Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
M.Daroonparvar ,A.Hlmr ,A.M.Ralls ,M.U.Farooq Khan ,A.K.Kasar ,R.K.Gupta ,M.Misra,S.Shao,P.L.Mnzs,N.Shamsai
a National Center for Additive Manufacturing Excellence, Auburn University, Auburn, AL 36849, USA
b Department of Mechanical Engineering, Auburn University, Auburn, AL 36849, USA
cDepartment of Materials Science and Engineering, College of Engineering, North Carolina State University, 911 Partners Way, Raleigh, NC 27695, USA
d Department of Mechanical Engineering, University of Nevada Reno, Reno, NV 89501, USA
e Department of Nuclear Engineering and Radiological Sciences, University of Michigan, Ann Arbor, MI 48105 USA
fDepartment of Chemical and Materials Engineering, University of Nevada Reno, NV 89501, USA
Abstract Nitrogen processed,cold sprayed commercially pure(CP)-Al coatings on Mg-based alloys mostly lack acceptable hardness,wear resistance and most importantly are highly susceptible to localized corrosion in chloride containing solutions.In this research,commercially pure α-Ti top coating having good pitting potential (~1293 mVSCE),high microhardness (HV0.025: 263.03) and low wear rate was applied on a CP-Al coated Mg-based alloy using high pressure cold spray technology.Potentiodynamic polarization (PDP) curves indicated that the probability of transition from metastable pits to the stable pits for cold spayed (CS) Al coating is considerably higher compared to that with the CS Ti top coating (for Ti/Al/Mg system).In addition,CS Ti top coating was in the passivation region in most pH ranges even after 48 h immersion in 3.5 wt% NaCl solution.The stored energy in the CS Ti top coating (as a passive metal) was presumed to be responsible for the easy passivation.Immersion tests indicated no obvious pits formation on the intact CS Ti top coating surface and revealed effective corrosion protection performance of the CS double layered noble barrier coatings on Mg alloys in 3.5 wt% NaCl solution even after 264 h.
Keywords: Ti coating;Mg alloys;Localized corrosion;Passivity;Dislocation density;Crystallite size.
1.Introduction
Magnesium and its alloys,owing to their low density and high strength-to-weight ratio,have received considerable interests for applications in the aerospace,sports,and electronics industries [1–5].Because of the crisis of energy in the world,lightweight materials have received extensive attention to decrease fuel consumption in the aviation and automotive industries [5].However,poor creep,wear as well as corrosion resistances of Mg-based alloys limit their different applications [4,6,7].Various conversion coatings,anodization process,plasma electrolytic oxidation (PEO),electrophoretic deposition,physical vapor deposition (PVD),electrodeposition,air plasma spray (APS),ions implantation methods,etc.,have been used to augment the corrosion and wear resistances of Mg-based alloys [8–23].The corrosion resistance of a substrate can be substantially ameliorated by a well adhered coating having low porosity.Nonetheless,few of the abovementioned coating techniques may fulfill these requirements[6,8].In contrast to high velocity oxy-fuel (HVOF) process that utilizes the mixture of thermal and kinetic energies,the cold spray technology (Fig.1) employs the kinetic energy to produce coating layers.In this process,fine powder particles(in the range of about 5–50 μm) with supersonic velocity(about 300–1200 m/s) impact the substrate surface.Hence,powder particles experience adiabatic heating and severe plastic deformation at very high shear rates.This causes particles to be flattened and strongly bonded to the beneath surface(via materials jet formation) (Fig.1c-f) [24–28].
In contrast to the other alternative thermal spray methods which might damage the heat-vulnerable metallic substrates microstructure such as Mg alloys,the cold spray process could circumvent this microstructural damage during process [8,16,26–31].Compared to the ones produced by other coating methods (e.g.,E-plating method,chemical conversion coatings,anodizing technique,etc.),the CS coatings were reported to substantially enhance the corrosion resistance of Mg-based alloys in a corrosive solution [32–36].
Al (as a noble barrier coating) with low density,good corrosion resistance,and low standard reduction potential difference with Mg-based alloys has been reported to decrease the corrosion rate of Mg-base alloys in a corrosive electrolyte[14,37–39].Dense Al-based coatings were applied on LA43M substrates using common cold spray process and then post treated by the shot peening process [40].In this process,deposited Al powder particles can absorb the kinetic energy of martensitic stainless-steel particles,and a large fraction of the deposited Al powder particles was densified using this postcold spray treatment.This led to the considerable reduction of porosity level in the shot-peened CS Al coating.Compared to the as-sprayed Al-coated LA43M sample,the shot peened Alcoated LA43M sample showed higher open circuit potential(OCP),lower corrosion current density and better impedance values.Moreover,in this work [40],no passivation region(i.e.,on the PDP curves) was observed for both CS Al (in as-sprayed condition) and shot peened CS Al-based coatings in 3.5 wt% NaCl solution [40].
The current CS Al-based coatings (when applied as a single coating using N2as propellant gas) on Mg-based alloys suffer from low hardness,high wear rate and are extremely vulnerable to the localized corrosions (e.g.,pitting corrosion)in severe corrosive environments [37,41,42].Adding ceramicbased particles into the Al-based powder particles was carried out to augment the mechanical properties of the CS Al-based coatings/deposits [43].Nevertheless,the interface between Al and the ceramic particles facilitated the corrosive electrolyte infiltration over time and declined the corrosion shielding performance of the CS Al-ceramic composite coatings on the Mg-based alloys during corrosion [43].
Titanium and its alloys are employed in the severe corrosive environment applications owing to their good mechanical properties and great corrosion resistance (caused by firm/dense protective oxide film formation on the surface)[44–46].In addition,Ti coating can be considered as a great candidate for the surface preservation of Al and Al-based alloys [46],as there is a small potential difference between Ti and Al [47].In this regard,as-deposited Ti coating considerably increased the pitting corrosion resistance and lowered the corrosion rate of Al-based alloy in chloride containing solution [47].Moreover,cold sprayed Ti coating showed better wear performances compared to the bare AA2024 alloy [48].
In the present research (stimulated by the presented literature),we established the CS double layered noble barrier coatings (Ti/Al) on magnesium-based alloys using cold spray process.The advantages of the bi-layered coatings in comparison with the mono layered coatings have also been described by the other researchers [49].Besides the tribology performance evaluation of the deposited CS coatings on Mg alloy,the pitting corrosion and general corrosion behaviors of the CS double layered Ti/Al and the mono layered aluminumbased coatings on Mg alloy were investigated using electrochemical corrosion tests (up to 48 h) and immersion tests (up to 264 h) in 3.5 wt% NaCl solution.The underlying mechanisms were then analyzed in detail.
