Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
Guangyu Li,Wenming Jiang,Feng Guan,Zheng Zhang,Junlong Wang,Yang Yu,Zitian Fan
State Key Laboratory of Materials Processing and Die &Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
Abstract Mg/Al bimetal combines the advantages of both aluminum and magnesium and has broad application prospects in automotive,aerospace,weapons,digital products and so on.The compound casting has the characteristics of low cost,easy to achieve metallurgical combination and suitable for the preparation of complex bimetallic parts.However,bimetallic joint strength is low due to differences of physical properties between Al and Mg,oxide film on metallic surface and interfacial Al-Mg IMCs,which is closely related to the interfacial microstructure and properties.Therefore,how to control the interface of the bimetal to achieve performance enhancement is the focus and difficulty in this field.At present,there are mainly the following strengthening methods.First,the “zincate galvanizing” and “electrolytic polishing+anodic oxidation” technology were exert on the surface of Al alloy to remove and break the oxide film,which improved the wettability between Al and Mg.Second,the undesirable Al-Mg IMCs were reduce or elimination by adding the interlayers (Zn,Ni and Ni-Cu).Thirdly,the evolution process of interfacial microstructure was changed and fine strengthening phases were formed by adding Si element to Al alloy or rare earth element to Mg alloy.Fourthly,mechanical vibration and ultrasonic vibration were applied in the process of the filling and solidification to refine and homogenize the interfacial structure.Finally,some other methods,including secondary rolling,thermal modification,heat treatment and constructing exterior 3D morphology,also can be used to regulate the interfacial microstructure and compositions.The above strengthening methods can be used alone or in combination to achieve bimetallic strengthening.Finally,the future development direction of the Mg/Al bimetal is prospected,which provides some new ideas for the development and application of the Mg/Al bimetal.
Keywords: Mg/Al bimetal;Preparation;Compound casting;Interfacial regulation;Interface strengthening;Research progress.
1.Introduction
With the rapid development of modern industry and the increasing requirements for environmental protection,people have put forward higher requirements for the comprehensive performance and lightweight of materials [1,2].Magnesium alloy (Mg alloy) and aluminum alloy (Al alloy),the two light engineering metals with the lowest density,have their advantages and disadvantages.Magnesium alloy has low density,excellent shock absorption,good electromagnetic shielding and machinability,but the corrosion and wears resistance are poor [3,4].Aluminum alloy has the advantages of wear resistance,corrosion resistance and good plasticity,but its density is higher than that of magnesium alloy [5–7].In many cases,it is difficult for a single material to meet the increasing demand for high comprehensive performance and lightweight at the same time.In order to solve this problem,the concept of“Mg/Al bimetal” has been put forward.
Mg/Al bimetal refers to the connection of aluminum alloy and magnesium alloy by mechanical or metallurgical bonding to make different positions meet different performance requirements,which will be able to realize the complementary advantages of the Al alloy and Mg alloy [8–10].It can not only meet the needs of comprehensive performance but also realize the lightweight,which has excellent application prospects in automobile,aerospace,weapons and equipment,pipeline transportation,construction engineering,digital 3C and other fields.
At present,the preparation methods of Al/Mg bimetal can be divided into solid-solid compound,solid-liquid compound and liquid-liquid compound,respectively,according to the state of the material before preparation,and each method has its own advantages,disadvantages and applicable fields,as listed in Table 1.Compound casting refers to utilize the casting technology,namely pouring the liquid metal on or around the solid alloy or pouring two kinds of liquid metal at the same time,to achieve the combination between Al alloy and Mg alloy,as exhibited in Fig.1.Its advantages are simple preparation process,easy to realize metallurgical bonding and small residual stress.Thus,it is suitable for the preparation of the complex parts with large size,large joint area and complex connection interface.
