Negative thermal expansion and phase transition of low-temperature Mg2NiH4
Qun Luo ,Qi Ci ,Qinn Gu ,Yu Shi ,Bin Liu ,Xun Quy Trn ,Syo Mtsumur,Tong-Yi Zhng,Kzuhiro Nogit,To Lyu,Qin Li,,*,Fushng Pn
a State Key Laboratory of Advanced Special Steel &Shanghai Key Laboratory of Advanced Ferrometallurgy &School of Materials Science and Engineering,Shanghai University, Shanghai 200444, China
b Materials Genome Institute, Shanghai University, Shanghai 200444, China
c Australian Synchrotron, ANSTO, 800 Blackburn Road, Clayton, Victoria 3168, Australia
d Department of Applied Quantum Physics and Nuclear Engineering, Kyushu University, Fukuoka 819-0395, Japan
e Nihon Superior Centre for the Manufacture of Electronic Materials (NS CMEM), School of Mechanical and Mining Engineering, The University of Queensland, Brisbane, Queensland 4072, Australia
fNational Engineering Research Center for Magnesium Alloy, Chongqing University, Chongqing 400044, China
Abstract The negative thermal expansion (NTE) phenomenon is of great significance in fabricating zero thermal expansion (ZTE) materials to avoid thermal shock during heating and cooling.NTE is observed in limited groups of materials,e.g.,metal cyanides,oxometallates,and metalorganic frameworks,but has not been reported in the family of metal hydrides.Herein,a colossal and continuous negative thermal expansion is firstly developed in the low-temperature phases of LT1-and LT2-Mg2NiH4 between 488 K and 733 K from in-situ transmission electron microscope (TEM) video,with the volume contraction reaching 18.7% and 11.3%,respectively.The mechanisms for volume contraction of LT1 and LT2 phases are elucidated from the viewpoints of phase transformation,magnetic transition,and dehydrogenation,which is different from common NTE materials containing flexible polyhedra units in the structure.The linear volume shrinkage of LT2 in the temperature of 488–553 K corresponds to the phase transition of LT2→HT with a thermal expansion coefficient of–799.7 × 10–6 K–1 revealed by in-situ synchrotron powder X-ray diffraction.The sudden volume contraction in LT1 between 488 and 493 K may be caused by the rapid dehydrogenation of LT1 to Mg2Ni.The revealed phenomenon in single composite material with different structures would be significant for preparing zero thermal expansion materials by tuning the fraction of LT1 and LT2 phases.
Keywords: Negative thermal expansion;Mg2NiH4;Phase transformation;In situ TEM;In situ XRD.
1.Introduction
Negative thermal expansion (NTE) materials can be assembled with materials exhibiting positive thermal expansion(PTE) to fabricate zero thermal expansion (ZTE) composites.The ZTE composites are able to avoid thermal shock during rapid heating or cooling in the fields of aerospace,optical components,medical instrument,etc.[1–3].The well-known phenomena of NTE from everyday life include the allotropic transformation of the metal tin (tin pest) and the liquid water between 273 K and 277 K.However,it is the discovery of ZrW2O8[4],with a coefficient of thermal expansion (CTE)of–27.3 × 10–6K–1,that enables the NTE materials to attract prominent attention.In the last two decades,the NTE phenomena have covered the areas of ferroelectrics,magnetic materials,semiconductors,and superconductors [5–7].Among the existing NTE materials,those demonstrating giant NTE are of fundamental interest for their potential and flexibility in fabricating ZTE composites [8,9].Azuma et al.[10]discovered colossal NTE in Bi0.95La0.05NiO3,associated with metal-insulator transition,and volume change was 2.6%with a CTE of–137×10–6K–1.A strong coupling between magnetism and lattice may also lead to large NTE,and the CTE of CuO nanoparticles is–110×10–6K–1[11].Up to now,some of the largest volume shrinkages include 6.7% in layered ruthenate [12]and 7.9% in Pb0.8Bi0.2VO3polycrystalline material [13].Their large volume shrinkages are adaptive for offsetting a wide range of volume expansion from diverse PTE materials.To date,the NTE phenomena are only discovered in limited groups of materials,e.g.,metal cyanides,oxometallates and metal-organic frameworks,following the mechanisms associated with phase transition,rotation of octahedra,and magnetic transition[14–19].There are no reports on the NTE phenomena in the family of metal hydrides.