2.Materials and methods
2.1. Powders and samples
Commercially pure (CP) Al powder with particle size range of 9–40 μm (H-15,produced by inert gas atomization,Valimet Inc,California),and plasma atomized CP-Ti grade 1 powder with particle size range of 10–45 μm(AP&C powder,Quebec,Canada)were utilized for the cold spray process.The samples (i.e.,substrates) were cut out of from a commercially available AZ31B Mg alloy plate (381 mm (L) × 455 mm(W) × 9.5 mm (H)).Before cold spray process,the Mg alloy substrates surface were grit blasted.
2.2. Cold spray deposition
High pressure cold spray system (Impact Innovation 5/11 system,GmbH Germany) at ASB Industries.Inc.was used to deposit metallic powder particles on the AZ31BMg alloy substrates (using the cold spray process parameters provided in Table 1).It should be noted that the substrate and the coatings temperature was retained at temperatures less than 65 °C during the entire spraying process.In the present research,mono-layered Al,and double layered Ti/Al coatings were deposited on the Mg-based alloy substrates.
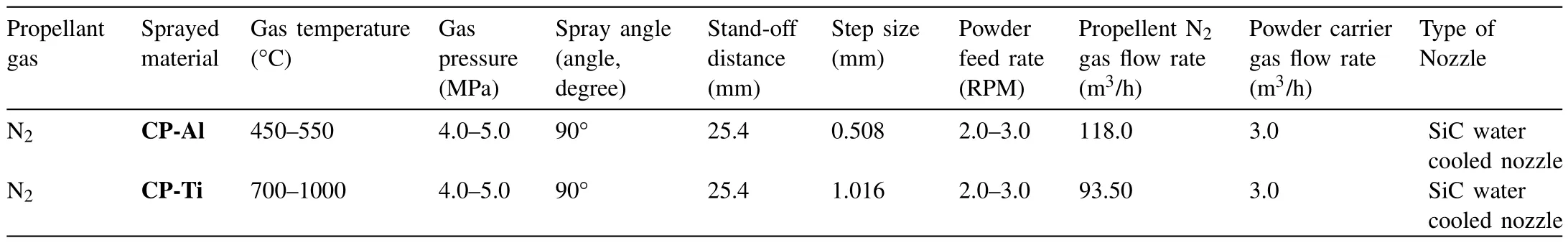
Table 1HPCS process parameters.
2.3. Characterization of feed-stock powders and as-sprayed coatings on Mg alloy
The microstructure of cross section of the CS coatings on the Mg-based alloys was analyzed using an optical microscope (Olympus IX70).Hence,coated specimens after cutting and mounting were ground and polished using Allied Metprep 3TMgrinder/polisher system (utilizing standard metallographic methods).Also,ImageJ software was used to analyze the porosity level of the as-sprayed coatings on Mg alloy(using ASTM E2109-01).Before and after immersion tests,TESCAN LYRA-3 model XUM integrated variable pressure FIB-FESEM (Focused Ion Beam-Field Emission Scanning Electron Microscope) and Hitachi TM-3030 Scanning Electron Microscope (SEM) equipped with Energy Dispersive Xray Spectroscopy (EDS) were used to investigate the surface morphology,cross-sectional microstructures,and the elemental analysis of the CS coatings on Mg-based alloy substrates.Besides,Rigaku Ultima IV X-ray machine was employed to analyze the structural phases(or constituent phases)of the CS coatings,uncoated Mg-based alloy substrate and feed stock powders.The surfaces of as-sprayed coatings and bare Mgbased alloys were ground up to 1200 grit size sandpaper before X-ray diffraction (XRD) analysis tests.During XRD analysis,the X-ray tube emitted Cu-Kαradiation with an excitation voltage of 40 kV and excitation current of 35 mA.Also,specimens were scanned at a rate of 1 deg/min with a step width of 0.04°.It should be noted that the obtained data were analyzed using X’Pert HighScore Plus software with ICDD database.Moreover,2θ(i.e.,diffraction angle) range of 20°–90° was used to collect diffraction patterns of various specimens.The average crystallite size and strain from each coating was determined from the collected X-ray spectra using the Williamson-Hall method [50].The broadened peaks of the X-ray diffractograms are explained by considering peak width as a function of 2θ.X-ray peak broadening due to small crystallites is considered to be inversely proportional to cosθand the strain of the lattice to tanθ,the broadening is described by Eq.(1).Where K is a shape factor,D is average crystallite size,andεthe lattice strain.
The X-ray data are plottedβtotalcosθvs.4sinθas a line,the slope is equivalent to the strain present in the lattice and the y-intercept may be used to calculate the crystallite size.
For metallic materials subjected to severe plastic deformation,dislocation density (ρ) was calculated by the following Eq.(2) [51]:
Where b is the magnitude of the Burgers vector for Al and Ti.As mentioned earlier,D andεare considered as average crystallite size and microstrian,respectively.D andεare calculated using Williamson–Hall (W-H) method [50,52],based on the Eq.(1).
The micro-hardness of the Mg alloy substrate and the CS coatings were measured using a Vickers micro-hardness-tester(Buehler Wilson-Tukon,1202)under the load of 0.245 N.Furthermore,ten measurements were carried out on each specimen and average was then considered as microhardness value.Rtec-Tribometer at room temperature (~25 °C and 40–50%relative humidity) was used to perform dry reciprocating sliding tests (according to ASTM G133).Before sliding tests,the surface of uncoated Mg-based alloys and all CS coatings were polished to attain an average surface roughness (Ra) of 0.2±0.05 μm.Moreover,E52100 steel-based ball (having 6.35 mm diameter) was employed as a counterpart in this experiment.It should be noted that all the reciprocating sliding experiments were done with a track length of 15 mm and 1 mm/s velocity (under a normal load of 4 N for a total distance of 1000 mm).After the experiments,an optical profilometer was used to capture the wear tracks on each specimen.Wear profiles were then investigated to calculate the wear volume (needed for Eq.(3)).The formula given by Eq.(3) was then used for calculation of the wear rate of the samples [53]:
The surface topography and the average surface roughness(Ra) of the CS coatings were investigated during profilometry by means of an Alicona-Infinite Focus 3D measurement system.This system has a non-contact optical measurement principle (based on the focus-variation).Before 3D surface profilometry,surface of specimens was cleaned up by using the deionized (DI) water (utilizing an ultrasonic cleaner).Also,the brightness and the contrast were fine-tuned at a range of focus.This can ensure all the features are within focus during the scans.Moreover,the lateral resolution was adjusted at 50 nm.Furthermore,the average surface roughness was determined by means of line scans (across the IFM scan).It should be noted that at least 5 readings were obtained to decrease the standard deviation for Ra.