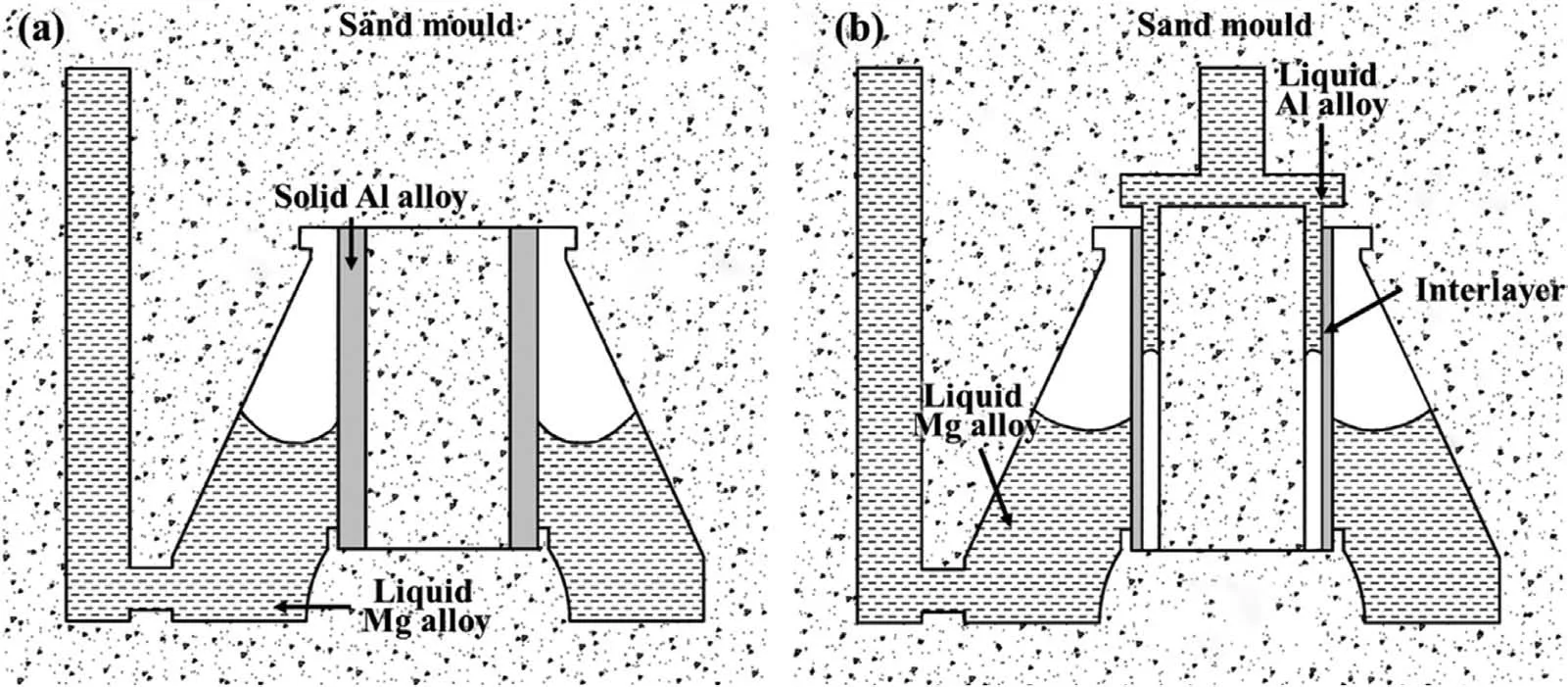
Fig.1.Schematic diagram of compound casting: (a) Solid-liquid compound;(b) liquid-liquid compound.