Mg alloys have attracted wide attention for their high specific strength and stiffness,large hydrogen storage capacity,and high theoretical specific capacity for batteries [20–23].The metal hydride Mg2NiH4is commonly used as a hydrogen storage medium [24–27].It crystallizes in a cubic structure at a high temperature phase (HT) above 510 K and undergoes a phase transition into two low-temperature variants[28],un-twinned LT1 (monoclinic,space group: C2/c) and twinned LT2 (orthorhombic,space group: Ia) [29,30]when it is cooled to the temperature below 510 K.Previous studies demonstrated that the LT1 phase shows metallic-like electrical conductivity,while the LT2 phase is an insulator [31].In addition,a diverse combination of the LT1 and LT2 variants could lead to a variation in conductivity,color,and hydrogen desorption rate of the mixture [29,32-34].The Mg2NiH4powder with a large amount of LT1 phase exhibited a gray color and a high hydrogen desorption rate.By contrast,an increased amount of LT2 in the mixture results in an orange color and substantially decreases the desorption rate of hydrogen [32].The differences in the physical properties of the Mg2NiH4composites are attributed to the difference in the structure of the LT1 and LT2 phases.
Here,a giant negative thermal expansion in the LT2-Mg2NiH4phase with an orthorhombic structure was directly observed by ultra-high voltage transmission electron microscopy (UHV-TEM),while positive thermal expansion was found in the LT1-Mg2NiH4phase with a monoclinic structure.In-situ synchrotron powder X-ray diffraction (SRPXRD) experiments and magnetic measurements were employed to examine the phase transformation and magnetic transition to determine the mechanism of thermal expansion/contraction.In addition,in view of the specific physical properties of Mg2NiH4corresponding to a certain combination of LT1 and LT2 phases,it is prospective to obtain controllable thermal expansion composites by tuning the LT1/LT2 ratio in the Mg2NiH4alloy.The rational design and fabrication of Mg2NiH4with optimized composition paves a critical way to unlock its potential for ZTE applications.
2.Experimental details
High-purity stoichiometric Mg2NiH4of LT1 intermetallic phase was obtained from Mg2Ni powder (containing~5.1 wt.% MgNi2minor phase) with the particle size of 75 μm.To prepare LT1-Mg2NiH4phase,the pristine Mg2Ni powder was activated at 623 K for 5 cycles by absorption of hydrogen at a pressure of 5 MPa and desorption of hydrogen in vacuum.The absorption time was 2 h and the desorption time was 1 h for each cycle.The powder was finally dehydrogenated,and the temperature was then lowered to 400 K in 5 h.When the temperature was stable,hydrogen was carefully added at a pressure of 5 MPa.The high-purity LT1-Mg2NiH4powder was obtained after raising to 450 K and preserving for 48 h.
High-purity stoichiometric Mg2NiH4of LT2 intermetallic phase was obtained from ball milled Mg2Ni powder.The rotation speed was 700 rpm and the ball to powder weight ratio was 20: 1.After milling for 15 h in an Ar atmosphere,the Mg2Ni powder was hydrogenated at 623 K for 2 h with the H2pressure of 2 MPa.The LT2-Mg2NiH4was then obtained by cooling the powder to room temperature.A phenomenon which is worthy to notice is that the XRD pattern of LT2-Mg2NiH4powder is almost not changed after preserving in air for 21 days (Supplementary Fig.S1),indicating the powder is insensitive to the air and moisture.
