LEV后的水凝胶表面致密光滑, 表明其网格结构和表面吸附点位已基本被LEV占据填满, 放大后的SEM照片(图1(D))可清晰地观察到其网状结构更紧实充盈, ZnFe2O4磁性纳米粒子的球形凸起状态也更明显. 进一步表明ZnFe2O4磁性纳米粒子可显著增加水凝胶比表面积和表面螯合点位数量, 使LEV迅速进入水凝胶内部, 缩短吸附平衡时间, 进而有效提升水凝胶吸附能力.
图2为材料的FT-IR,XRD,VSM和TGA曲线,由图2(A)可见,在ZnFe2 04的FT-IR谱中,位于561. 33,447. 26 cm-l处的峰为ZnFe2 04中Fe()的特征吸收峰[ll].在CMC/PVA/PAA水凝胶的FT-IR谱中,位于3 557. 96,l 727. 70 cm-l处的峰为CMC中O "H和C=O的红外特征峰,位于2 929,l 256. 65 cm-l处的峰为CMC中CH:的不对称伸缩振动和面外摇摆振动峰[lO],位于l 551. 80,1 "436. 31 cm-l处的峰为PAA中 "COOH和 "COO "基团的红外特征峰[12].在CMC/PVA/PAA/ZnFe2 04水凝胶的FT-IR谱中:位于3 469. 81 cm-l处的峰为CMC/PVA/PAA水凝胶在3 557. 96 cm-l处较强的() "H伸缩振动峰位移至此处,且变为中低强度峰,这是由于CMC和PVA中存在不同强度的O "H伸缩振动,导致() "H的存在形式增多,使O "H特征峰变宽、偏移;同时,交联反应中O "H不断消耗也是该峰变宽变弱的原因[13];位于I 166. 08 cm-l处的峰为ZnFe2 04的Fe()C伸缩振动峰;位于509. 71,417. 26 cm-l处的峰为Fe O的红外特征峰,与ZnFe2 04中对应的峰相比发生了蓝移.综上可见,ZnFel 04成功负载于CMC/PVA/PAA水凝胶表面,与SEM表征结果一致,在吸附LEV后的CMC/PVA/PAA/ZnFe2()。水凝胶FT-IR谱中:在3 300 cm-l处出现了来自LEV的 "NH红外吸收峰[4 在l 721. 64 cm-l处原属于PAA中COOH的C=O特征吸收峰明显增强,说明在吸附过程中,酸性条件使水凝胶中部分COO官能团已逐步转化为COOHD-l,证明LEV已成功吸附于CMC/PVA/PAA/ZnFe,()。水凝胶内部.
由图2(B)可见,在ZnFe,04的XRD谱中,在20=35。,43。,53。,56。,62。,74。处有明显特征衍射峰,这与ZnFe2 04的PDF标准卡片(PDF#22-1012)相符,在CMC的XRD谱中,由于CMC的半结晶性,因此在20=22。处出现明显的特征衍射峰[16].在CMC/PVA/PAA水凝胶和CMC/PVA /PAA/ZnFe2 04水凝胶的XRD谱中,在20=22。处出现来自CMC明显变宽变弱的特征衍射峰,说明CMC与PVA和PAA发生了交联反应,导致其晶型能力降低,从而转为非晶体性结构[17].同时,在CMC/PVA/PAA/ZnFe2 04水凝胶的XRD谱中,在20=35.2。~74。处也可观察到与ZnFe2 04的XRD谱相比明显变弱的特征衍射峰.该现象表明ZnFe2 04磁性纳米粒子均匀掺杂于CMC/PVA/PAA/ZnFe2 04水凝胶网络中,与SEM和FT-IR结论一致.
由图2(C)可见,吸附LEV前盾CMC/PVA/PAA/ZnFe2 04水凝胶的磁滞回线关于原点对称,无剩磁和矫顽力,说明制备的水凝胶具有超顺磁性,无磁滞现象,即水凝胶在外加磁场作用下具有磁性,撤掉磁场,磁性消失,该特性有利于水凝胶的分离回收,水凝胶的最大磁饱和强度随磁场强度的增加而增大,最大磁饱和强度为5. 89 A/m;吸附LEV后,水凝胶的最大磁饱和强度降低至1.91 A/m.这是由于吸附反应导致LEV与ZnFe2 04发生配合,水凝胶表面由ZnFe2 04粒子产生的吸附位点减少,使水凝胶磁饱和强度降低[l8].
由图2(D)可见,CMC/PVA/PAA/ZnFe,04水凝胶的热分解可分为3个阶段:第一阶段
(34~311℃),水凝胶失去部分质量,质量损失率为28. 29%,这是由于随着温度升高,水凝胶内部失去大量结合水所致;第二阶段(311~537℃),水凝胶失质量现象明显,其内部大量含氧官能团发生热降解,导致该过程质量损失率可达37. 02%;第三阶段(537~800℃),水凝胶内部仍存在一定数量小分子挥发性产物,该过程质量损失率约为11. 750/,此时水凝胶内部大分子碳链结构开始热解,水凝胶逐渐碳化.