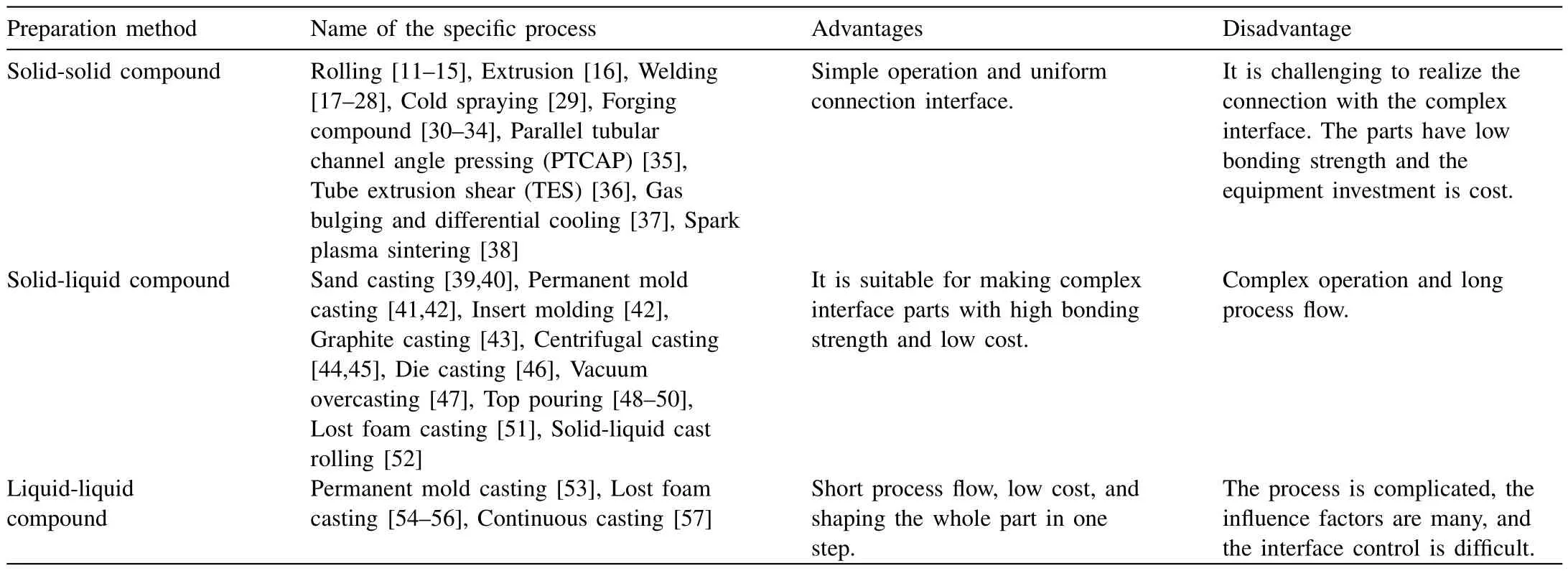
Table 1Advantages and disadvantages of the different preparation methods of the Al/Mg bimetal.
However,there are some problems in the preparation process of the Mg/Al bimetal with compound casting.First,due to the difference in physical properties between aluminum and magnesium,the interface is easy to produce stress concentration and cracking [58].Second,although it is easy to achieve metallurgical bonding,the interface layer is mainly composed of Al-Mg IMCs (Al12Mg17,Al3Mg2,Al30Mg23,etc.).These IMCs belong to the brittle and hard phase and have poor resistance to plastic deformation,which is easy to crack under external force [59].Third,for compound casting,the surface of solid inlay has oxide films which are difficult to remove.On the one hand,these oxide films reduce the wettability between the solid inlay and liquid metal.On the other hand,the oxide films will react with the liquid metal to form oxide inclusion defects at the interface.
Under the effect of the above problems,the performance of the Mg/Al bimetal prepared by compound casting cannot meet the requirements of practical use at present,so it needs to be further improved.The performance of the Mg/Al bimetal is mainly related to the Al-Mg interface.Therefore,how to regulate the interface of the Mg/Al bimetal is the focus and hotspot of current research.This paper first introduces the preparation methods and existing problems of the Mg/Al bimetal fabricated by compound casting.Then,the research on regulating and strengthening the Mg/Al bimetallic interface is summarized,and several potential bimetallic strengthening methods are proposed.
