金鸡菊苷与CYP3A4/CYP2D6的结合特性和稳定性

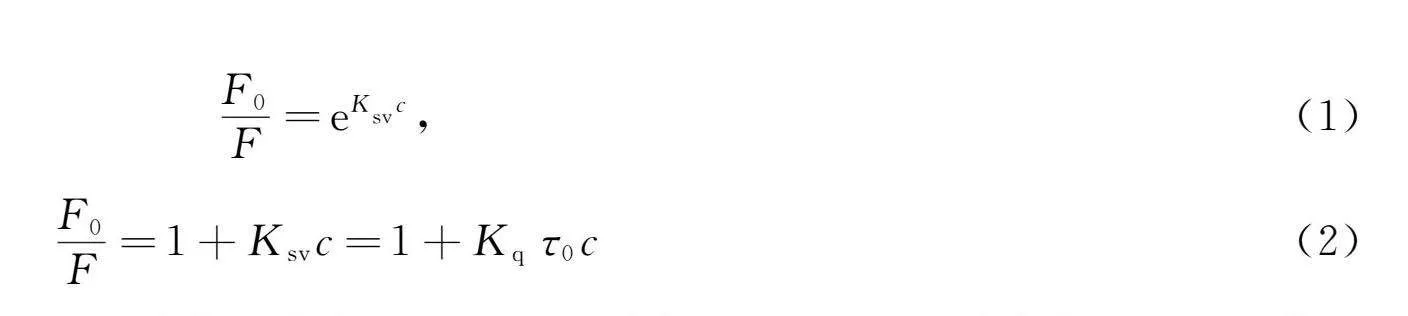






摘要: 采用光谱分析和计算机模拟技术研究金鸡菊苷与CYP3A4/CYP2D6的结合特性和稳定性. 结果表明: 金鸡菊苷以静态猝灭为主、 "动态猝灭为辅的方式猝灭细胞色素P450同工酶(CYPs)的固有荧光; 金鸡菊苷与CYP3A4的结合力大于与CYP2D6的结合力; "金鸡菊苷与CYPs发生相互作用形成复合物; 金鸡菊苷与CYPs结合, 导致CYPs二级结构发生改变; "金鸡菊苷主要通过氢键和范德华力与CYPs结合; 金鸡菊苷与两种CYPs形成的复合物稳定.
关键词: "金鸡菊苷; 细胞色素P3A4; 细胞色素P2D6; 结合特性; 稳定性
中图分类号: O65""文献标志码: A""文章编号: 1671-5489(2024)06-1491-08
Binding Characteristics and Stability of Coreopsin with CYP3A4/CYP2D6
LI Li, "LI Yuan, TAO Yanzhou, "LIAN Di, CUI Jingjing, "DU Yutong
(College of Chemistry, "Changchun Normal University, "Changchun 130032, "China)
Abstract: """The binding characteristics and stability of coreopsin with CYP3A4/CYP2D6 was studied by "using spectroscopy analysis and computer simulation techniques. The results show that the intrinsic fluorescence of cytochrome P450 proteins (CYPs) is quenched mainly by static quenching and supplemented by dynamic quenching. The binding capacity of coreopsin with CYP3A4 is greater than that of CYP2D6. The coreopsin interacts with "CYPs to form a complex. The binding of coreopsin to CYPs leads to changes in "the secondary structure of CYPs. The coreopsin mainly binds to CYPs through hydrogen bonds and van der Waals forces. The "complex formed by coreopsin and two types of CYPs is stable.
Keywords: "coreopsin; "cytochrome P3A4; "cytochrome P2D6; binding characteristics; """stability
0"引"言
CYP3A4和CYP2D6为CYP450代谢酶中两种重要亚型酶, 分别参与50%和25%以上的临床药物代谢[1-2]. 研究已证明由同一种细胞色素P450同工酶(CYP)代谢的两种以上药物同时服用, 可导致药物相互作用, 增加联合用药风险\一些黄抑制CYP3A4或CYP2D6活性而导致相互作用的研究已引起人们广泛关注、两色金鸡菊具有预防糖尿病、降血压、抗氧化和抗炎等多种生物活性,其主要活性成分为黄酮,金鸡菊背(图1)是一种来源于两色金鸡菊的黄酮类成分,研究金鸡菊苷与CYP3A4/CYP2D6相互作用对预知其与某些药物是否发生相互作用具有重要意义.
本文通过光谱分析CYP3A4/CYP2D6与金鸡菊苷结合前后的特征光谱及二级结构的变化. 利用计算机模拟金鸡菊苷与两种CYPs最佳结合模式, 及其形成复合物的稳定性. 提示金鸡菊苷与一些由CYP3A4和CYP2D6负责代谢的药物同时服用可增加药物相互作用风险.
1"实"验
1.1"材料与仪器
CYP3A4和CYP2D6购于美国Sigma公司, 金鸡菊苷购于宝鸡辰光生物科技有限公司, 其他试剂均为国产分析纯试剂.
F-7000型荧光光谱仪(日本岛津公司), MOS-500型圆二色谱仪(法国Biologic公司), Cary300型紫外-可见分光光度计(美国安捷伦公司).