2.2 "吸附时间、温度、ph值子强度对吸附的影响
在20~240 min内,考察吸附时间对CMC/PVA/PAA/ZnFe2 04水凝胶吸附性能的影响,结果如图3所示,由图3可见,在吸附初期的40 min内,水凝胶对LEV的吸附速率迅速增大,此时吸附量(Q。)可达吸附总量的80%,这种快速的动力学过程主要归因于水凝胶表面大量的活性点位以及高浓度LEV的吸附驱动力[19].但随着吸附反应进行,水凝胶表面吸附点位被大量占据,且体系中LEV浓度降低,导致吸附阻力增大,从而吸附速率显著降低,在200 min时基本达到吸附平衡,其平衡吸附量约为405 mg/g,LEV去除率(R)约为81%.不同吸附材料对LEV的吸附性能比较列于表2.由表2可见,与其他材料相比,CMC/PVA/PAA/ZnFe2 04水凝胶的吸附性能优势明显.
在15~55 ℃内考察环境温度对CMC/PVA/PAA/ZnFe2O4水凝胶吸附性能的影响, 结果图4所示. 由图4可见: 当环境温度为15 ℃~25 ℃时, 水凝胶对LEV的吸附速率趋于稳定, 在25 ℃时达到最佳吸附效果, 其吸附量约为405 mg/g; 当环境温度大于25 ℃时, 凝胶对LEV的吸附速率逐步降低. 这是因为适当升高环境温度能激发LEV分子在溶液中的热运动而增加其与水凝胶有效官能团的接触几率, 从而提高水凝胶的吸附能力[27],但过高的环境温度会导致水凝胶内部结构坍塌,表面张力减弱,吸附位点减少,且部分已吸附在水凝胶表面的LEV出现解吸现象,从而水凝胶对LEV的吸附量有所下降28].由于环境温度变化对水凝胶吸附性能的影响不显著,因此适宜水凝胶吸附的温度范围相对较宽,但其吸附过程仍可定义为放热反应,升高环境温度不利于吸附反应进行.
在pH=I~II内,考察溶液pH值对CMC/PVA/PAA/ZnFe2 04水凝胶吸附性能和Zeta电位的影响,结果如图5所示,由图5可见:水凝胶表面电位随溶液pH值的升高而显著减小,在溶液pHgt;I后出现等电点,其表面转变带有负电荷;水凝胶对LEV的吸附量随溶液pH值的升高呈先增大后减小的趋势,在pH=5时具有最佳吸附性能,吸附量约为365 mg/g,去除率约为73%. LEV的pK;,,和pK;。。分别为5.97和8.28[29],当溶液pH=I时,水凝胶交联网络中的 "COO-和 "NH:发生质子化,分别转变为 "COOH和 "NH3+,此时带正电荷的水凝胶表面与溶液中带正电荷的LEV+发生排斥反应,从而导致水凝胶对LEV的吸附量相对较低30],当溶液pHgt;I时,H+的竞争作用随其数量的减少而下降,水凝胶表面也因出现等电点而带有负电荷,此时带负电荷的水凝胶与溶液中带正电荷的LEV+发生静电吸引,水凝胶吸附量显著增大,在pH=5时静电引力作用达到最大,此时吸附效果最佳31],当5lt;pHlt;9时,LEV以两性离子形式存在,LEV+数量减少,水凝胶吸附量下降,但 "COO-向 "COOH转化的缓冲行为导致其吸附量的下阵趋势并不明显‘30].当pHgt;9时,水凝胶表面 COOH和 "NH::发生去质子化,转化为 "COO-和 "NH:,此时LEV主要以LEV-存在,与带有相同负电荷的水凝胶产生静电排斥,导致吸附量下降显著30].
考虑实际环境水样成分的复杂性与非均质性,通过实验筛选抗生素废水中普遍存在的共存离子,评估其强度对CMC/PVA/PAA/ZnFe,()。水凝胶吸附性能的影响,结果如图6所示,共存离子价态对水凝胶吸附性能影响程度大致表现为Mg2~-≈Ca2+ gt;Na+,表明共存离子水合半径越大,对水凝胶吸附性能影响越大31],但随着共存离子(Na+,Mg2+,Ca2+)的离子强度增加,水凝胶对LEV的吸附量和去除率均呈下降趋势,表明高浓度离子对水凝胶吸附LEV具有明显的阻碍作用,这是因为吸附过程在溶液pH=5时进行,此时LEV主要以LEV+存在,共存阳离子会与LEV+竞争水凝胶表面的有限吸附位点,从而使LEV+与水凝胶的接触几率降低[201;同时,增大共存离子的离子强度会使水凝胶表面双电层变薄,并与LEV之间产生静电屏蔽作用[31],从而导致水凝胶对LEV的吸附效果下降,该现象进一步验证了静电吸附对水凝胶与LEV之间起主导吸附作用,与FT-IR表征和pH值对水凝胶吸附性能影响的结论一致.