2.Preparation methods of the Mg/Al bimetal fabricated by compound casting
The compound includes sand casting,permanent mold casting,insert molding casting,graphite casting,centrifugal casting,die casting,lost foam casting,vacuum overcasting,top pouring,and solid-liquid cast rolling.Each method is described in detail below.
2.1. Sand casting
Sand casting is the most used casting method,and the principle of preparing Mg/Al bimetal by sand casting is shown in Fig.2.The polished solid inlay was first fixed in the sand mold,and carbon dioxide gas was injected as a protective gas before pouring.The liquid alloy was then poured into the mold cavity to achieve the combination between aluminum and magnesium.

Fig.2.Schematic diagram of the sand compound casting [39].
E.Hajjari et al.[39]used pure Al and pure Mg,respectively,as the solid inlays and poured another metal to obtain the Mg/Al bimetallic castings.Studies show that when pure Mg is used as an inlay,the metallurgical bonding cannot be formed in the bimetallic castings,and there are gaps at the interface,as indicated in Fig.3(a).Only when the pure Al is used as an inlay,the metallurgical reaction layer can be formed at the interface,but there are a few hole defects at the interface layer,as exhibited in Fig.3(b).The bimetallic interface layer consists of Al3Mg2near the Al matrix,Al12Mg17+δ-Mg eutectic structure near the Mg matrix and Al12Mg17between them,as held up in Fig.3(c).

Fig.3.Microstructure of the Mg/Al bimetal prepared by sand casting: (a) casting Al melt around Mg insert;(b) casting Mg melt around Al insert;(c)interfacial SEM image of Fig.3(b) [39].
Hajjari et al.[40]pointed out in another study that there were net-like periodic antiphase domains (APDs) in the reaction layer of Al12Mg17,as displayed in Fig.4.The size of the APDs is not constant,which depends on the amount of deviation from the stoichiometric proportion (SP) of Al12Mg17.With the increase of the deviation from SP,the size of APDs decreases and the density of the antiphase boundaries (APBs)increases.

Fig.4.TEM results of Al12Mg17 reaction layer: (a,b) Bright field image in two different regions;(c) HRTEM image of APD and APB;(d,e) SAED images for a single domain and multiple domains in Fig.4(a),respectively;(f) A larger image of Fig.4(e) [40].
2.2. Permanent mold casting
The permanent mold casting is also a common and simple method for preparing the Mg/Al bimetals,and the principle is held up in Fig.5.Liu et al.[41]first inserted the pure Al inlay into a cylindrical steel mold with a diameter of 36 mm and a height of 90 mm,and them were preheated at 300 °C.Pure magnesium was then melted and poured into a steel mold under vacuum,which is cooled to obtain an Mg/Al bimetallic casting.

Fig.5.Schematic diagram of the permanent mold casting [41].
He et al.[42]also studied the microstructure,mechanical properties and corrosion property of laser-sprayed Al/AZ91D bimetallic composite prepared by the permanent mold casting,and the preparation principle was exhibited in Fig.6.First,a layer of pure aluminum coating with a thickness of 5±1 mm was sprayed on the steel mold by arc spraying,and then the AZ91D magnesium melt at 720 °C was poured into the steel mold with a preheating temperature of 250 °C.After cooling,the steel mold was removed to obtain the bimetallic materials.The results show that the interfacial layer of the bimetal is also composed of Al3Mg2,Al12Mg17and Al12Mg17+Mg eutectic structure.There are four forms of Al12Mg17+Mg eutectic structure: (a) feather-like structure,(b) honeycomb structure,(c) radial structure and (d) interdendritic structure,and Fig.7 shows the relationship between the SEM morphology and the composition of Mg element.The reason may be related to the uneven distribution of Al,Mg and O elements.The hardness of the four eutectic structures is different,increasing from 66 HV to 144 HV successively.