2.4. Corrosion tests
Prior to corrosion tests,surface layers (i.e.,rough,and porous top layers) of the CS coatings were eliminated [37].SiC abrasive papers (up to 1200 grit size sandpaper) were used to grind up the surface of the specimens(i.e.,both coated and uncoated specimens).These samples were then cleaned with ethanol by using an ultrasonic cleaner for about 5 min.
2.4.1.Potentiodynamic polarization(PDP)tests on the coated and uncoated specimens
A flat cell using a VMP-300 Bio-logic potentiostat following a three-electrode setup were used to perform the potentiodynamic polarization(PDP)tests.In this experiment,the used three-electrode setup (in a flat cell) was comprised of a standard calomel electrode (as a reference electrode),platinum electrode (as a counter electrode) and sample (as a working electrode).Prior to the PDP tests,to permit the system to attain an equilibrium in the electrolyte,the open circuit potential(OCP) was monitored for 1 h.Also,a scan rate of 1 mV/s from 200 mV below OCP to a current limit of 1 mA/cm2(or a potential limit of 2.5 VSCE) at room temperature were considered as PDP test parameters in the current research.The corrosion current density was determined via Tafel extrapolation of the cathodic polarization curve [42,54–57].
2.4.2.Electrochemical corrosion tests for 48 h
In these tests,the used three-electrode setup (in a flat cell)also consisted of a standard calomel electrode (as a reference electrode),platinum electrode (as a counter electrode)and sample (as a working electrode).The open circuit potential (OCP) was observed for 48 h (for bare and coated samples) in 3.5 wt% NaCl electrolyte.Also,EIS (electrochemical impedance spectroscopy) test was performed after 48 h immersion in 3.5 wt% NaCl electrolyte.EIS was done in the frequency range from 100 kHz to 10 mHz at OCP.For each EIS scan,ten measurements were recorded per decade,with an average of at least three points per measurement.Furthermore,sinusoidal AC perturbation with an amplitude of 10 mV (rms) was considered for EIS tests.EC-Lab V11.21 and Z-view software were utilized to analyze the EIS data.To substantiate the repeatability of the results,all the electrochemical corrosion tests were done three times.The specimens were rinsed with DI water and then dried in air after immersion tests for 48 h in a corrosive electrolyte.
2.4.3.Immersion tests for 264 h
To further illuminate the efficacy and corrosion protective performance of the CS double layered coatings on Mg-based alloys in chloride containing solutions,the immersion test was performed on the coated samples in 3.5 wt% NaCl solution for 264 h.The coated specimens were first mounted.The mounted samples were then ground and polished.The interface between the coating and the mounting material was sealed by a resin+hardener.An ultrasonic cleaner (for 5 min) was used to clean the surface of the samples with ethanol before tests.Also,the specimens were washed with water (i.e.,DI) and afterwards dried up in air after the immersion tests in 3.5 wt% NaCl solution for 264 h.
3.Results and discussion
3.1. Characterization of feed-stock powders and cold sprayed coatings
Commercially pure-Al (with particle size range of+9/-40 μm),and commercially pure-Ti(having particle size range of +10/-45 μm) powders all showed spherical morphology (Fig.2a and b).SEM images of the mono layered CS Al and double layered CS Ti/Al coatings surface (before surface grinding and polishing) are shown in Fig.2c and d.Compared to CS Al coating (with average surface roughness(Ra) of about 2.81 μm,Fig.3b),lower Ra(about 2.23 μm)obtained for CS Ti/Al coating (Fig.3a) could be attributed to the intense plastic deformation of Ti powder particles during high pressure cold spray process (especially when high cold spray process parameters are used).The microstructure of cross section of the CS coatings on the Mg-based alloys are depicted in Fig.4 (before and after etching).

Fig.3.Surface topography of as-sprayed coatings (a) Ti/Al coated Mg alloy surface,and (b) Al coated Mg alloy surface.

Fig.4.Photomicrographs (optical microscopy) from (a,b) Al layer on Mg alloy at 50X and 200X magnifications,(c-g) Ti/Al layers on Mg at 50X,100X,200X,200X,and 400X magnifications,respectively (in as-sprayed condition).
Compared to previous works which reported higher levels of porosities (between 2.7% and 10.2%) for the cold sprayed,warm sprayed and plasma sprayed Ti coatings [58,59],cold sprayed Ti coating (Fig.4c,d,f and g) in this work showed a near-dense structure having about 0.50±0.20% porosity level.Also,extremely restricted micro-pores and the local deformation of Ti powder particles were conspicuous in the CS Ti top coating microstructure,as shown in Fig.4f and g.In the literature,rapid degradation of Mg-based alloy substrate(after 24 h of immersion in 3.5 wt% NaCl solution) was related to the presence of interconnected porosities in the warm sprayed Ti coating’s structure (with about 3.8–5.4% porosity level) [59].In the current research,compared to the CS Ti top coating (Fig.4d),CS Al coating showed higher level of porosities(about 1.10±0.20%),Fig.4a and b.Tao et al.[37]reported the existence of numerous micro-defects (i.e.,micro pores and cracks mostly at the inter-particle boundaries)in the CS Al coating microstructure on a Mg-based alloy substrate.The expansive formation of micro-defects at the inter-particle boundaries was attributed to the lower degree of localized plastic deformation (stimulated by localized heating,stresses)during the cold spray process [37].However,compared to the commercially pure Al bulk substrates,CS Al coatings having high compactness (i.e.,low porosity) and sub-micron sized grains were reported to significantly enhance the corrosion resistance in a corrosive solution [37,41].
In the current research,it is obviously seen that Ti layer has deeply penetrated the Al layer with severe plastic deformation (Fig.4c,d,e).This was accompanied by noticeable densification of softer underneath layer (i.e.,Al) by hard particles (i.e.,Ti) with high kinetic energy (due to hammering or tamping effect).