To alleviate sample damage by electron beam,in-situ ultra-high vacuum transmission electron microscope (UHVTEM) observation of the Mg2NiH4powder was performed using a JEM-1300NEF (JEOL,Japan) at an acceleration voltage of 1250 kV with an EM-F00113 (JEOL,Japan) heating holder and high-resolution video recorder under a vacuum of 1.4 × 10–6Pa.Since Mg2Ni powder was used for the homogeneous absorption of hydrogen,TEM was used to measure the size of a particle that may include void space/cracks.For in-situ synchrotron powder X-ray diffraction (SR-PXRD) experiments,the sample was measured under vacuum (1.0 × 10–2Pa) and heated from 300 to 733 K with a hot air blower (at the powder diffraction beamline,Australian Synchrotron,ANSTO).The X-ray beam wavelength was 0.7754 ˚A,and the heating rate was 50 K·min–1.While the sample was isothermally held at each measuring temperature for 5 min,the corresponding diffraction pattern was simultaneously recorded.Rietveld refinement was conducted with TOPAS 5.0 software (Bruker-AXS) to obtain the crystal structures of the LT1 and LT2 phases.The following crystallographic information files (CIFs) were used: #162,411(Mg2Ni,P6222),#151,374(MgNi2,P63/mmc),#162,412(HT Mg2NiH4,Fm-3 m),#201,606 (LT1 Mg2NiH4,C2/c).A proposed structure for LT2 Mg2NiH4(Pcc2) by Cuevas et al.[30]was used as the starting model.The Mg2NiHx(x=0–0.3) has the same structure type but with a larger unit cell compared to Mg2Ni [35].Temperature dependence of magnetization for LT1-and LT2-Mg2NiH4is measured by a physical property measurement system(Quantum Design PPMS-9).The applied field is 0.5 T,and the temperature range is 300–573 K.Magnetic hysteresis loops are measured at 300 K to determine the room-temperature magnetism of the two phases.
3.Results
3.1. Negative thermal expansions of LT1 and LT2-Mg2NiH4
The overall area variation in the temperature range of 300–773 K for LT1-and LT2-Mg2NiH4is illustrated in Fig.1a,according to the recorded video under TEM (the TEM image shows the projection area of particles).The screenshots of the video are given in Supplementary Fig.S2 and S3.In order to determine the area change ratio,the original area of the particles at 323 K is used as the reference (S0) and the area change is determined to be 100.8%S0and 102.3%S0for the LT1 and LT2 particles at 488 K,respectively,indicating the small thermal expansion of volume.However,both LT1 and LT2 particles show a total negative thermal expansion from 488 to 773 K.

Fig.1.(a) Area change of LT1 and LT2 particles determined from TEM images in the temperature range of 300–723 K,(b) the calculated volume change of LT1 and LT2 particles (The coefficient of thermal expansion (CTE), β,is indicated in this figure),(c) the CTE of LT1 and LT2 compared with other typical metals [36–41]and compounds [42–44].(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)
An overall NTE phenomenon was observed in the LT2 particles at the temperature of 488–723 K.An almost linear shrinkage of 3.5% S0occurred between 488 and 553 K,and then two significant decreases in area of about–2.7% and–1.2% S0were observed near 553 K and 608 K,respectively.When the temperature was over 613 K,the LT2 phase displayed a continuous NTE again.The coefficient of thermal expansion (CTE,β) can be defined as
whereΔVandΔTare the changes in volume and temperature,respectively.Therefore,the area change ratio is converted to volume change based on the following equation,assuming that the particle is a cubic in order to simplify the calculation
whereS0andSare the area at 323 K and any other temperature determined from TEM images.The volume change ratio is shown in Fig.1b.It suggests that the two linear volume shrinkages occur at 488–553 K and 613–723 K in LT2 particles with the thermal expansion coefficientβ1=–799.7 × 10–6K–1andβ2=–85.7 × 10–6K–1.This is the first observation of metal hydride with the total contraction reaching 11.3% V0for the LT2 phase,which is comparable to the largest negative thermal expansion value reported in the perovskite compound containing octahedra units [13].