1.2"方"法
1.2.1"荧光光谱
将金鸡菊苷滴入CYPs(11 μmol/L)中, 使金鸡菊苷终浓度分别为0,4.6,13.79,22.96,32.12,41.25,50.37 μmol/L. 分别于298,303,310 K孵育30 min. 荧光测定条件: 激发和发射狭缝宽度为5 nm, 激发波长为280 nm, 波长为295~450 nm. 于298 K, Δλ=15,60 nm测定同步荧光光谱, 样品浓度与荧光光谱的样品浓度相同. 扫描范围为220~400 nm, 激发波长为210~350 nm, 增量为2 nm. 测量三维荧光光谱时的CYPs和金鸡菊苷浓度分别为11,50.37 μmol/L.
1.2.2"紫外光谱
将金鸡菊苷加入CYPs(11 μmol/L), 得到与1.2.1节中相同浓度的金鸡菊苷溶液, 进行紫外光谱测试, 以相同浓度的金鸡菊苷为空白. 紫外光谱的波长为200~400 nm, 狭缝宽度为1 nm.
1.2.3"圆二色光谱
CYPs浓度为11 μmol/L, 分别配制n(金鸡菊苷)∶n(CYPs)=0∶1,4.6∶1,9.2∶1的溶液用于测定CD光谱, 采用Dichroweb计算CYPs二级结构变化.
1.2.4"计算机模拟
从PDB(protein data bank)下载CYP3A4(1TQN)和CYP2D6(3QM4)模板, "在King Draw中优化金鸡菊苷3D结构, 利用Autodock 4.0对接, 对接盒子尺寸为5.0 nm×5.0 nm×5.0 nm, 格框间距为0.037 5 nm, 对接次数为100次. 采用PyMOL(1.7.2)处理结果.
利用 Gromacs 2020.6进行动力学模拟. 选择 AMBER99SB-ILDN 和Gaff作为CYPs和金鸡菊苷力场. 建立 TIP3P模型, 建立水盒子, 添加Na+平衡体系; 将分子对接结果作为初始结构, 采用最快下降法进行最大步数(50 000步)的能量最小化. 