This observed behavior is mostly detected when hard particles (having high kinetic energy) are applied on the soft substrates or deposits (e.g.,Al) [25,60,61].It is predicted that the densified Al coating (Fig.4e) could protect the AZ31BMg alloy from corrosive electrolyte if it (i.e.,electrolyte) passes through Ti top coating during long immersion time.High pressure-CS Ti top coating could also increase the micro-hardness (HV0.025) to 263.03 for Ti/Al coated Mgbased alloy surface from 56.43 for Al coated Mg-based alloy surface.The increased microhardness observed for the Ti top coating could be ascribed to the appreciable multiplication of dislocations caused by severe plastic deformation of powder particles (specially at inter-particle boundaries) during the cold spray process and lower porosity level in the coating microstructure as well [41,62].XRD scan results of uncoated Mg-based alloy,feedstock powder particles used in this work and as-sprayed Al and Ti/Al coatings are depicted in Fig.5.

Fig.5.XRD patterns of (a) uncoated Mg-based alloy,(b,c) as-sprayed Al and Ti/Al coatings,and feedstock powder particles used in this research work.
As shown in Fig.5a,α-Mg phase with H.C.P crystal structure was detected as major phase constituent for uncoated Mg-based alloy.Moreover,the analogous phase structure and crystal planes (Fig.5b,and c) were identified for both feedstock powder particles and cold sprayed coatings/deposits.In fact,considerable peening influence of the particles during the CS process as well as low processing temperature of this method would result in the retention of the original phases and crystalline planes of the feed-stock powder particles in the resultant cold sprayed coatings/deposits.Also,no phase transformation and/or oxidation were detected in the CS coatings in the present work,Fig.5b and c.In another study,the presence of Ti-based oxides (e.g.,TiO) were indicated in the XRD patterns of warm sprayed (WS) Ti coatings which were deposited using powder particles heated at high temperatures[63].In the case of flame spray (FS) processing,the oxide,nitride,and carbide phases were detected in the wire flame sprayed Ti coatings.These phases were reported to diminish the Ti coatings protection performance in NaCl solution.Therefore,these coatings are typically sealed with epoxy or Si resin [59].
Compared to the XRD scan of CS Al coating in the present work (Fig.5c),the obvious peak broadening in XRD scan of CS Ti top coating (Fig.5b) can be majorly ascribed to the severe plastic deformation of powder particles during CS process [64,65],supplementary information (Fig.S1).Based on the Eq.(1),the values ofεand D were obtained through the slope and Y-intercept of the fitted W-H plots (Fig.6,and Table 2).

Table 2Average crystallite size,microstrain and dislocation density of CS coatings as calculated from the W-H plots (Fig.6).
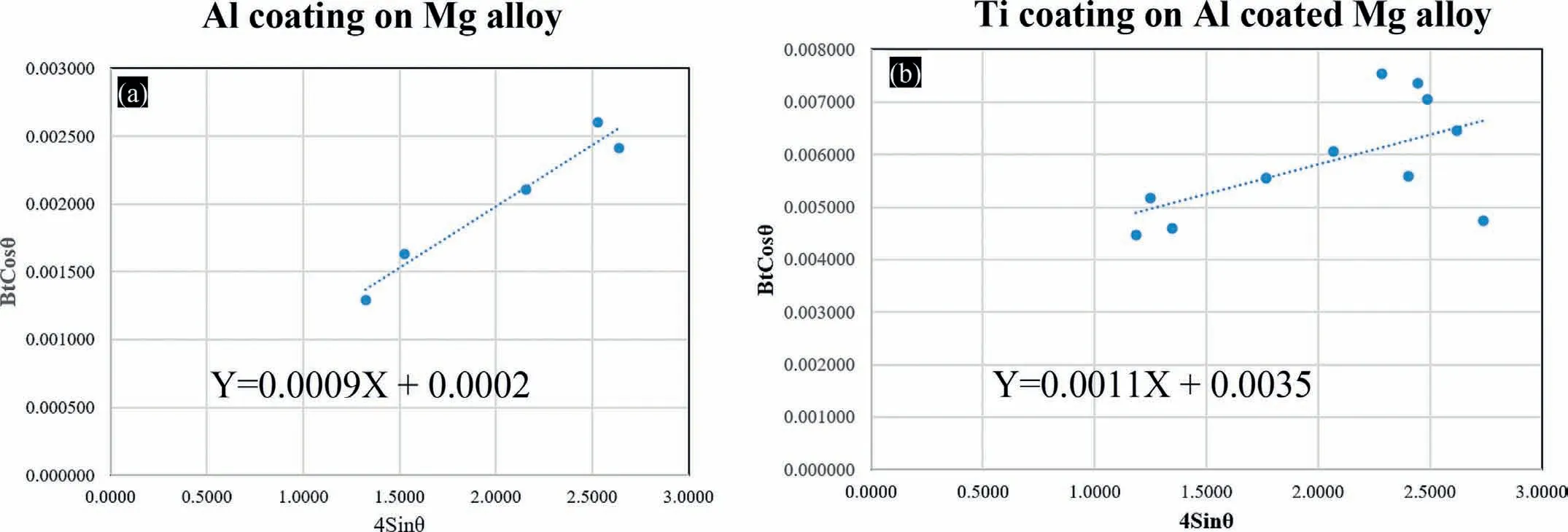
Fig.6.W-H plots of CS coatings,(a) Al coating,(b) Ti top coating.

Fig.7.(a-c) Wear tracks of bare and CS coated Mg alloys (2D and 3D surface profiles).

Fig.8.(a-d) Microhardness,COF,wear volume and wear rate of bare and CS coated Mg alloys.
The dislocation density (ρ) values of CS Ti and Al coatings were 3.39 × 1014m-2and 1.39 × 1013m-2,respectively (using Eq.(2)).Compared to the CS Al coating(Fig.6a),a steeper slope of the W-H plot for the CS Ti top coating (Fig.6b) indicates a large amount of microstrain caused by the severe plastic deformation (SPD).The presence of crystal defects (especially dislocations) which formed during the CS process can cause this microstrain in the structure [66].It should be noted that the increase in microhardness is consistent with that of dislocation density after applying Ti coating on the Al coating in this research.More grains in a much smaller area can be accommodated when the crystallite size decreases.Therefore,the migration of dislocations inside the grain structure of the nanocrystalline materials can be obstructed by the grain boundaries of such closely packed grain structures.Also,the material’s plastic flow is decreased by this considerable accumulation of dislocations.This can lead to the enhancement of the material’s strength and microhardness.In fact,the reduction of crystallite size is reported to increase the mechanical properties of polycrystalline materials [67,68].