2.3 "水凝胶对LEV的吸附动力学
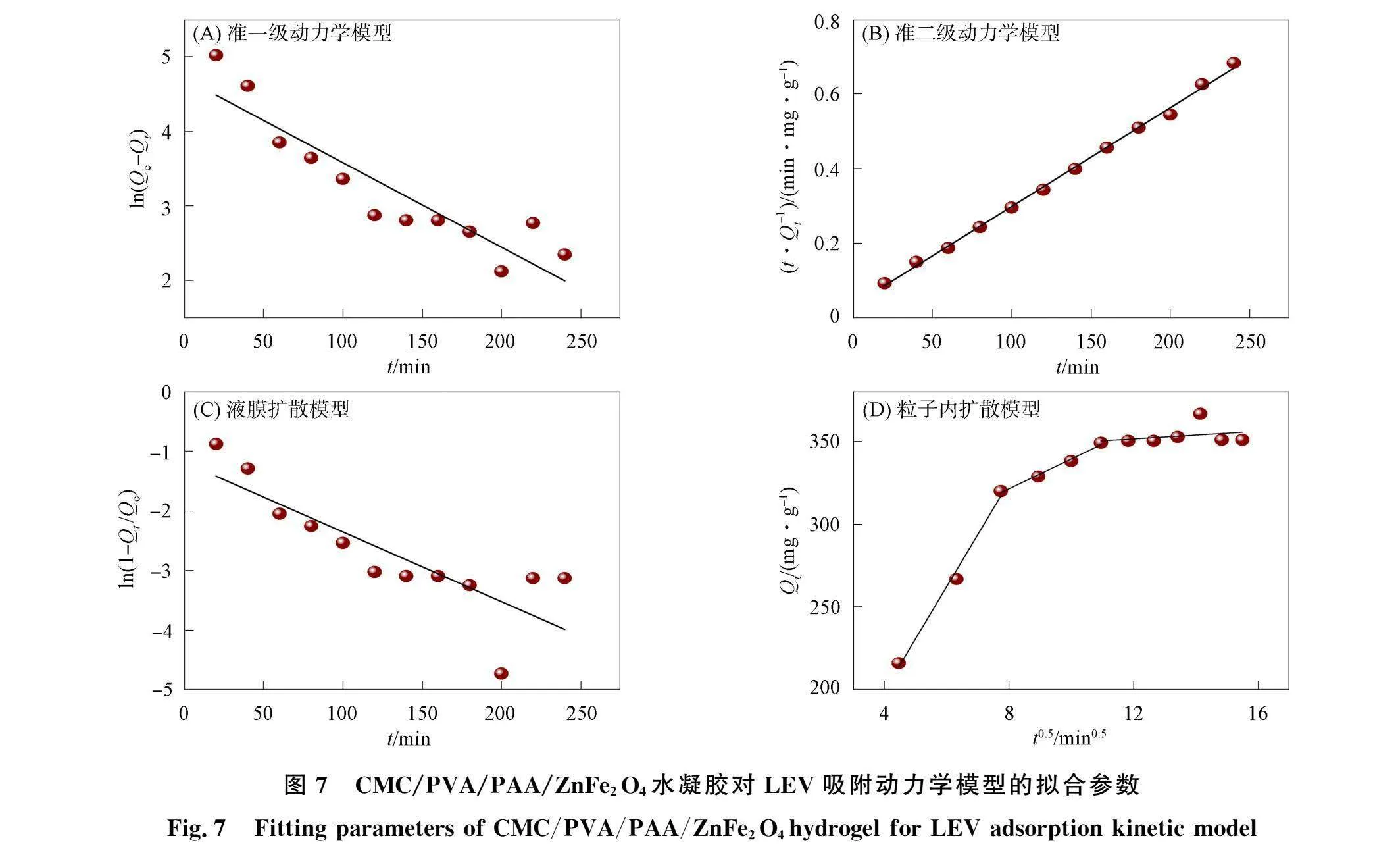
采用准一级反应动力学、准二级反应动力学、液膜扩散模型和粒子内扩散模型对CMC/PVA/PAA/ZnFe2 04水凝胶吸附LEV的过程进行拟合‘32],其吸附动力学拟合结果如图7和表3所示,
拟合结果表明,水凝胶对LEV的准二级比准一级反应动力学模型拟合相关系数更优,且准二级反应动力学模型拟合得到的理论平衡吸附量(377. 36 mg/g)与实测值(405 mg/g)更接近,说明水凝胶对LEV的吸附更遵循准二级反应动力学模型,即吸附过程主要为化学吸附,由于吸附过程可能由一个或多个速率控制,单凭准一级或准二级反应动力学模型无法完整判断整个吸附过程,因此通过液膜扩散和粒子内扩散拟合进一步分析LEV与水凝胶之间的吸附机制.根据水凝胶对LEV的液膜扩散和粒子内扩散模型拟合数据,水凝胶对LEV的吸附分为快速表面吸附、颗粒内扩散及吸附和脱附平衡动态过程3个阶段,每个阶段拟合得到的吸附量Q,与£0.5均呈线性相关,其边界层扩散速率常数K1d
和颗粒内扩散速率常数k2d远大于吸附和脱附平衡动态速率常数k3d和液膜扩散常数Kf,且各自拟合相关系数也符合上述结果,说明以边界层扩散为主的快速表面吸附和颗粒内扩散过程是水凝胶吸附LEV主要的控速步骤.但准一级动力学和准二级动力学的拟合曲线均未通过坐标原点,因此存在如液膜扩散等其他因素共同影响CMC/PVA/PAA/ZnFe2 04水凝胶与LEV之间的相互作用.