宏观正则系综(NVT)和等温等压(NPT)平衡采用优化体系步长为2 fs, 总时长为100 ps. 模拟温度为298 K, 模拟时间为100 ns, 时间间隔为2 fs. 通过均方根偏差(RMSD)、 "均方根浮动(RMSF)和回旋半径(Rg)评估稳定性.
2"结果分析与讨论
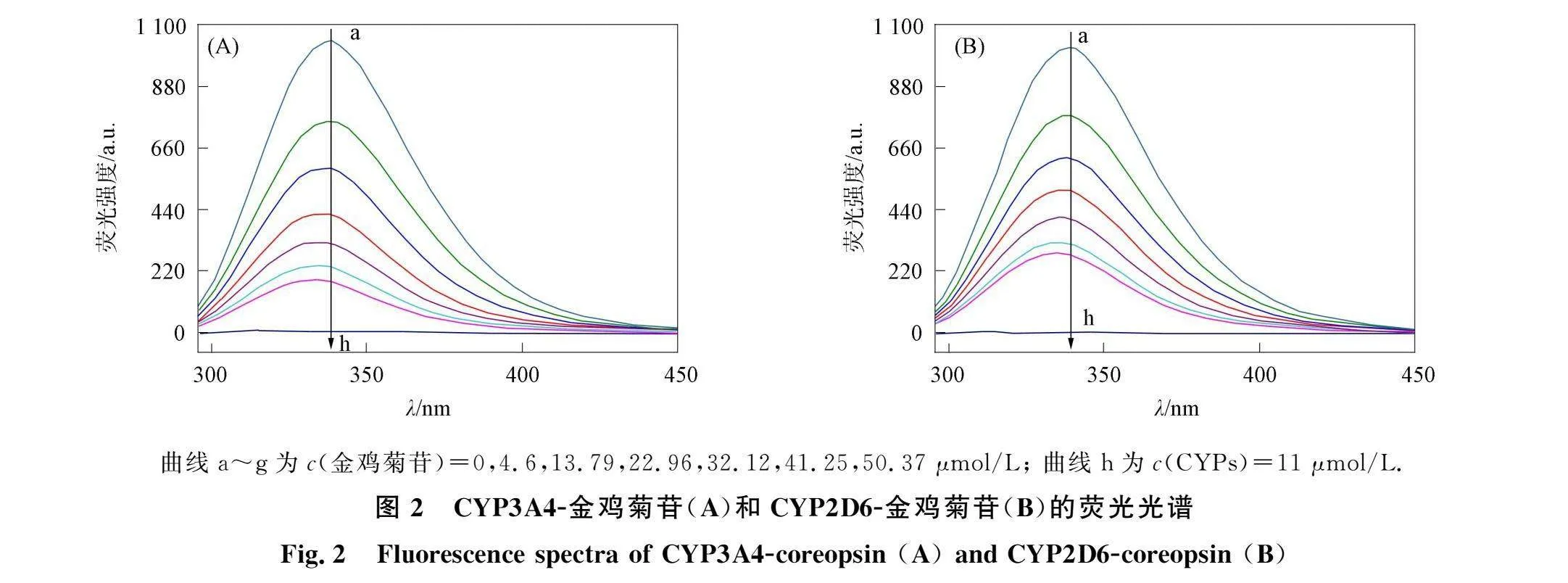
2.1"荧光猝灭机理
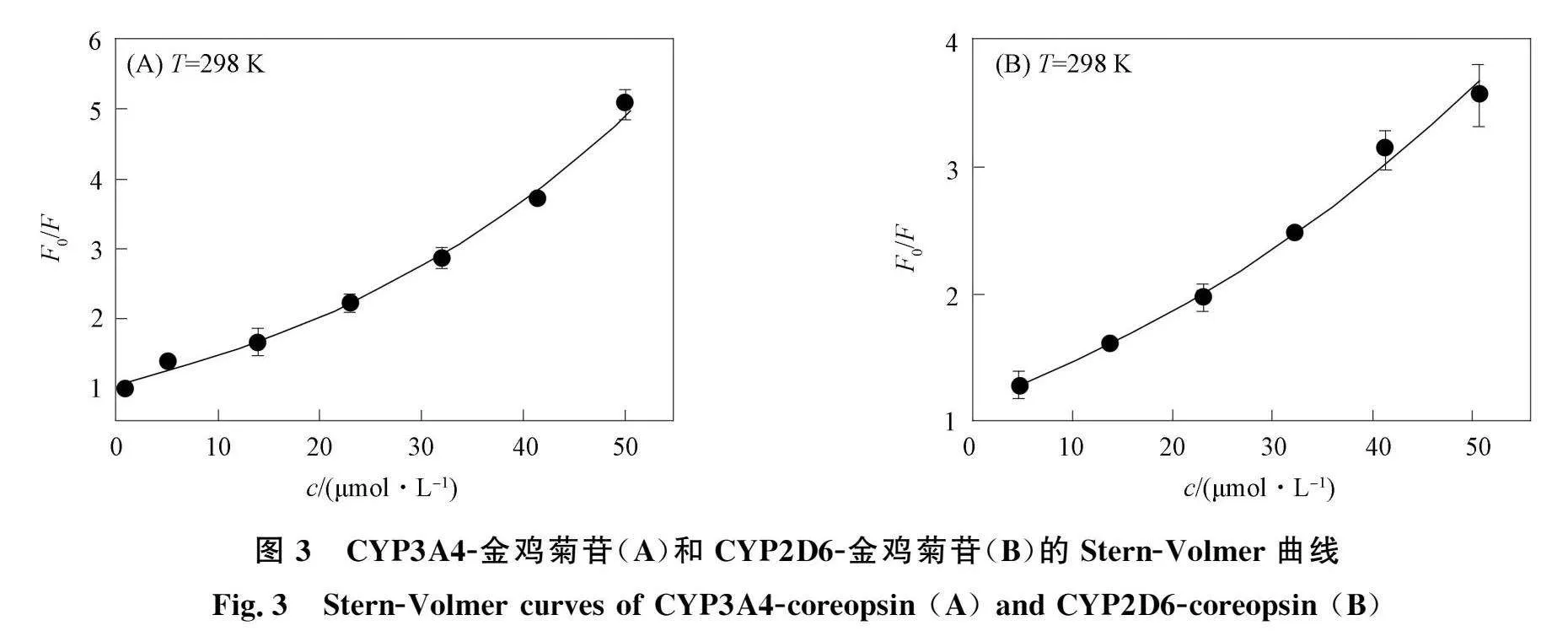
CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的荧光光谱如图2所示. 由图2可见, 两种CYPs在338 nm附近出现强荧光发射峰, 其中金鸡菊苷的荧光强度很低, 可忽略. 加入金鸡菊苷降低了CYPs的荧光强度, 并导致CYPs的峰蓝移, 表明金鸡菊苷与CYPs结合可有效猝灭CYPs内源性荧光, 使发射基团处于更疏水环境[8]. CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的Stern-Volmer曲线如图3所示. 由图3可见, 金鸡菊苷的浓度c与F0/F的曲线弯向y轴, 并向上伸展, 表明金鸡菊苷对CYPs的猝灭机制是混合型猝灭. 利用
F0F=eKsvc,(1)
F0F=1+Ksvc=1+Kqτ0c(2)
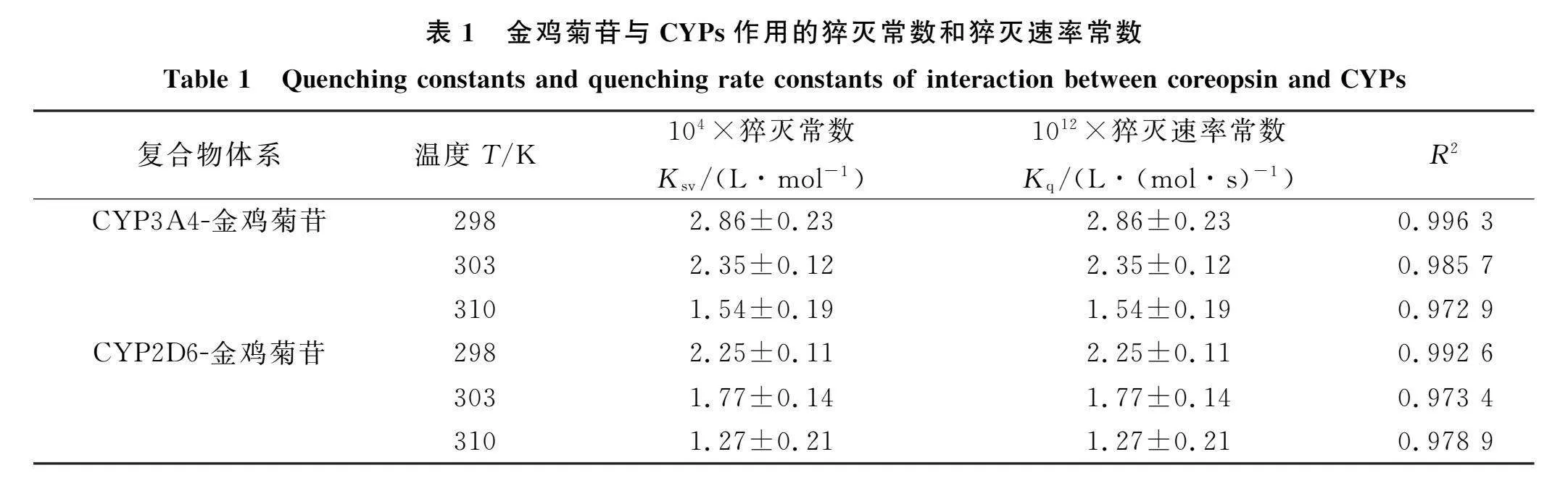
计算Ksv和Kq, 结果列于表1, "其中c为金鸡菊苷浓度, F0和F分别表示CYPs和金鸡菊苷-CYPs的荧光强度, Ksv和Kq分别表示Stern-Volmer猝灭常数和猝灭速率常数, τ0 表示CYPs平均寿命(τ0=10-8"s). 由表1可见, Ksv值随温度的升高而降低, "Kq高于最大动态猝灭常数(2×1010"L/(mols)), 表明反应体系猝灭方式主要为静态猝灭[9].