In the present research,the Ti/Al coating having a surface with higher hardness provided lowest wear rate compared to the other samples (Figs.7 and 8).Khun et al.[53]also reported higher wear resistance for the cold sprayed pure Ti coatings (tested against the steel ball) in comparison with Ti-6Al-4V substrate.In fact,cold work (strain) hardening during the cold spray and considerable formation of wearresistant oxide layers on the wear tracks of the CS Ti coatings during wear tests were reported to be majorly accountable for this wear resistance improvement.Furthermore,in another research,the wear behavior of a bare Al-based alloy (AA2024)was considerably improved by the CS Ti coatings [48].
3.2. Corrosion performance of coated and uncoated samples
3.2.1.Short-term electrochemical corrosion behavior
Surface of bare,mono layered Al coated,and double layered Ti/Al coated Mg-based alloys were ground and polished to Ra<1 μm (prior to corrosion tests).Fig.9 shows the short-term electrochemical corrosion experiment results of bare Mg-based alloy and coated Mg-based alloys in 3.5 wt%NaCl solution.The OCP of specimens against time is indicated in Fig.9a.The uncoated Mg-based alloy and mono layered Al coated Mg alloys showed relatively constant OCP value.After 1 h in 3.5 wt% NaCl solution,potential (mV vs.SCE) value for the uncoated Mg-based alloy and mono layered Al coated Mg alloys was stabilized at~-1590 mV and~-727 mV,respectively.The Al coating displays noticeable OCP oscillation amplitude (Fig.9b).This was correlated to the breakdown of the passive film [69].OCP value for the Ti/Al coated Mg-based alloy was stabilized at around-250 mV.It is evidently observed that the mono layered Al coated sample is more active than the double layered Ti/Al coated sample in 3.5 wt% NaCl solution.
The potentiodynamic polarization curves of uncoated Mgbased alloy and coated Mg-based alloys (after 60 min,when the OCP was stabilized) in 3.5 wt% NaCl electrolyte are shown in Fig.9c.Potentiodynamic polarization curve of the double layered Ti/Al coated Mg-based alloy (its anodic branch) displays the typical spontaneous passivation and pitting characteristics that is almost analogous to the passivation behavior of the bulk titanium in 3.5 wt% NaCl electrolyte [70].The CS titanium coating on the Al coated Mg-based alloy maintained linear passivation (i.e.,passive layer formation) even at higher potentials.This behavior was retained up to (or before) breakdown (pitting) potential:Eb=~1293 mVSCEin a chloride containing solution.
Corrosion current density (icorr) and corrosion potential(Ecorr) were elicited from the potentiodynamic polarization curves (Fig.9c) and presented in Table 3.The icorrof the Ti/Al coated Mg alloy was negligibly lower than that of the Al coated Mg alloy.It is hypothesized that the substantial replication of dislocations,stimulation of lattice micro-strain,refined-crystals,and residual stresses in the microstructure of CS coating/deposit have been caused by the considerable plastic deformation of titanium powder particles upon high velocity impingement on the underneath layer during the cold spray process [71–74].The higher corrosion current densities of the CS coatings were attributed to the existence of much more active sites for corrosion which were a result of the intense degree of plastic deformation during the cold spray process.Nevertheless,the Ti/Al coated Mg alloy showed highest Ecorr(-355 mVSCE) compared to the Ecorrof the other samples(Table 3).This indicates that the Ti/Al coated Mg alloy has lowest corrosion vulnerability in comparison with the other samples in 3.5 wt% NaCl solution.Moreover,the CS Ti top coating (as a passive metal) with high pitting corrosion resistance (Eb-Ecorr) could considerably lower the active dissolution of the CS Al coated AZ31B Mg alloy in the corrosive electrolyte.The stored energy in the CS coatings/deposits(such as niobium as a passive metal) was reported to help and facilitate the passivation in a corrosive solution [75].

Table 3Results of potentiodynamic polarization tests of uncoated and coated Mg alloys in 3.5 wt% NaCl solution.
To describe the likelihood of transition from the metastable pits to the stable pits,the value of the pitting potentials difference (Eb-Em) is generally used.Emwas considered as the metastable pitting potential (Fig.10),and the potential where the current increased continually was designated as the stable pitting potential (Ebor breakdown potential).In fact,the higher value of (Eb-Em) signifies lower transition probability [76,77].This indicates that the probability of transition from metastable pits to the stable pits for the CS Al coating (Eb-Em=~17 mVSCE) is considerably higher compared to the CS Ti top coating (for the Ti/Al/Mg system,Fig.10A,Eb-Em=~974 mVSCE),in the current research.

Fig.10.Magnified passive regions of potentiodynamic polarization curves of coated Mg-based alloys (obtained from Fig.9c,d).
The long-term corrosion performance of CS coatings is very sensitive to the presence of through-thickness pores or gaps.Therefore,this will be comprehensively investigated in the next sections of this research paper.
3.2.2.Electrochemical corrosion behavior(i.e.,OCP and EIS tests for 48 h)
The corrosion behavior of coated and uncoated Mg-based alloys in 3.5 wt% NaCl electrolyte was further explicated using an immersion test for 48 h.Fig.11 indicate the surface morphology of polished samples (i.e.,coated,and uncoated Mg-based alloys) after 48 h of immersion.As shown in Fig.11a,the uneven-corrosion products along with some microcracks are seen on the uncoated Mg-based alloy surface after 48 h of immersion.These corrosion products were majorly composed of Mg,O,Cl,along with Al,Na and C elements (Fig.11b–e).The existence of Mg,O and Cl elements (Fig.11C) could be ascribed to the likely presence of MgCl2and Mg(OH)2phases in the corrosion products[78,79].In contrast to bare Mg alloy surface covered with the loose corrosion products,the mono layered CS Al coating surface (having Ra of 74.17 nm) showed noticeable signs of localized corrosion (i.e.,corrosion pits) after 2 days of immersion.Interestingly,scratches (i.e.,grinding tracks caused by the CS coatings surface grinding before the experiments)are not detectable (Fig.11f) on the Al coating surface after immersion test.This observation may be related to the influence of general corrosion on the most surface area of the CS Al coating.It appears that some pits (caused by the pitting corrosion) are filled with corrosion products (i.e.,oxygen-rich corrosion products) which might be composed of hydroxides and/or oxides (Fig.11h and i).As shown in Fig.11g,and i,Al and O as well as other elements such as Cl,Na,C,and Si were identified as the constituent elements (Fig.11j-l) of the corrosion products on the corroded surface of the mono layered CS Al coating.Also,magnesium element (originated from the AZ31B Mg alloy substrate) was not detected on the mono layered CS Al coating surface after 48 h immersion.In fact,the Mg-based alloy substrate surface can be isolated from the chloride containing electrolyte by the mono layered CS Al coating (in the current research) during the immersion test for two days.