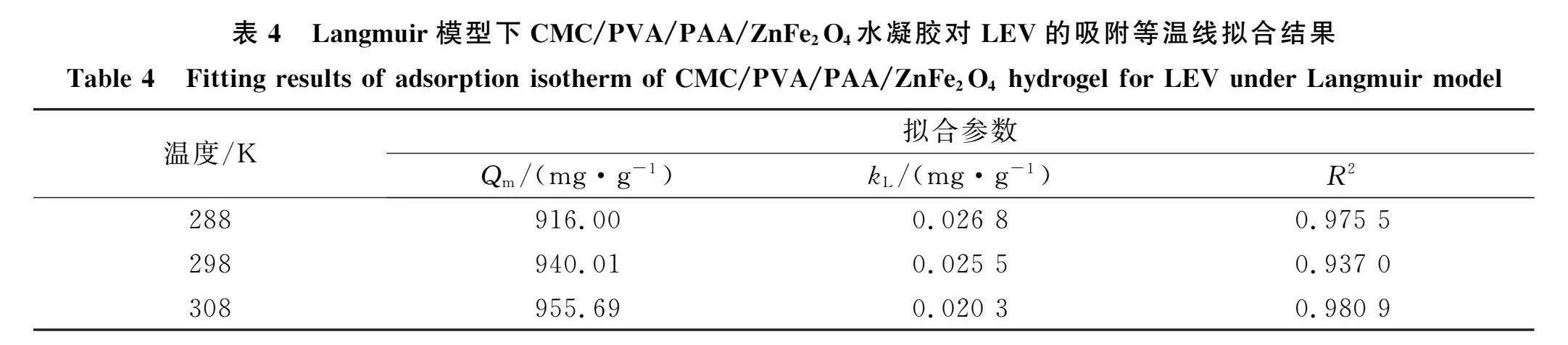
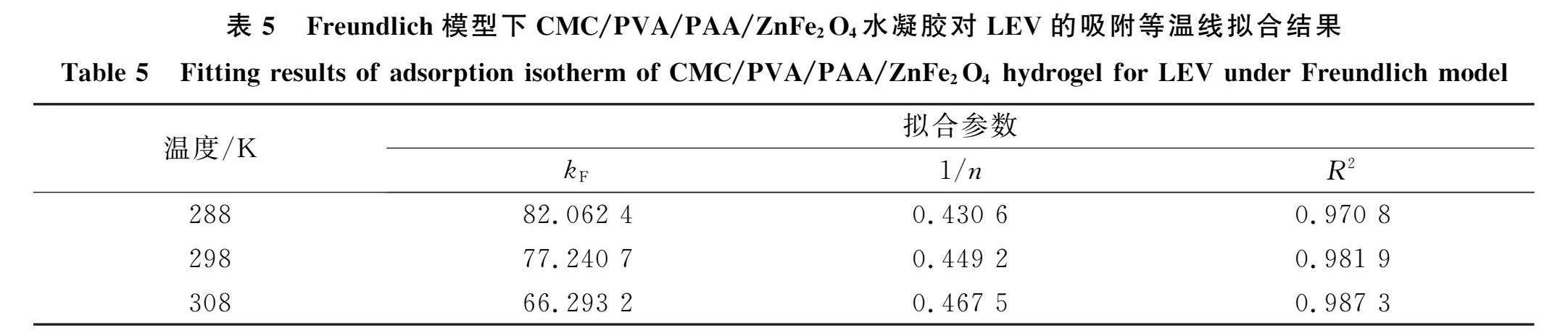
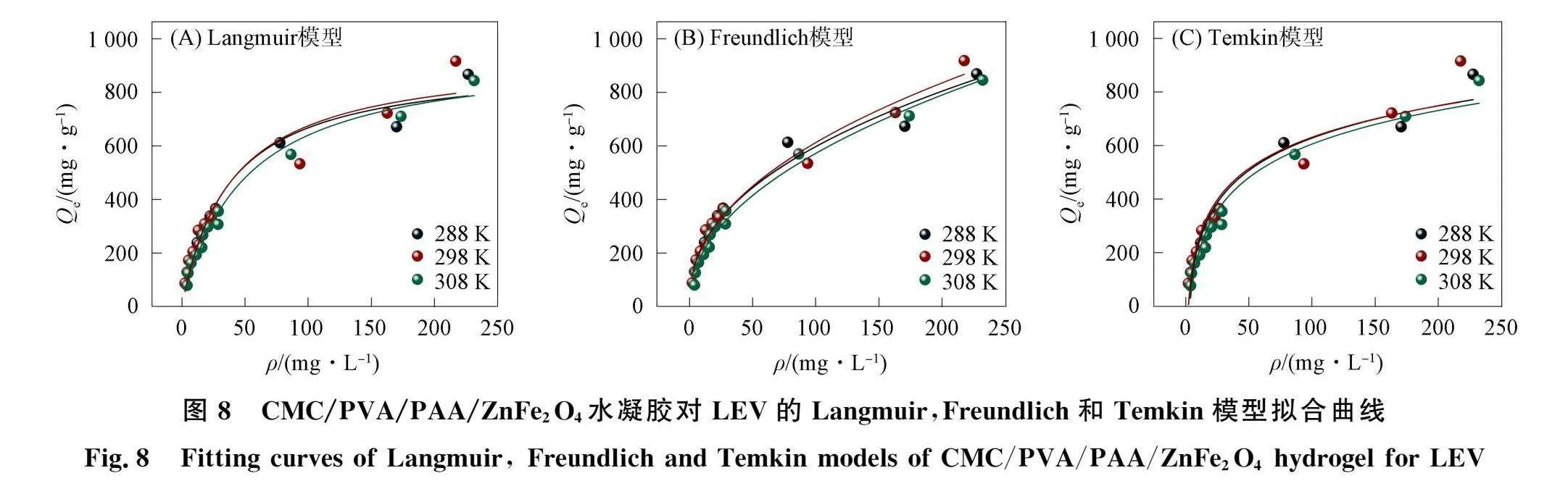
2.4 "水凝胶对LEV的吸附等温线与热力学参数采用Langmuir, Freundlich和Temkin模型对不同初始质量浓度的LEV(20,30,40,50,60,100,200,300,400 mg/L)在CMC/PVA/PAA/ZnFe70。水凝胶上吸附量变化的实验数据进行拟合[33],拟合结果如图8和表4~表6所示.由图8可见,水凝胶对LEV的吸附量在LEV质量浓度较低时增加较
迅速,之后随着LEV初始质量浓度增大,其吸附量增加趋势变缓并逐渐达到平衡吸附,表明初始质量浓度变化对LEV和水凝胶之间的传质驱动力起一定促进作用,从而增加了LEV相水凝胶的相互作用;当水凝胶质量不变,初始质量浓度逐渐增大的LEV使其在水凝胶表面的覆盖率增大,导致溶液中游离的LEV向水凝胶内部更深孑L径的传质受到阻力,使这些游离的LEV难以被水凝胶顺利捕获,从而导致水凝胶对LEV的吸附量呈先快速增加后放缓的状态34], 由表4~表6可见,在不同温度(288,289,308 K)下,水凝胶对LEV的Freundlich吸附等温模型均优于Langmuir和Temkin吸附等温模型的拟合相关系数R2,表明Freundlich吸附等温模型更适合解释水凝胶对LEV的吸附过程,即吸附过程是多相多分子层吸附30],其中,Freundlich模型拟合得到的n值可衡量吸附过程的吸附动力和吸附位能,l/n值越小,说明水凝胶吸附性能越好,吸附越易进行20].不同温度下水凝胶对LEV的拟合结果l/n值均小于l,表明该吸附反应能自主进行;随着吸附温度逐渐升高,l/n值逐渐增大,从而验证了环境温度对水凝胶吸附性能影响的结论,即升高温度会增加吸附难度. CMC/PVA/PAA/ZnFe,()。