曲线a~g为c(金鸡菊苷)=0,4.6,13.79,22.96,32.12,41.25,50.37 μmol/L; 曲线h为c(CYPs)=11 μmol/L.
CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的Benesi-Hildebrand曲线和Van’t Hoff图如图4所示. 由图4可见, "1/(F-F0)与浓度的倒数呈线性相关, 与浓度平方的倒数呈非线性, 说明CYPs-金鸡菊苷的化学计量比是1∶1.
根据
1F-F0=1F1-F0+1KbF1-F0cn, (3)
ln Kb=-ΔH0RT+ΔS0R,(4)
ΔG0= ΔH0-TΔS0=-RTln Kb(5)
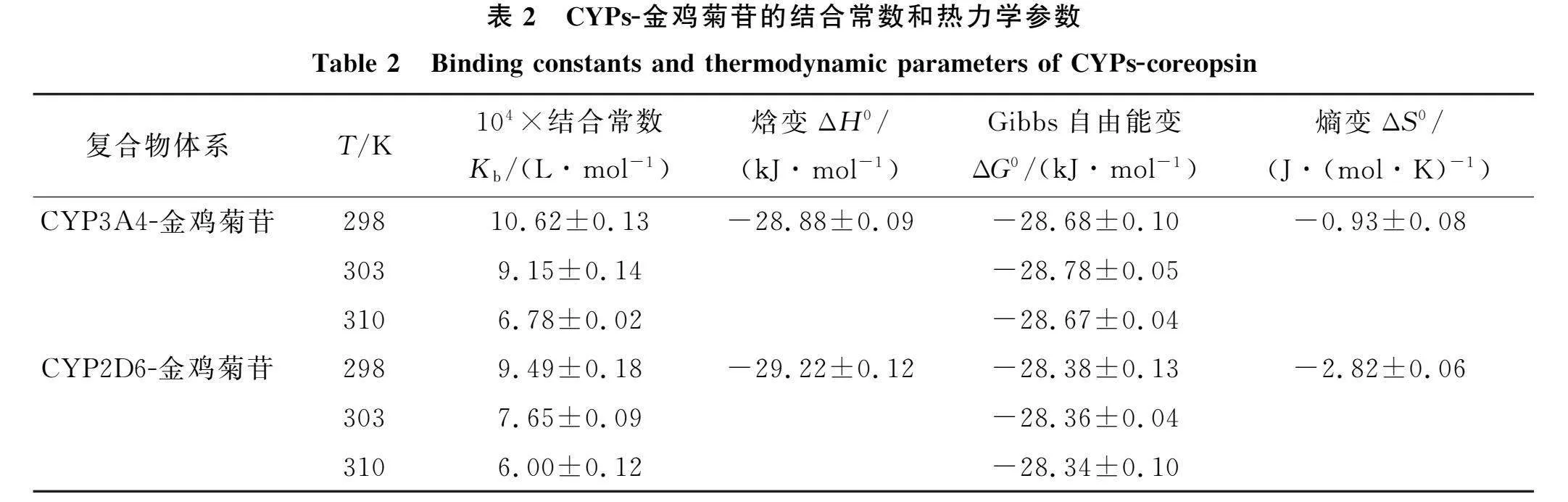
计算结合常数、 反应的Gibbs自由能变(ΔG0)、 焓变(ΔH0)和熵变(ΔS0), 计算结果列于表2. 其中, F1为金鸡菊苷过量时CYPs的荧光强度, "Kb和n表示结合常数和结合位点数, R为热力学常数, T为温度(K). 