An abrupt shrinkage of 18.7% V0occurred in the LT1 particle at 493 K,but contrary to the behavior in LT2,the volume of the LT1 particle began to increase progressively when the temperature was raised from 493 to 723 K.The positive CTE wasβ3=33.4 × 10–6K–1for LT1 phase at this temperature range which is comparable to the CTEs for common metals[36–41](magnesium,aluminum and titanium,as listed in Table S1 in Supplementary material).
Fig.1c shows the CTEs for several metals and typical NTE materials compared with the CTEs of LT1 and LT2.It is interesting that the LT1 and LT2 phases possess opposite thermal expansion behaviors in the temperature range of 493–723 K,although both phases exhibit an NTE phenomenon as a whole during the heating process.The LT2 shows a much larger CTE than typical NTE materials in a large temperature range of 503–723 K [42–44].
It is worth noting that the contraction seems to be reversible,as shown in the TEM images in Supplementary Fig.S4 and S5 where the volumes of LT1 and LT2 particles almost recovered to their original volume when they were cooled down to room temperature.The area of the LT1 particle after heating is 1.6% larger than that of the particle before heating,and the LT2 particle is 1.1% larger.The reversible phase transformation of LT-and HT-Mg2NiH4is also observed in the literature [35,45].Since the LT1 and LT2 alloy do not contain a framework structure,the observed NTE behavior may be related to phase transformation,magnetic transition,or dehydrogenation.
3.2. Phase transformation upon heating process
Fig.2a–c shows the TEM images of a LT1 particle,taken at 300 K,493 K,and 733 K,accompanied by the corresponding selected area electron diffraction (SAED) patterns(Fig.2d–f) during the in-situ observation.493 K is the critical temperature right after abrupt shrinkage in the LT1 particle.However,polycrystalline diffraction rings of the LT1 phase were identified for both particles at 300 K and 493 K indicating that phase transformation of LT1→HT or dehydrogenation reaction hardly occurs below 493 K.A few diffraction rings of LT1 are missing in the patterns at 493 K due to the volume contraction that altered the orientation of the planes.Hence,the NTE phenomenon of LT1-Mg2NiH4at 493 K originates possibly from the structural change rather than the phase transformation.The TEM image of the LT1 particle at 733 K is shown in Fig.2c,from which dehydriding pores are recognized,and the diffraction rings of Mg2Ni are identified in the corresponding SAED patterns,indicating the LT1 phase has partially released hydrogen.The rings of the MgO phase are due to the minor phase of MgO thin film on the surface of the LT1 particle,which is inevitable during fabrication.

Fig.2.TEM images of an LT1-Mg2NiH4 particle at (a) 300 K,(b) 493 K,and (c) 733 K.The SAED patterns at (d) 300 K,(e) 493 K,and (f) 733 K correspond to the images of (a–c).The original area of the particles is indicated as S0 in a.The gray area is the reseaux on the Cu mesh in (a–c).The solid line in (a) is the border of the LT1 particle.The dash lines in (b) and (c) are the borders of the particle in (a).
A similar phenomenon was observed in the LT2 phase as well.Fig.3a–c shows the TEM images and the corresponding SAED patterns of LT2-Mg2NiH4particles,taken at 300 K,553 K,and 733 K.The slow volume shrinkage occurred from 483 K,and the area reduction of the particle was 3.9% at 553 K in comparison with theS0at 300 K.Diffraction rings and a set of single-crystal diffraction spots of LT2 are shown in the SAED patterns of Fig.3e.The thermal contraction of the particle causes the orientation change of grains and randomly satisfy the Bragg condition [35].Diffraction rings of the HT phase are included in the patterns taken at 553 K,indicating a phase transformation of LT2→HT occurred prior to this shrinkage.These rings of the HT phase disappeared at 733 K as shown in Fig.3f,accompanied by the emergence of those of the Mg2Ni phase,suggesting a dehydriding process of HT→Mg2Ni in the temperature range of 553 to 733 K.Therefore,it can be inferred that the negative thermal expansion of LT2 at 488–553 K originates from the phase transformation of LT2→HT,and the volume change at 553–733 K is related to the dehydriding.