水凝胶对LEV的吸附热力学参数拟合结果列于表7.由表7可见:在不同温度(288,289,308 K)下,水凝胶吸附LEV的AG均为负值,表明水凝胶对LEV的吸附是一种自发吸附行为[];当环境温度从288 K升高至308 K时,AG的绝对值呈先增大后减小的趋势,表明适宜的环境温度可增大吸附推动力,提高水凝胶吸附LEV的自发趋势,但温度过高会阻碍吸附反应进行;AH表示水凝胶和LEV发生吸附反应所需热能,该吸附过程拟合得到的AH为负值,表明水凝胶吸附LEV为放热反应,即升高温度不利于反应进行;拟合得到的AS为正值,表明吸附过程导致固液界面无序度增加,吸附LEV后水凝胶内部结构变化明显,反应体系自由度显著变大36].
当热力学过程拟合得到AG=0~-20 kj/mol,且AHlt;84 kj/mol时,表明该热力学过程存在物理吸附‘36],上述热力学拟合数据符合该结论.在2.3节中的动力学拟合结果也证明化学吸附是水凝胶对LEV的主控吸附步骤,因此水凝胶对LEV的吸附是以化学吸附为主,物理吸附为辅,由二者共同控制的吸附机制.
2.5 "水凝胶对LEV的脱附再生
LEV在/PVA/PAA/ZnFe2 04水凝胶上的脱附再生效果如图9所示.由图9可见,水凝胶
对LEV的吸附量随脱附次数的增加逐渐降低,这可能是由于在吸附解吸过程中,由CH30H生成的HCOOH无法对吸附位点解吸完全,从而导致部分吸附位点缺失[37].同时,由于解吸溶液中的NaOH降低了体系酸度,导致H+数量减少,使水凝胶内部 "NH。质子化程度减弱,因此无法生成足量的NH3~-与吸附的LEV+发生充分排斥反应38].经5次吸附解吸实验后,水凝胶对LEV的吸附量从405 mg/g降至343. 96 mg/g,但其吸附效率仍可达到初次吸附效率的84%,表明制备的纤维素基磁性水凝胶具有良好的重复使用性能,是一种具有实际应用前景的处理抗生素废水吸附剂.