由表2可见, "Kb值在104~105"L/mol之间, 表明金鸡菊苷与CYPs间存在中等及较强的亲和力. Kb值与温度呈负相关, 说明温度升高, "CYPs-金鸡菊苷复合物的稳定性降低. CYP3A4-金鸡菊苷强于CYP2D6-金鸡菊苷的结合能力\
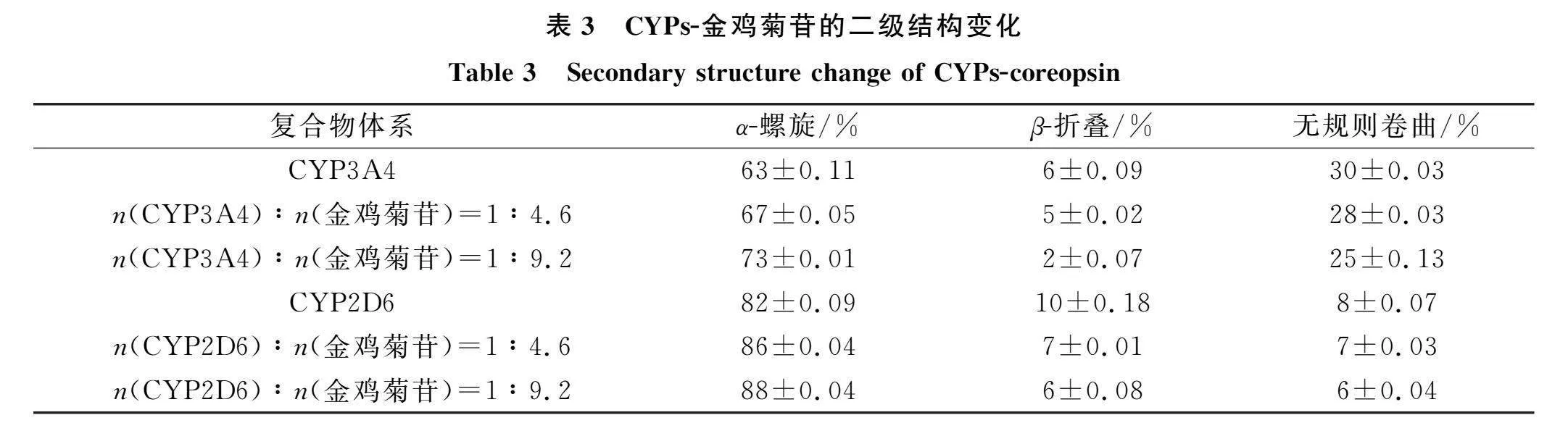
2.2"CYPs二级结构变化
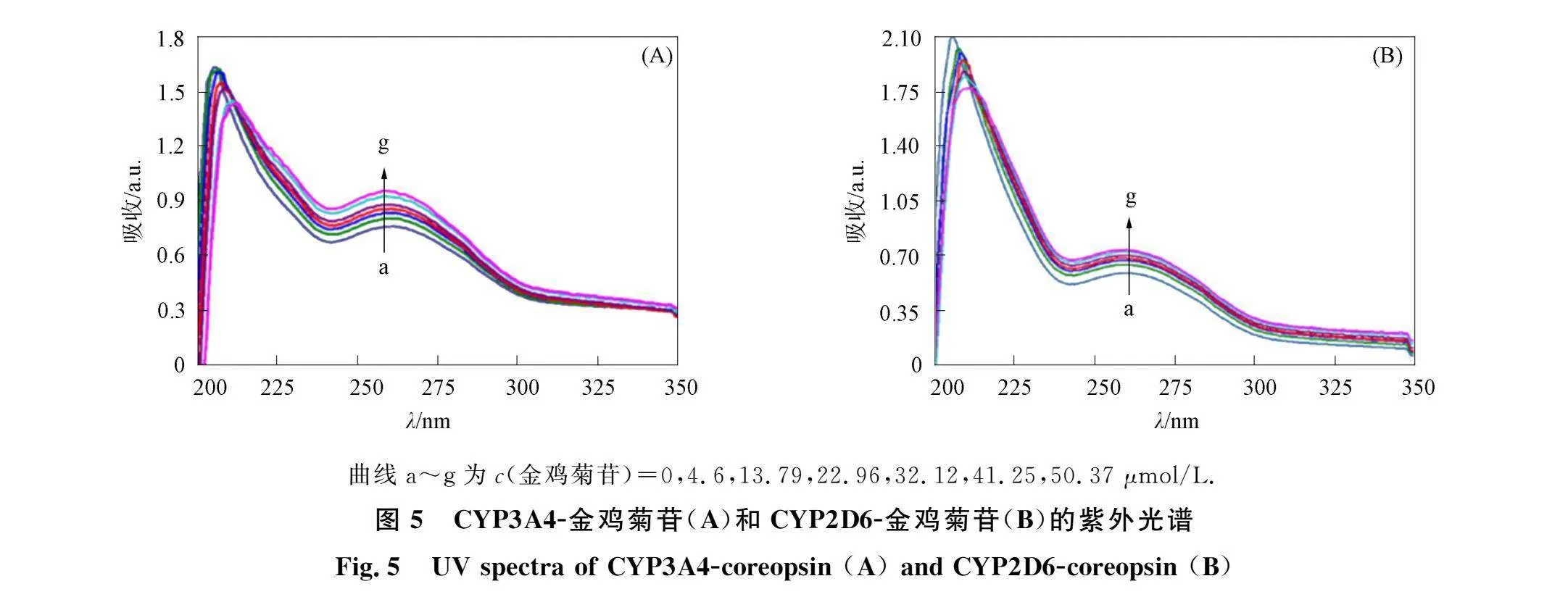
图5为CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的紫外光谱. "由图5可见, 随着金鸡菊苷浓度的增大, 位于206 nm处的峰强度下降并伴随明显红移, 位于258 nm处的峰未移动但强度显著增加. 两个复合体系的紫外光谱的变化趋势相似, "表明两种CYPs-金鸡菊苷复合物形成导致蛋白多肽链改变\
曲线a~g为c(金鸡菊苷)=0,4.6,13.79,22.96,32.12,41.25,50.37 μmol/L.