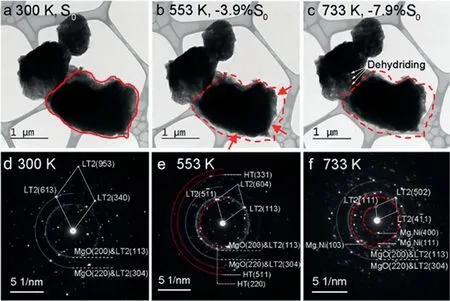
Fig.3.TEM images of LT2-Mg2NiH4 particles at (a) 300 K,(b) 553 K,and (c) 733 K.The SAED patterns at (d) 300 K,(e) 553 K,and (f) 733 K correspond to the images of (a–c).The original area of the particle is indicated as S0 in (a).The gray area is the reseaux on the Cu mesh in (a–c).The solid lines in (a)are the border of the LT2 particle.The dash lines in b and c are the borders of the particle in (a).
In order to find out the detailed phase transformation process during the heating process,in-situ SR-PXRD patterns of LT1-and LT2-Mg2NiH4samples were collected as shown in Fig.4.Two stages are exhibited upon the heating process in the LT1 sample and the phase transformation relates to dehydrogenation at 513 K.However,a multi-stage transition appeared in the LT2 sample,involving an obvious low temperature (LT)-high temperature (HT) phase transition starting at 493 K and dehydrogenation starting at 533 K.There is no phase transition and dehydrogenation from 323 to 488 K for both LT1 and LT2 samples (as shown in Fig.S6).In addition,the mass spectrometer did not detect any H2gas release from the end of the sample capillary before the high temperature phase transformation during the in-situ PXRD setup.
The Rietveld refinement results of in-situ SR-PXRD patterns of the LT1 sample are shown in Fig.5a–c,which provides the phase change close to the critical temperature.Fig.5d shows the evolution of phase constituents with the increase of temperature.There are two phases,LT1 and MgNi2,below 503 K.MgNi2is a minor impurity phase in the pristine Mg2Ni powder,which does not absorb hydrogen at the current experimental condition [46].A small amount of LT1(~9.4 wt.%) transformed to HT at 513 K,and meanwhile,the LT1 phase released hydrogen to form Mg2Ni (with a little solid solution of H).The LT1 was depleted at 553 K,while the formed HT continued to desorb hydrogen until 603 K.The SR-PXRD results have good consistency with in-situ TEM results that LT1→HT and dehydrogenation reactions begin above 493 K.However,the TEM results illustrated that the NTE occurred before the phase transition and dehydrogenation.
The Rietveld refinement results of in-situ SR-PXRD patterns of the LT2 sample are shown in Fig.6a–e,which provides the phase change below and above the critical temperatures of 493 K,533 K and 683 K.Fig.6f shows the evolution of phase constituents with the increase of temperature.It contains 29.1 wt.% LT1 and 6.9 wt.% MgNi2in LT2 samples at room temperature,which are the impurity phases for this sample.High purity LT2 samples can hardly be obtained because LT1 is more stable than LT2 in the conventional hydrogenation process.When the temperature is heating to 493 K,both LT2 and LT1 start to transform to HT which can be seen from the suddenly sharp diffraction peaks marked by pink arrows as shown in Fig.6b.LT1 almost disappeared at 553 K,but LT2 still existed until 683 K,as shown in Fig.6f,which indicates that LT2 is more stable than LT1 at high temperature.The Mg2Ni emerged at 533 K and the increasing intensity of its diffraction peaks are marked by purple arrows in Fig.6c and d,which indicates the dehydrogenation of LT2 and HT.The temperature of hydrogen release is higher than that of the LT1 sample (513 K),which also suggests the good stability of LT2 at high temperatures.The dehydrogenation reaction of LT2 and HT continued until 683 K.The comprehensive phase transition and dehydrogenation reaction in the LT2 sample occurred during the temperature range of 493–723 K,which may contribute to the negative thermal expansion of LT2.