Fig.11.Morphological characteristics of polished surface of bare and coated AZ31B Mg alloys after immersion in 3.5 wt% NaCl solution for 2 days,(a) bare AZ31B Mg alloy surface,(b,e) EDS analysis of area A,(c,d) EDS analysis of area B,(f) polished surface of Al coated AZ31B Mg alloy after immersion test in 3.5 wt% NaCl solution for 2 days,(g,j) EDS analysis of area A’,(h,k) EDS analysis of area B’,(i,l) EDS analysis of area C’,(m) polished surface of Ti/Al coated AZ31B Mg alloy after immersion test in 3.5 wt% NaCl solution for 2 days,(n,o) EDS analysis of area A”.
Compared to the CS Al coating after immersion test(Fig.11f),perceptible corrosion pits and the other forms of localized corrosions were not seen on the corroded Ti top coating surface after 48 h of immersion test (Fig.11m).Also,scratches (grinding tracks) are still identifiable (Fig.11m,inset) on the Ti top coating surface in the Ti/Al/Mg system.This shows that the CS Ti top coating with high passivation propensity not only improve the surface hardness and wear resistance of the CS Al coating,but also substantially mitigate the pitting corrosion problem associated with commercially pure Al coatings in chloride containing solutions.
As shown in Fig.11n,and o,Ti and O elements were identified as major constituent elements of the corrosion surface film on the Ti top coating surface after 48 h immersion.These detected elements can be most probably related to the existence of titanium-oxides [45]on the Ti coating surface after immersion test.Interestingly,Mg and Al elements (i.e.,from Mg alloy substrate and Al underneath coating,respectively) were not identified on the Ti coating surface after 48 h of immersion.This indicates that Al coated Mg alloy surface can be isolated from the chloride containing solution by the Ti top coating after 2 days of immersion test.However,in another research,galvanic cell formation between the AZ91E Mg substrate and warm sprayed (WS) Ti-based coatings was reported [59].This phenomenon caused the appreciable corrosion products formation and accumulation at the interface between the WS Ti-based coatings and Mg-based alloy substrate.Also,abrupt crack/separation of the WS Ti-based coatings were seen just after 24 h immersion in 3.5 wt% NaCl electrolyte [59].On the contrary,in the current research,no visible change was observed in the double layered Ti/Al coatings (even after 48 h immersion) compared to the double layered Ti/Al coated Mg-based alloy (before immersion test),Fig.12.In fact,the Ti top coating (in the present research)with even some un-connected micropores (at the inter-particle boundaries) and high surface activity (compared with bulk titanium [80]) can improve the corrosion behavior of the current mono layered Al coated Mg-based alloys in chloridecontaining corrosive solutions.

Fig.12.Photomacrographs of immersed coated and uncoated Mg-based alloys in 3.5 wt% solution for 48 h.No visible change was observed in the Ti/Al coating on Mg alloy (after 2days of immersion) compared to unexposed as-polished Ti/Al coated Mg alloy.*Ra: Surface Roughness Average.
As depicted in Fig.13,the OCP of the doble layered CS Ti/Al coating immediately diminished (nearly after 1 h immersion).This could boil down to the existence of permeable micropores on the surface layers of the CS Ti top coating which might have provided paths for the corrosive electrolyte to permeate over immersion time.However,the continuous increase of OCP (nearly after 23 h immersion)could be imputed to the reaction dynamic equilibrium between the CS Ti top coating or deposit and the corrosive electrolyte as well as the corrosion product formation which may hamper the further permeation of the electrolyte into the interior zones of the CS Ti top coating [81].Moreover,formation of the stable oxide along the interparticle boundaries of CS Ti deposit was reported to impede the additional aggressive attack of corrosive solution over immersion time[81].
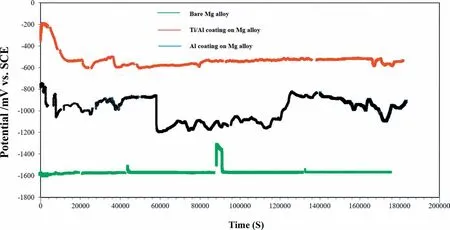
Fig.13.OCP curves of coated and uncoated Mg-based alloys in 3.5 wt% NaCl solution for 48 h.
It was also noticed that the mono layered CS Al coated Mg-based alloy is still more active than the double layered CS Ti/Al coated Mg-based alloy,Fig.13.This indicates that the cold sprayed Ti top coating can conspicuously reduce the activity (propensity to corrosion) of the mono layered CS Al coated Mg-based alloy in 3.5 wt% NaCl solution (Fig.13).Compared to mono layered coatings,the advantages of bilayered coatings are already reported in the previous research[49].
In comparison with OCP value of bare Mg alloy (~-1569 mVSCE) after 48 h of immersion in a chloride containing solution,the OCP values for the double layered Ti/Al and the mono layered Al coated Mg-based alloys were about-538.27 mVSCEand-911.51 mVSCE,respectively.This implies that CS Ti coating on the Al coated Mg alloy (having highest Rp) has the lowest tendency to corrosion (from thermodynamic point of view) compared to the mono layered CS Al coating.Also,according to the obtained potential (~-538 mVSCE) for the Ti/Al coating on Mg alloy and E-pH(Pourbaix) diagram for Ti (Fig.14,[82]),it is postulated that the CS Ti coating is in the passivation region in most pH ranges even after 48 h of immersion in 3.5 wt% NaCl solution.As previously mentioned,the stored energy in the CS coatings/deposits (especially passive metals) is reported to assist the easy passivation [75],Figs.10 and 14.