酪氨酸(Tyr)和色氨酸(Trp)为蛋白主要荧光团, 同步荧光光谱在Δλ=15,60 nm处可监测Tyr和Trp变化. 图6为CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的同步荧光光谱. 由图6可见, 随着金鸡菊浓度的增大, 两种CYPs的荧光强度有规律地降低. "Tyr和Trp发生微小蓝移, 表明Tyr和Trp残基暴露在亲水性较低的环境中. 荧光猝灭比(RSFQ)分析表明, 金鸡菊苷对CYPs荧光猝灭主要来自Trp的贡献\FF0.(6)曲线a~g的c(金鸡菊苷)=0,4.6,13.79,22.96,32.12,41.25,50.37 μmol/L.
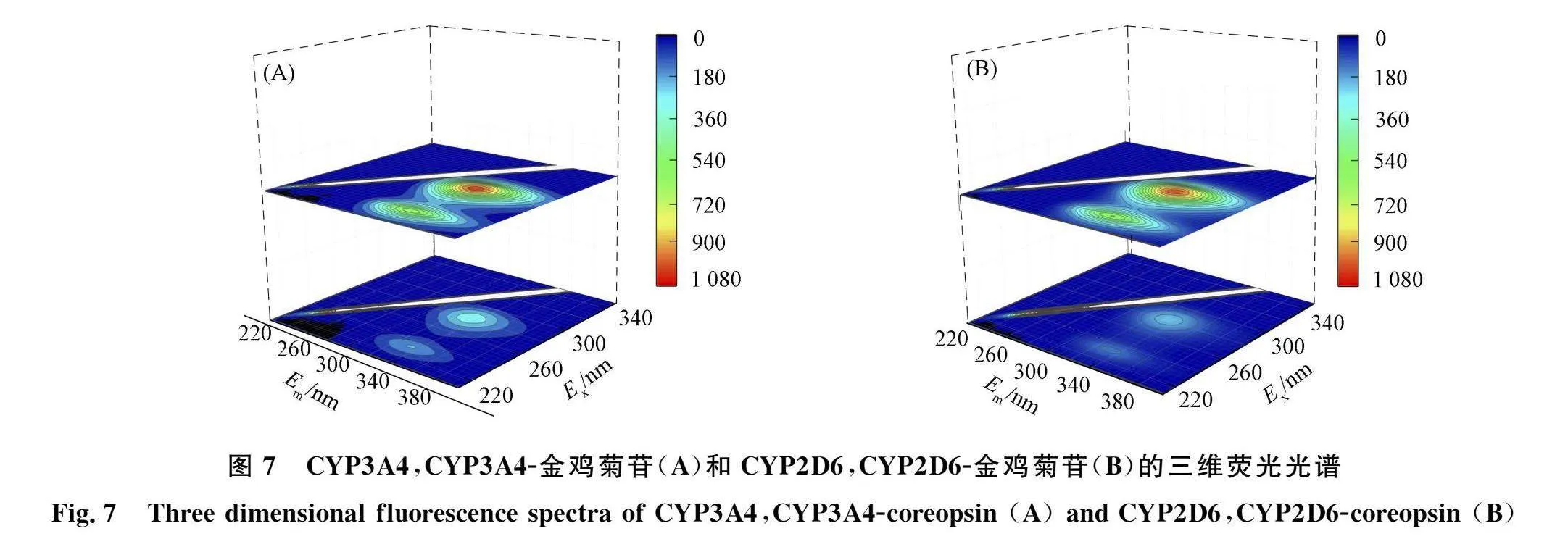
CYP3A4,CYP3A4-金鸡菊苷和CYP2D6,CYP2D6-金鸡菊苷的三维荧光光谱如图7所示. 由图7可见, 金鸡菊苷与CYPs结合后, 峰1的荧光强度显著下降并出现微小蓝移, 表明金鸡菊苷导致Trp和Tyr微环境疏水性增强. 峰2的荧光强度显著下降并发生微小蓝移, 表明金鸡菊苷和CYPs结合导致酶肽链改变.