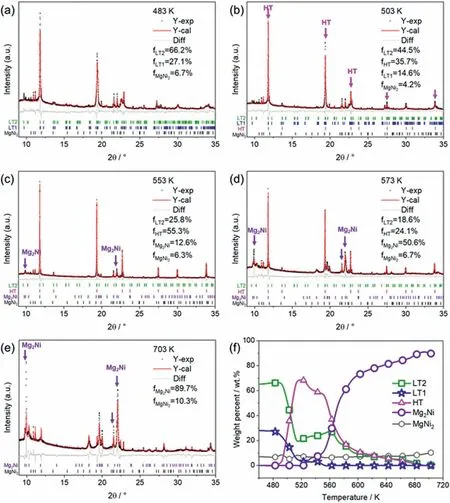
Fig.6.Rietveld refinement results of the SR-PXRD patterns for LT2-Mg2NiH4 at (a) 483 K,(b) 503 K,(c) 553 K,(d) 573 K,and (e) 703 K and (f) the evolution of phase constituent (wt.%) with the increase of temperature.
3.3. Magnetic transition upon heating process
The temperature dependence of inverse magnetic susceptibility,χ–1,for the LT1 and LT2 powder is illustrated in Fig.7a.A magnetic transition occurred at 493 K and 553 K for LT1 and LT2 powder,respectively.The room-temperature magnetic hysteresis loops for the LT1 and LT2 powder are shown in Fig.7b and c.Both phases exhibited ferromagnetism at room temperature.The unsaturated magnetization at high magnetic fields is due to the paramagnetic signal from the MgNi2impurity phase [47].Moreover,the segments of theχ–1/Tcurves at the temperature over 530 K and 579 K for LT1 and LT2,respectively,are well fitted by the Curie-Weiss law.The equation of the Curie-Weiss law is

Fig.7.Magnetic properties of LT1 and LT2 powder as a function of temperature:(a)Temperature dependence of inverse magnetic susceptibility,χ–1,for the LT1 and LT2-Mg2NiH4 powders.Fitting curves are based on the Curie-Weiss law.Magnetic hysteresis loop of (b) LT1-and (c) LT2-Mg2NiH4 powder at 300 K.
whereCis the Curie constant,andθpis the Weiss temperature.The solid lines in Fig.3a are the fitting curves of theχ-1data by the Eq.(3) atT>530 K for LT1 andT>579 K for LT2.The curie constantCis fitted to be 4.2 × 10–5and 1.2 × 10–3emu·K·mol-1for polycrystalline LT1 and LT2,respectively.
The downturn deviation from the Curie–Weiss law observed below 493 K and 553 K for LT1 and LT2,respectively,also proves the magnetic transition.Hence,it is suggested a magnetic transition occurred in both LT1 and LT2 phases when the temperature increased,and the onset transition temperatures were 493 K and 553 K,respectively.It is reasonable to assume that the NTE phenomenon has a magnetic origin,as the temperature of the sudden volume contraction coincided with that of the magnetic transition.
4.Discussion
The phase transformation upon heating process of LT1 seems ordinary only containing LT1→Mg2Ni and LT1→HT from 513 K which is higher than the temperature (493 K)of the abrupt volume shrinkage in TEM images.The inconsistence between the two temperatures suggests the sudden NTE may be independent of the phase transformation.However,the agreement of the magnetic transition temperature of LT1 with that of the abrupt volume shrinkage indicates that the NTE phenomenon of LT1 may be highly coupled with the magnetic transition.The low-temperature ferromagnetic configuration possesses ordered spins,while the spins are disordered in the high-temperature paramagnetic configuration.The disappearance of the net magnetic moment could reduce the volume [48].Such magneto-volume effect (MVE),a change in volume associated with the variation of magnetic moments,has been reported in diversified systems containing magnetic elements,including R2Fe17(R=rare earth),La(Fe,Si,Co)13,and Mn3AN (A=Ga,Zn,Cu,etc.).Typical R2Fe17alloys,Y2Fe17and Pr2Fe17,with hexagonal and rhombohedral structures,respectively,are ferromagnetic below the magnetic ordering temperature,and the NTE phenomenon occurs at this temperature[49,50].The NTE in antiperovskite Mn3AN originates from the ferro-/paramagnetic or antiferro-/paramagnetic transitions,in which strong spin-lattice coupling plays important roles [51].And the NTE phenomenon in the La(Fe,Si)13-type alloys follows this mechanism as well [52].However,there is still a mystery that the cell volume of LT1 shows a slight increase with the temperature rising from 483 to 513 K and an extensive increase from 513 to 543 K,as shown in Fig.8a.That is to say,no reduction of lattice parameters is accompanied by the ferro-/paramagnetic transition.Therefore,the large sudden NTE for LT1 needs to be further investigated.