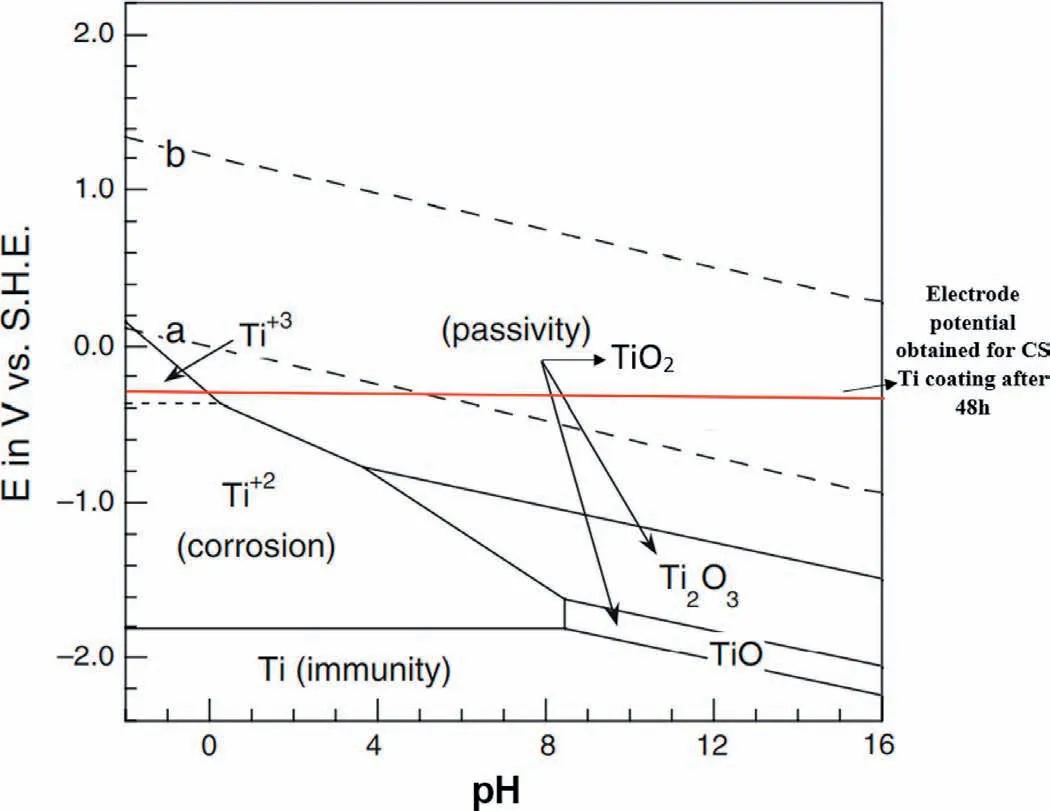
Fig.14.Pourbaix diagram (E-pH) for Ti at 25 °C [modified from 76].
It was also speculated that dislocations (inside grains) or high residual compressive stresses enhances the corrosion resistance of titanium by either supplying nucleation sites (for passive films) or augmenting the interfacial adherence of the passive film on the surface during corrosion [83,84].
For further elucidation of the influence of CS coatings on the corrosion resistance of AZ31B Mg alloy,electrochemical impedance spectroscopy (EIS) of coated and uncoated Mg alloys was measured after 48 h immersion in 3.5 wt% NaCl solution.Nyquist plots are shown in Fig.15a,b.The equivalent electrical circuits (Fig.15c–e) models were employed to fit the impedance spectra.One capacitive loop at high frequency and one inductive loop at low frequency were considered for the Nyquist plot of bare Mg alloy (Fig.15e).This assumption is in harmony with the previous researches [42,85].

Fig.15.Nyquist plots (a,b) of coated and un-coated Mg alloys at OCP after 48 h immersion in 3.5 wt% NaCl solution.(c-d) Electrical equivalent circuits(EECs) models to fit the impedance spectra of Al coated Mg alloy (c),Ti/Al coated Mg alloy (d),and bare Mg alloy (e).
Two capacitive loops at high and low frequency regions were considered for the coated AZ31B Mg alloys.The impedance spectra of coated Mg alloys were acceptably fitted with the electrical equivalent circuits (EECs,Fig.15c,d).Used EECs models for fitting the impedance spectra of uncoated AZ31B Mg alloy,Al,and Ti/Al coated AZ31B Mg alloys are presented in Fig.15c–e.EEC for bare AZ31B Mg alloy is comprised of Rct(charge transfer resistance across the electric double layer at the electrode/electrolyte interface),Rs(solution resistance),Qdl(CPE related to electrical double layer),L (adsorption inductance) and RL(adsorption resistance) elements.On the other hand,EEC for Al coated AZ31B Mg alloy is constituted by Rs,Rct,Qdl(CPE related to electrical double layer (inner)),Zw(Warburg diffusive impedance),Qc(constant phase element for surface film (outer)),and Rc(resistance which includes the resistance of the oxide film/corrosion products and the electrolyte resistance inside the pores [86]) elements.An approximately straight line at low frequency range which is the characteristic of diffusion-controlled reaction[87,88],was observed for the Al coated Mg alloy (Nyquist spectra,Fig.15b)in chloride containing solution [89–91].However,EEC for Ti/Al coated Mg alloy consists of Rs,Qc,Rc,Qdl,elements and Rctelement which is not in series with Zwelement(Fig.15d).The impedance spectra of all samples were perfectly fitted to the above-mentioned EECs.Likewise,with regard to the non-ideality of the systems,the capacitors were replaced with the constant phase elements(CPE)for all samples[90].
Rctcan predominantly control the rate of electrochemical processes at the electrode/electrolyte interface (across the electrical double layer) [92,93].Therefore,larger Rctcan considerably decelerate the electrochemical dissolution process and could result in higher corrosion resistance [40].Bare AZ31B Mg alloy with the lowest Rctshowed the highest corrosion rate between the three (3) samples.This could be attributed to the low protective performance of the formed corrosion surface film on the Mg alloy surface in corrosive solution.However,Rctvalues for Al,and Ti/Al coated Mg alloys were 11591Ωcm2,and 35,463Ωcm2,respectively.This indicates that Mg alloy could be protected by Al and Ti/Al coatings.Nevertheless,Rctvalue for the Ti/Al coated Mg alloy is roughly 3 times the value of Rctfor the Al coated Mg alloy.Moreover,The Nyquist spectra for Ti/Al coated Mg alloy didn’t show straight line at low frequency range(characteristic of diffusion-controlled reaction).The above-mentioned results reveal that the cold sprayed Ti (with noticeable passivation behavior (Fig.9c)) top layer not only lowers the wear rate of Al coated Mg alloy,but also can improve the corrosion behavior of Al coated Mg alloy in the chloride containing solutions.
The polarization resistance at the corrosion potential (Rpol)was employed to characterize the corrosion resistance.Based on the Eqs.(4)–(6) and EECs models in Fig.15c–e,Rpolvalues for uncoated and coated Mg alloys were calculated after 48 h immersion in 3.5 wt% NaCl solution [94,95].
Ti/Al coated Mg alloy showed higher corrosion resistance than Al coated Mg alloy even after 48 h immersion in 3.5 wt% NaCl solution (Table 4).It is predicted that this significant improvement could also be maintained even during longer immersion times in chloride containing solutions.