图8为CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的CD光谱. 由图8可见, 在209,222 nm处有两条负CD谱带, 属于α-螺旋典型特征, 两条负带强度随金鸡菊苷的加入而下降. CYPs-金鸡菊苷的二级结构变化列于表3. 由表3可见, 当金鸡菊苷比例增加时, 两种CYPs的α-螺旋变化明显, 表明金鸡菊苷主要通过增加α-螺旋改变CYPs构象[13]
2.3"计算机模拟
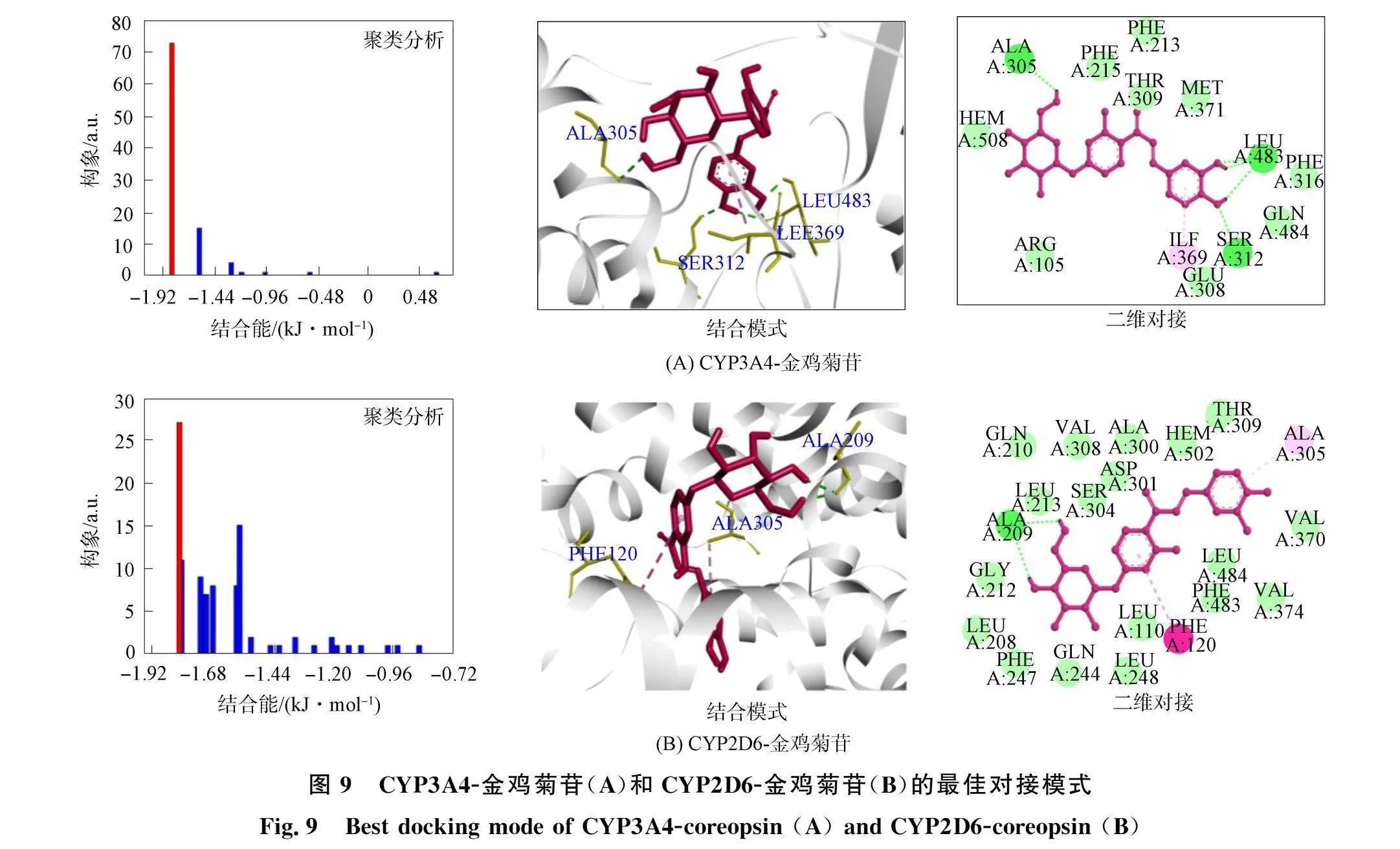
根据最多亲和构象簇和最小能量选择最佳对接模式, 结果如图9所示. CYP3A4/CYP2D6-金鸡菊苷对接能量分别为-32.65,-31.52 kJ/mol, 与荧光测量ΔG略有不同\由图9可见, 金鸡菊苷与CYP3A4中的ALA305 (0.255 nm )、 "SER312 (0.402 nm)和LEU483 (0.349,0.347,0.475 nm )形成5个氢键. 金鸡菊苷与CYP2D6中的ALA209 (0.348 nm)和ALA209 (0.351 nm)形成2个氢键. 因为ΔE2(范德华能、 氢键能和脱溶剂自由能)大于ΔE3(静电能), 表明两种复合物主要通过氢键和范德华力形成[10]
图10为CYP3A4-金鸡菊苷和CYP2D6-金鸡菊苷的模拟动力学图,由图10可见,CYPs与金鸡菊苷结合后,RMSD在0.1~0.3mm内波动,表明金鸡菊苷与CYPs形成稳定复合物,RMSF可分析蛋白柔韧性和氨基酸运动变化、CYPs与金鸡菊苷结合后,部分氨基酸残基略高于游离CYPs的RMSF值,表明金鸡菊增强了这些残基的柔韧性,而部分残基略小于游离CYPs的RMSF值,表明金鸡菊苷使这些残基的自由度降低,CYPs与金鸡菊苷结合后,在0~100ns时间内,R值在一定范围内波动,当复合物略大于游离CYPs的R,值时,说明金鸡菊使CYPs结构变松数:当复合物略小子游离CYPs的R,值时,说明金鸡使CYPs结构变得更紧密“,计算机模拟结果表明,加入金鸡菊改变了CYPs的二级结构.
综上所述, 金鸡菊苷对CYPs表现出静态猝灭为主、 动态猝灭为辅的猝灭机制; "金鸡菊苷与CYPs结合, 改变了CYPs的二级结构; 金鸡菊苷通过氢键和范德华力与CYPs形成复合物, 复合物体系稳定. 研究结果表明, 金鸡菊苷与一些由CYP3A4/CYP2D6负责代谢的药物同时服用可增加药物相互作用风险.
参考文献
[1]"WILKINSON G R. Cytochrome P4503A (CYP3A) Metabolism: "Prediction of in vivo Activity in Humans[J]. Journal of Pharmacokinetics and Biopharmaceutics, "1996, "24(5): 475-490.