Fig.8.Lattice parameters of (a) LT1 cell at 483–543 K,(b) LT2 cell at 483–683 K,(c) HT cell in LT2 sample at 493–683 K,and (d) Mg2Ni cell in LT2 sample at 533–703 K.
The situation is complicated for LT2 because it contains multiple reactions from 493 to 703 K,such as LT2→HT,LT2→Mg2Ni and HT→Mg2Ni.To study the structural change in the heating process,the cell volume and lattice parameters of LT2 and relevant phases around the temperature of NTE are calculated as shown in Fig.8b–d,according to the Rietveld refinement results.For the orthorhombic LT2 structure,the cell volume shows a small reduction at 493–513 K where the HT phase rapidly forms,then monotonically increases across the magnetic transition temperature of (543–563 K) until the LT2 phase disappears.The cell parameter and volume of the formed HT increase linearly with temperature,while the cell volume of Mg2Ni decreases as temperature increases from 553 K and becomes stable above 683 K,as shown in Fig.8c and d,respectively.The volume contraction of Mg2Ni was determined by the both decrease ofaandc.The temperature of 683 K coincides with the end of the dehydrogenation reaction,indicating the cell volume change of Mg2Ni has relations with phase transformation and a solid solution of hydrogen atoms.
In order to determine whether the NTE of the LT2 sample is caused by phase transition,the total volume change of relevant phases needs to be considered,including LT2,LT1,HT and Mg2Ni.The MgNi2phase is not considered because it didn’t participate in the phase transition of LT2.The total volume of the sample (V) is
whereVLT2,VLT1,VHTandVMg2Niare the volumes of LT2,LT1,HT and Mg2Ni,respectively.The volume of phase is related to the cell volume of phase (Vc,phase) and phase mole content (fphase),
without considering the contribution of grain boundaries,microstructure defects,etc.fphasecan be calculated by
wherefwis the weight content of the phase,andMphaseis the atomic weight of the phase.For Mg2Ni,the solid solution of H atoms is not considered.Therefore,substituting Eq.(5) and(6) into Eq.(4),one can obtain
TheVis the total volume of sample.However,the volume change obtained from TEM images is the area (twodimension) difference compared with the original sample.Supposing the particle of the sample has a cubic shape,the projection area of the sample should be
Setting the original area of a sample at 323 K as theS0,the area change ratio at any temperature is
Thefw,phaseandVc,phasecome from the Rietveld refinement results,which are shown in Fig.6f and Fig.8b–d.TheMphaseis constant in the heating process and the values are 890.7,890.2,445.3 and 645.6 g·mol–1for LT2,LT1,HT and Mg2Ni,respectively.Furthermore,the LT2 content (63.8%)in the original sample should be considered because the volume change induced by the phase transition of LT1 phase in this sample would cause some error.Therefore,the final area change ratio of LT2 phase should be
Similarly,the area change ratio of LT1 phase also can be calculated usingfw,phaseandVc,phasefrom the Rietveld refinement results.
The predicted area change ratio is shown as Fig.9 with comparing the area change from TEM observation.The predicted totalΔS/S0of LT1 and LT2 phases induced by phase transition during heating process are–13.0% and–9.3%,respectively,which are consistent with the values observed from in-situ TEM.