3 "结 "论
综上所述,本文采用水溶液聚合法成功制备了CMC/PVA/PAA/ZnFe2 04水凝胶,用于对LEV的吸附研究,得到如下结论.
1)材料表征结果表明,CMC/PVA/PAA/ZnFe2 04水凝胶结构稳定,具有良好的超顺磁性,当CMC/PVA/PAA/ZnFe70。水凝胶在最佳吸附条件(吸附时间4h、环境温度25℃、溶液pH=5)时,对LEV最大吸附量可达405 mg/g.高浓度共存离子(Na~-,Mg2+,Ca2+)对水凝胶吸附LEV具有明显的阻碍作用,其影响程度大致表现为Mg-+~Ca2+ gt;Na+.
2) CMC/PVA/PAA/ZnFe204水凝胶对LEV的吸附主要以化学吸附为主,边界层扩散和颗粒内扩散是主要控速步骤,且吸附过程是自发进行的多相多分子层吸附的放热反应.在最佳吸附条件下,CMC/PVA/PAA/ZnFe2 04水凝胶经5次吸附解吸后,其吸附效率仍可达到原吸附效率的84%,说明其具有良好的循环再生利用性能.
3)本文所用污染物为实验室配置的单一模拟污染水体,且配置浓度大于实际水体,为以后开展实际水体研究提供了理论依据.
参考文献
[1]"LIU Y, "GUO H G, "ZHANG Y L, "et al. Heterogeneous Activation of Peroxymonosulfate by Sillenite Bi25FeO40: "Singlet Oxygen Generation and Degradation for Aquatic Levofloxacin [J]. Chemical Engineering Journal, "2018, "343: "128-137.
[2]"ASMAA K M, "MOHAMED E M. Encapsulation of Starch Hydrogel and Doping Nanomagnetite onto Metal-Organic Frameworks for Efficient Removal of Fluvastatin Antibiotic from Water [J]. Carbohydrate Polymers, "2020, "245: "116438-1-116438-10.
[3]"XU Z Y, "XIANG Y J, "ZHOU H, "et al. Manganese Ferrite Modified Biochar from Vinasse for Enhanced Adsorption of Levofloxacin: "Effects and Mechanisms [J]. Environmental Pollution, "2021, "272: "115968-1-115968-8.
[4]"LENG "L J, "WEI L, "XIONG Q, "et "al. Use of Microalgae Based Technology for the Removal of Antibiotics from Wastewater: "A Review[J]. Chemosphere, "2019, "238: "124680-1-124680-14.
[5]"LIU "J M, "JI Z Y, "SHI Y B, "et al. Effective Treatment of Levofloxacin Wastewater by an Electro-Fenton Process with Hydrothermal-Activated Graphite Felt as Cathode[J]. Environmental Pollution, "2020, "266: "115348-1-115348-9.
[6]"ZHAO "J, "YANG X, "LIANG G W, "et al. Effective Removal of Two Ffluoroquinolone Antibiotics by PEG-4000 Stabilized Nanoscale Zero-Valent Iron Supported onto Zeolite (PZ-NZVI)[J]. Science of the Total Environment, "2020, "710: "136289-1-136289-13.
[7]"GE "W J, "SHUAI J B, "WANG Y Y, "et "al. Progress on Chemical Modification of Cellulose in “Green” Solvents[J]. Polymer Chemistry, "2022, "13: "359-372.
[8]"张陆雨. 竹纳米纤维素基自愈合双交联水凝胶的制备及其应用基础研究[D]. 长沙: 中南林业科技大学, "2022.
(ZHANG L Y. Basic Research on Preparation and Application of Bamboo Nano Cellulose Based Self-healing Double Crosslinked Hydrogel[D]. Changsha: Central South University of Forestry and Technology, "2022.)
[9]"MA "Y F, "LI M, "LI P, "et al. Hydrothermal Synthesis of Magnetic Sludge Biochar for Tetracycline and Ciprofloxacin Adsorptive Removal[J]. Bioresource Technology, "2021, "319: "124199-1-124199-10.
[10]"吴淑茗, "刘钰成, "吴佳斌, "等. 玉米淀粉/羧甲基纤维素生物复合凝胶的制备及吸附行为研究[J]. 塑料科技, "2023, "51(3): "73-78.
(WU S M, "LIU Y C, "WU J B, "et "al. Preparation and Adsorption Behavior of Corn Starch/CMC Biomass Composite Hydrogel[J]. Plastics Sceince and "Technology, "2023, "51(3): "73-78.)
[11]"CHEN "X, "HUANG Z, "LUO S Y, "et al. Multi-functional Magnetic Hydrogels Based on Millettia speciosa Champ Residue Cellulose and Chitosan: "Highly Efficient and Reusable Adsorbent for Congo Red and Cu2+"Removal[J]. Chemical Engineering Journal, "2021, "423: "130198-1-130198-13.