3.3. Immersion tests for 264 h: evaluation of corrosion protection performance of cold sprayed coatings
To further explicate the efficacy and protective performance of the CS double layered coatings on the Mg-based alloy substrates in chloride containing solutions,the immersion test [96–98]was performed on the coated samples in 3.5 wt% NaCl solution for 264 h.Fig.16 demonstrate the surface morphological characteristics of Ti/Al and Al coated Mg alloys after immersion tests in chloride containing solutions for 264 h.At the beginning of the immersion test,no appreciable corrosion was seen for the CS Al coating on Mg alloy.Nevertheless,part of the CS Al coating (in the present research) finally ruptured after 264 h of immersion(Fig.16a and s) in 3.5 wt% NaCl solution.This observation could be related to the galvanic cell formation between the Mg alloy substrate and CS Al coating (as a noble barrier coating),corrosion products formation and their pile up at the interface during immersion time.In fact,exacerbating the localized corrosion and the presence of interconnected porosities in the CS Al coating might have led to this occurrence for the Al coating after 264 h.In another research,compared to friction stir-spot-processed Al coating on AZ91D Mg Alloy,corrosion product layers which were composed of Mg and O elements were also observed at the as-cold sprayed Al coating/AZ91D Mg alloy substrate interface after corrosion test [99].Furthermore,the formation of loose and expanded corrosion products at the cold sprayed Cu coating (as a noble barrier coating)/Mg alloy substrate interface was reported for both as sprayed and ultrasonic shot peened Cu coatings on Mg alloy [100].This gradually ruptured the Cu coating and finally led to the coating failure during the immersion test in 3.5 wt% NaCl solution [100].In the present research,CS Al coating having low compactness and through thickness porosities could not isolate the Mg alloy from the corrosive solution.In fact,the corrosive medium slowly infiltrated into the coating through some channels(e.g.,pores,crevices,interparticle boundaries with low quality,etc.,) and finally reached the Mg alloy substrate during immersion(Fig.17a,b).Al,Mg(from the Mg-based alloy substrate),O as well as other elements such as Cl and Na were found on the mono layered CS Al coating surface (Fig.16d and e) after 264 h of immersion.

Fig.16.SEM images and EDS analysis of CS Al coating surface after 264 h of immersion test in 3.5 wt% NaCl solution (a–c),EDS spectrum and quantification results (d–g) of regions A and B in Figs.16b,16c,SEM images and EDS analysis of CS Ti/Al coating surface after 264 h of immersion test in 3.5 wt% NaCl solution (h–j),EDS spectrum and quantification results (k–r) of region A and spots 1–3 in Fig.16j,photo macrographs and stereomicroscope micrographs of samples before and after long-term immersion tests for 264 h of immersion in 3.5 wt% NaCl solution (s).

Fig.17.Schematic illustration of corrosion mechanism for CS Ti/Al (a) and CS Al coatings (b,c) on Mg alloy during the immersion tests in 3.5 wt% NaCl solution.
In contrast to the CS Al coating on Mg alloy,any apparent corrosion pits and the other localized corrosions events were not observed on the CS Ti coating (for the Ti/Al/Mg system)after immersion test for 264 h (Fig.16h-1r and 16 s).Also,scratches (i.e.,grinding tracks caused by the coating surface preparation) were still observable after 264 h of immersion in 3.5 wt% NaCl solution (Fig.16j,s).Moreover,Ti and O elements (as main constituent elements of the corrosion products) that might originate from the presence of Ti-oxides [44]were detected on the CS Ti top coating surface after 264 h of immersion (Fig.16j).Furthermore,Mg element (from Mg alloy substrate) and Al element from the CS Al underneath coating were not identified on the Ti top coating surface.In contrast to CS Al coating on Mg-based alloy (Figs.16s and 17c),no obvious change was seen on the Ti top coating surface (after 11 days of immersion,Fig.16h-r and s).It is also clearly seen that the multilayered coating (i.e.,Ti/Al coating)is still attached to Mg alloy.This could be related to the lack of corrosion products formation and accumulation at the Ti layer/Al layer,and Al layer/AZ31B Mg alloy interfaces (in Ti/Al/Mg system) after immersion test in 3.5 wt% NaCl solution.Actually,HPCS Ti top coating (in the present research)with a high passivation tendency and pitting corrosion resistance can substantially alleviate the problems accompanied by the CS Al coating (having through thickness porosities and inter-particle boundaries with low quality) and is able to isolate the Al coated Mg alloy from chloride containing solutions for up to 264 h.
4.Conclusions
Compared to the Mg bulk and the mono layered CS Al coating surfaces,the surface having higher hardness i.e.,double layered CS Ti/Al coating resulted in considerably lower wear rate.This revealed that the CS Ti top coating could raise the surface hardness as well as the wear resistance of the mono layered Al coated Mg-based alloy.The CS Ti top coating (in the Ti/Al/Mg system) presented wide passive window even at higher potentials (up to pitting potential: Eb=~1293 mVSCE) in a chloride containing solution.The pitting corrosion resistance (Eb-Ecorr) was the highest for the double layered CS Ti/Al coated Mg-based alloy compared to the Al coated Mg alloy in 3.5 wt% NaCl solution.Potentiodynamic polarization curves also showed the probability of transition from metastable pits to the stable pits for the CS Al coating is considerably higher compared to the CS Ti top coating(for the Ti/Al/Mg system).Also,according to the obtained potential (~-5387 mVSCE) for the double layered CS Ti/Al coating on Mg-based alloy and E-pH diagram for Ti,it is postulated that the CS Ti coating is in the passivation region in most pH ranges even after 48 h of immersion in 3.5 wt%NaCl solution.In fact,the stored energy in the cold sprayed Ti top coatings (as a passive metal) has probably assisted the easy passivation.Apparent corrosion pits and the other localized corrosions were not observed on the CS Ti top coating(for the Ti/Al/Mg system) even after a long-term immersion test for 264 h.The above-mentioned results disclosed that the CS Ti top layer not only lessens the wear rate of the mono layered Al coated Mg-based alloy,but also can substantially improve the localized corrosion behaviors of the mono layered Al coated Mg-based alloys in the chloride containing solutions even after 11 days.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
RKG would like to acknowledge the financial support received from the National Science Foundation (NSF-CMMI 2131441) under the direction of Dr.Alexis Lewis.The authors would like to acknowledge the ASB Industries,Inc for providing the specimens.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.09.008.
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
- Achieving high ductility and strength in magnesium alloy through cryogenic-hot forming