[2]"INGELMAN-SUNDBERG M. "Genetic Polymorphisms of Cytochrome P450 2D6 (CYP2D6): "Clinical Consequences, "Evolutionary Aspects and Functional Diversity[J]. The Pharmacogenomics Journal, "2005, "5(1): "6-13.
[3]"WANG Y R, "WANG C X, "WANG S H, "et al. Cytochrome P450-Based Drug-Drug Interactions of Vonoprazan in vitro and in vivo[J]. Frontiers in Pharmacology, "2020, "11: "53-62.
[4]"GUO Y, "CHEN Y, "TAN Z R, "et al. Repeated Administration of Berberine Inhibits Cytochromes P450 in Humans[J]. European Journal of Clinical Pharmacology, "2012, "68(2): "213-217.
[5]"NIU L F, "DING L N, "LU C Y, et al. Flavokawain A Inhibits Cytochrome P450 in vitro Metabolic and Inhibitory Investigations[J]. Journal of Ethnopharmacology, "2016, 191: "350-359.
[6]"姚新成, 田丽萍, 秦冬梅, 等. "两色金鸡菊化学成分及生物活性研究进展[J]. 西北药学杂志, "2014, "29(6): "655-658. (YAO X C, "TIAN L P, "QIN D M, "et al. Research Advances in the Chemical Constituents and Biological Activities of Corepsis tinctoria Nutt[J]. Northwest Journal of Pharmacy, "2014, "29(6): "655-658.)
[7]"姜保平, 许利嘉, 贾晓光, 等. 两色金鸡菊的化学成分和药理作用研究进展[J]. 现代药物与临床, 2014, 29(5): "567-573. (JIANG B P, "XU L J, "JIA X G, "et al.Research Progress on the Chemical Constituents and Pharmacological Activities of Coreopsis tinctoria [J]. Modern Drugs amp; Clinic, "2014, 29(5): "567-573.)
[8]"XIONG G Y, "GAO X Q, "WANG P, "et al. Comparative Study of Extraction Efficiency and Composition of Protein Recovered from Chicken Liver by Acid-Alkaline Treatment[J]. Process Biochemistry, "2016, "51(10): "1629-1635.
[9]"REN S C, "LI K K, "LIU Z L, "et al. Research on the Influences of Five Food-Borne Polyphenols on in vitro Slow Starch Digestion and the Mechanism of Action[J]. Journal of Agricultural and Food Chemistry, "2019, "67(31): "8617-8625.
[10]"卫莺. 有机锡抗癌化合物与CYP3A4代谢酶的相互作用[D]. 太原: "山西医科大学, "2017.
(WEI Y. Interaction of Organotin Anticancer Compounds with CYP3A4 Metabolic Enzymes[D]. Taiyuan: "Shanxi Medical University, "2017.)
[11]"ZHU M Q, "WANG L J, "WANG Y, "et al. Biointeractions of Herbicide Atrazine with Human Serum Albumin: "UV-Vis, "Fluorescence and Circular Dichroism Approaches[J]. International Journal of Environmental Research and Public Health, "2018, "15(1): "116-1-116-16.
[12]"METI M D, "XU Y, "XIE J F, "et al. Multi-spectroscopic Studies on the Interaction between Traditional Chinese Herb, "Helicid with Pepsin[J]. Molecular Biology Reports, "2018, "45(6): "1636-1646.
[13]"XU Y J, "DAI T T, "HUANG K C, "et al. Analyses on the Binding Interaction between Rice Glutelin and Conjugated Linoleic Acid by Multi-spectroscopy and Computational Docking Simulation[J]. Journal of Food Science and Technology, "2020, "57(3): "886-894.
[14]"ZENG H J, "HU G Z, "YOU J, "et al. Spectroscopic and Molecular Modeling Investigation on the Interactions between Hyaluronidase and Baicalein and Chrysin[J]. Process Biochemistry, "2015, "50(5): "738-745.
[15]"SHAO Y X, "ZHAO P, "LI Z, "et al. The Molecular Basis for the Inhibition of Human Cytochrome P450 1A2 by Oroxylin and Wogonin[J]. European Biophysics Journal, "2012, "41(3): "297-306.
[16]"BHARDWAJ P, "BISWAS G P, "MAHATA N, "et al. Exploration of Binding Mechanism of Triclosan towards Cancer Markers Using Molecular Docking and Molecular Dynamics[J]. Chemosphere, "2022, "293: "133550-1-133550-10.
[17]"PAUL S K, "SADDAM M, "RAHAMAN K A, "et al. Molecular Modeling, "Molecular Dynamics Simulation, "and Essential Dynamics Analysis of Grancalcin: "An Upregulated Biomarker in Experimental Autoimmune Encephalomyelitis Mice[J]. Heliyon, "2022, "8(10): "e11232-1-e11232-14.
[18]"WU X Q, "ZHANG G W, "HU M M, "et al. Molecular Characteristics of Gallocatechin Gallate Affecting Protein Glycation[J]. Food, Hydrocolloids, "2020, "105: "105782-1-105782-11.
[19]"ZHOU B J, "ZHOU H, "XU L N, "et al. An Insight into the Interaction between Indisulam and Human Serum Albumin: "Spectroscopic Method, "Computer Simulation and in vitro Cytotoxicity Assay[J]. Bioorganic Chemistry, "2022, "127: "106017-1-106017-13.
(责任编辑: 单"凝)