[12]"韩珊珊, "陈元涛, "张炜, "等. Cu(Ⅱ)交联羧甲基纤维素水凝胶制备及对铀的吸附[J]. 环境科学学报, "2024, 44(3): "83-94. "(HAN S S, "CHEN Y T, "ZHANG W, "et "al. The Preparation of Cu(Ⅱ) Cross Linked Carboxymethyl Cellulose Hydrogels and Its Adsorption for Uranium[J]. Acta Scientiae Circumstantiae, "2024, "44(3): "83-94.)
[13]"张宏宇, "田锦涛, "毕程程, "等. β-环糊精/聚乙烯醇/丙烯酸水凝胶对左氧氟沙星吸附性能及机理研究[J]. 化学研究与应用, "2022, "34(11): "2636-2646. (ZHANG H Y, "TIAN J T, "BI C C, "et ""al. Study on the Adsorption Performance and Mechanism of Levofloxacin by β-Cyclodextrin/Polyvinyl Alcohol/Acrylic Acid Hydrogel[J]. Chemical Research and Applications, "2022, "34(11): "2636-2646.)
[14]"ZHANG "Y, "LI Y H, "WANG Y Y, "et ""al. Adsorption of Levofloxacin by Ultraviolet Aging Microplastics [J]. Chemosphere, "2023, 343: "140196-1-140196-9.
[15]"WANG "Z R, "JIANG H M. Comparative Study on Characteristics and Mechanism of Levofloxacin Adsorption on Swine Manure Biochar [J]. Bioresource Technology, "2022, "351: "127025-1-127025-10.
[16]"KONG "Q P, "ZHANG H Z, "WANG P G, "et ""al. NiCo Bimetallic and the Corresponding Monometallic Organic Frameworks Loaded CMC Aerogels for Adsorbing Cu2+: "Adsorption Behavior and Mechanism [J]. International Journal of Biological Macromolecules, "2023, "244: "125169-1-125169-12.
[17]"MICHEAL "B, "IMBERTY A, "HEGGSET E B, "et al. Adsorption Characterization of Various Modified β-Cyclodextrins onto TEM PO-Oxidized Cellulose Nanofibril Membranes and Cryogels [J]. Sustainable Chemistry and Pharmacy, "2021, "24: "100523-1-100523-11.
[18]"冯琬淇, "哈尼夏·巴合提, "葛雨璇, "等. 磁性PASP/PAM半互穿水凝胶的制备及性能 [J]. 化工进展, "2023, "42(6): "3130-3137. (FENG W Q, "HANISHA B, "GE Y X, "et ""al. Preparation and Properties of Magnetic PASP/PAM Semi-interpenetrating Hydrogels [J]. Chemical Industry and Engineering Progress, "2023, "42(6): "3130-3137.)
[19]"郑云香, "高艺伦, "李宴汝, "等. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能 [J]. 化工进展, "2024, 43(8): 4542-4549. (ZHENG Y X, "GAO Y L, "LI Y R, "et ""al. Preparation and Adsorption Properties of Aaminotriacetic Anhydride Modified Porous Double-Network Hydrogels [J]. Chemical Industry and Engineering Progress, "2024, 43(8): 4542-4549.)
[20]"刘自超, "任亚男, "周文静, 等. 左氧氟沙星在铁氧化物表面的吸附: 动力学和pH的影响 [J]. 农业环境科学学报, "2023, "42(2): "362-372. (LIU Z C, "REN Y N, "ZHOU W J, "et "al. Adsorption of Levofloxacin on the Surface of Iron Oxides: "Kinetics and Effects of pH [J]. Journal of Agro-Environment Science, "2023, "42(2): "362-372.)
[21]"RAHMAN "N, "RAHEEM A. Mechanistic Investigation of Levofloxacin Aadsorption on Fe(Ⅲ)-Tartaric Acid/Xanthan Gum/Graphene Oxide/Polyacrylamide Hydrogel: Box-Behnken Design and Taguchi Method for Optimization [J]. Journal of Industrial and Engineering Chemistry, "2023, "127: "110-124.
[22]"刘桂燕, "车晖贤, "宾万艳, "等. 氮掺杂生物质炭吸附抗生素左氧氟沙星的研究 [J]. 炭素技术, "2024, "43(2): "43-48. (LIU G Y, "CHE H X, "BIN W Y, "et al. Research on the Adsorption of Antibiotic Levofloxacin by N-Doped Biochar [J]. Carbon Techniques, "2024, "43(2): "43-48.)
[23]"刘松林. 柿生物质功能材料对左氧氟沙星的吸附行为研究[D]. 桂林: 桂林电子科技大学, "2023.
(LIU S L. Study on the Adsorption Behavior of Persimmon Biomass Functional Materials for Levofloxacin[D]. "Guilin: Guilin University of Electronic Science and Technology, "2023.)
[24]"吴钦岳. 制药污泥制备生物炭吸附和催化降解左氧氟沙星的研究[D]. 无锡: 江南大学, "2023.