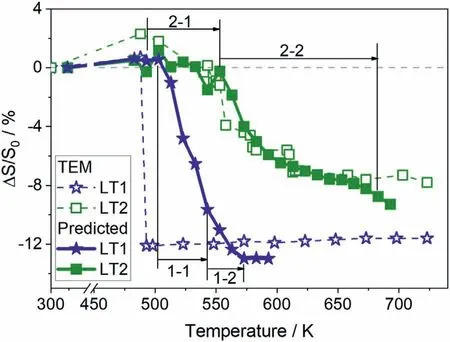
Fig.9.Prediction of the area change caused by phase transition in LT1 and LT2 samples.
The tendency of volume change of LT2 agreed well with the results obtained from TEM images,with a slight decrease at 493–553 K and a relatively larger decrease at 553–683 K.The two intervals correspond to the phase transition of LT2→HT in (2–1) temperature range and the dehydrogenation of LT2 and HT in (2–2) temperature range.The initial temperature of LT2→HT reaction is posterior to the temperature of shrinkage in the TEM experiment.
As for the LT1 sample,the dehydrogenation of LT1 and phase transition of LT1→HT simultaneously occur in the temperature range of (1–1) which would lead to a rapid reduction of volume,and then a small quantity of HT releases hydrogen in (1–2) temperature range contributes to further volume contraction.Although the predicted totalΔS/S0is close to the TEM observation value,the shrinkage temperature lags behind the TEM results.The abrupt volume shrinkage for the TEM sample occurred at 488 K,while the LT1→HT and dehydrogenation of LT1 started from 503 K for the in-situ SR-PXRD sample.The delay may be due to the electron irradiation in TEM causing the earlier dehydrogenation of LT1.
The different volume contraction mechanisms are revealed for LT1 and LT2 phases.However,it should be noted that the dehydrogenation causing volume shrinkage is very different from the NTE phenomenon.The dehydrogenation is a chemical reaction and hydrogen release usually leads to volumetric shrinkage.Therefore,the real NTE only occurred at 488–553 K of the LT2 sample which was caused by the phase transition of LT2→HT.An opposite thermal behavior is found in the LT1 and LT2 phases between 493 K and 553 K.With the elevated temperature,the LT1 sample expands,and the LT2 sample contracts to compensate for the volume change of LT1.As a result,the total volume can remain unchanged,which reaches zero thermal expansion.It is believed that by tuning the fraction of LT1 and LT2 based on a single material,a new and general way of exploring controllable thermal expansion applications is opened.
5.Conclusions
In summary,we present the experimental evidence for thermal contraction in LT1-and LT2-Mg2NiH4phases with systematic studies of the technique for fabricating pure LT1-,LT2-Mg2NiH4,and controllable mixture compounds.The LT1 and LT2 showed volume contraction up to 18.7 and 11.3%respectively at an elevated temperature,and the large negative thermal expansion phenomenon was found in LT2 phase.Detailed mechanisms for the volume contraction were studied for the LT1 and LT2 phases at different temperature ranges.The negative thermal expansion in the LT2 phase was attributed to the phase transformation of LT2→HT at 488–553 K,and the continuous volume contraction at higher temperature is caused by dehydrogenation of LT2 and HT.While the dehydrogenation should be responsible for the abrupt volume contraction in the LT1 phase.LT2 is more stable than LT1 at high temperature.An opposite thermal behavior is found in the LT1 and LT2 phases between 493 K and 553 K,which is expected to open a new and general way of exploring controllable thermal expansion applications by tuning the fraction of LT1 and LT2.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
Part of the experiment was done at PD beamline,Australian Synchrotron,ANSTO.This work was supported by the National Key Research and Development Program of China(2021YFB3701001),the National Natural Science Foundation of China (51871143),Shanghai Engineering Research Center for Metal Parts Green Remanufacture (No.19DZ2252900)from Shanghai Engineering Research Center Construction Project and Shanghai Rising-Star Program (21QA1403200).We acknowledge Mr.Hiroshi Maeno’s assistant in UHV-TEM experiments.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.09.012.
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