(WU Q Y. Study on the Adsorption and Catalytic Degradation of Levofloxacin by Biochar Prepared from Pharmaceutical Sludge[D]. Wuxi: Jiangnan University, "2023.)
[25]"王磊, "王方园, "戴胜伟, "等. 硫酸改性火山石对水中左氧氟沙星的吸附研究 [J]. 环境保护科学, "2022, "48(2): "89-95.
(WANG L, "WANG F Y, "DAI S W, "et al. Study on the Adsorption of Levofloxacin in Water by Volcanic Rocks "Modified by Sulfuric Acid "[J]. "Environmental Protection Science, "2022, "48(2): "89-95.)
[26]"AL-JABARI "M H, "SULAIMAN S, "ALI S, "et al. Adsorption Study of Levofloxacin on Reusable Magnetic Nanoparticles: "Kinetics and Antibacterial Activity [J]. Journal of Molecular Liquids, "2019, "19: "111249-1-111249-9.
[27]"LIN "L, "LI X, "SHI C L, "et ""al. Desorption of Hydrolyzed Poly(AM/DMDAAC) from Bentonite and Its Decomposition in Saltwater under High Temperatures [J]. E-Polymers, "2019, "19(1): 527-534.
[28]"刘宛宜, "王天野, "王铖熠, "等. 聚(丙烯酸酸-co-丙烯酰胺)水凝胶对阳离子染料亚甲基蓝和孔雀石绿吸附性能的研究 [J]. 分析化学, "2019, "47(11): "1785-1793. (LIU W Y, "WANG T Y, "WANG C Y, "et ""al. Study of Adsorption Properties of Cationic Dyes Methylene Blue and Malachite Green by Ploy(acrylate-co-acrylamide) "Hydrogel[J]. Chinese Journal of Analytical Chemistry, "2019, "47(11): "1785-1793.)
[29]"PIC "Y, "ANDREU V. Fluoroquinolones in Soil-Risks and Challenges [J]. Analytical and Bioanalytical Chemistry, "2007, "387(4): "1287-1299.
[30]"MAHMOUD "M E, "SAAD S R, "EI-GHANAM A M, "et al. Developed Magnetic Fe3O4-MoO3-AC Nanocomposite for Effective Removal of Ciprofloxacin from Water [J]. Materials Chemistry Physics, "2021, "257: "123454-1-123454-13.
[31]"YU "F, "LI Y, "HAN S, "et al. Adsorptive Removal of Ciprofloxacin by Sodium Alginate/Graphene Oxide Composite Beads from Aqueous Solution [J]. Journal of Colloid and Interface Science, "2016, "484: "196-204.
[32]"侯晓蕊. SBA-3的控制合成及其吸附性能研究[D]. "太原: 太原理工大学, "2022. (HOU X R. Study on Controlled Synthesis and Adsorption Property of SBA-3 [D]. Taiyuan: Taiyuan University of Technology, "2022.)
[33]"ARAUJO "C S T, "ALMEIDA I L S, "REZENDE H C, "et ""al. Elucidation of Mechanism Involved in Adsorption of Pb(Ⅱ) onto Lobeira Fruit (Solanum lycocarpum) Using Langmuir, "Freundlich and Temkin Isotherms [J]. Microchemical Journal, "2018, "137: "348-354.
[34]"SINGLA "P, "GOEL N, "SINGHAL S. Affnity of Boron Nitride Nanomaterials towards Antibiotics Established by Exhaustive Experimental and Theoretical Investigations [J]. Chemical Engineering Joumal, "2016, "299: "403-414.
[35]"HU "Y Y, "PAN C, ZHENG X H, "et al. Removal of Ciprofloxacin with Aluminum-Pillared Kaolin Sodium Alginate Beads (CA-Al-KABs): "Kinetics, "Isotherms, "and BBD Model [J]. Water, "2020, "12: "905-1-905-20.
[36]"SHADPOUR "M, "VAJIHEH B, "FERESHTEH M. Adsorptive Performance of Alginate/Carbon Nanotube-Carbon Dot-Magnesium Fluorohydroxyapatite Hydrogel for Methylene Blue-Contaminated Water [J]. Journal of Environmental Chemical Engineering, "2021, "9(2): "105170-1-105170-11.
[37]"王忠凯, "季军荣, "汤睿, "等. 双有机改性磁性膨润土对Cu(Ⅱ)和Zn(Ⅱ)的吸附 [J]. 高校化学工程学报, "2022, "36(2): "276-286. "(WANG Z K, "JI J R, "TANG R, "et "al. Preparation of Dual "Organic Modified Magnetic Bentonite for Cu(Ⅱ) and Zn(Ⅱ) "Adsorption[J]. Journal of Chemical Engineering of Chinese Universities, "2022, "36(2): "276-286.)
[38]"MO "Y Y, "VINCENT T, "GUIBAL E, "et "al. Se(Ⅵ) Sorption from Aqueous Solution Using Alginate/Polyethylenimine Membranes: "Sorption Performance and Mechanism [J]. International Journal of Biological Macromolecules, "2020, "147: "832-843.
(责任编辑: 单"凝)