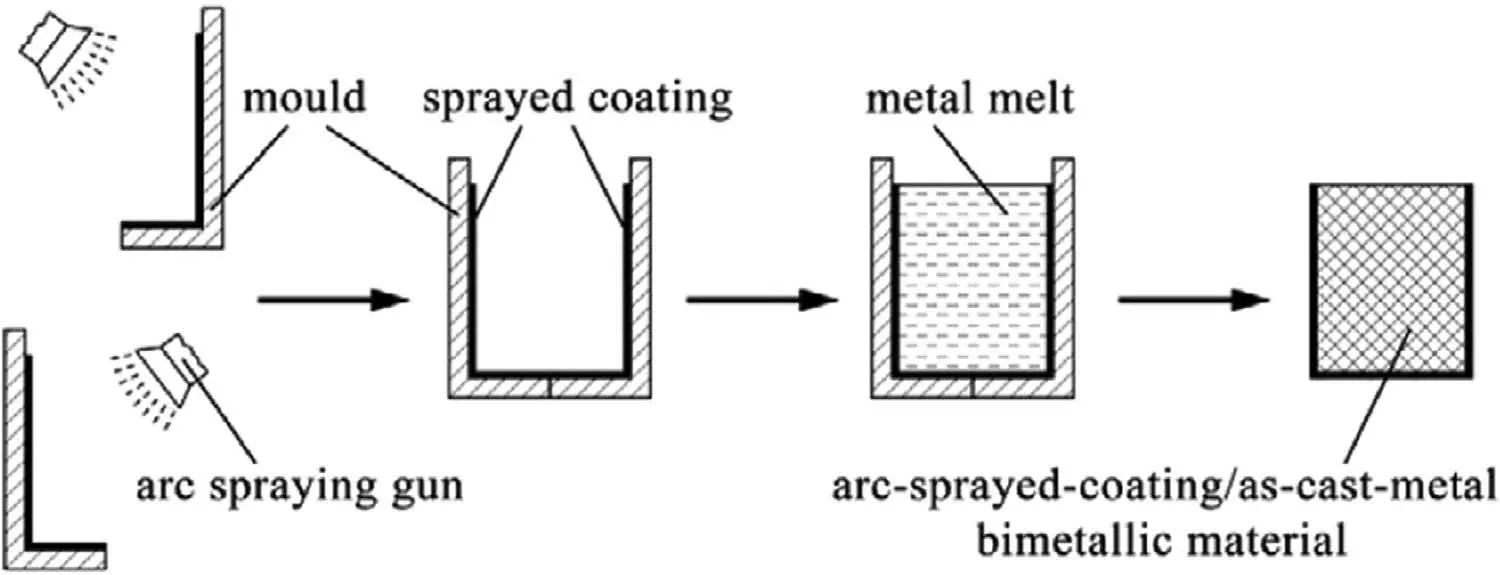
Fig.6.Preparation schematic of the laser-sprayed Al/AZ91D bimetallic composite [42].

Fig.7.The relationship between the SEM morphology and the composition of Mg element: (a) Feather-like structure;(b) honeycomb structure;(c) radial structure;(d) interdendritic structure;(e) schematic diagram of composition and morphology [42].
2.3. Insert molding casting
The insert molding casting also uses the metal as a mold,and the difference is the order of the inlays and liquid metal placed into the model.Liu et al.[60]prepared the Al 6061/AZ31 composite material by the insert molding casting,and the schematic diagram is displayed in Fig.8.Firstly,the Al 6001 inlay and steel mold were preheated for 30min at 450 and 500 °C,respectively.Then the molten AZ31 alloy was poured into the steel mold at 680 °C,and the Al 6001 inlay was inserted into the AZ31 melt from the top.Finally,the whole structure was cooled in the cooling water together.It has been shown that metallurgical connections can be formed between Al 6001 and AZ31,and the phase composition of the interfacial layer is the same as that of the bimetals prepared by the sand casting above,as demonstrated in Fig.9.Moreover,there are also some pore defects found in the interface layer.
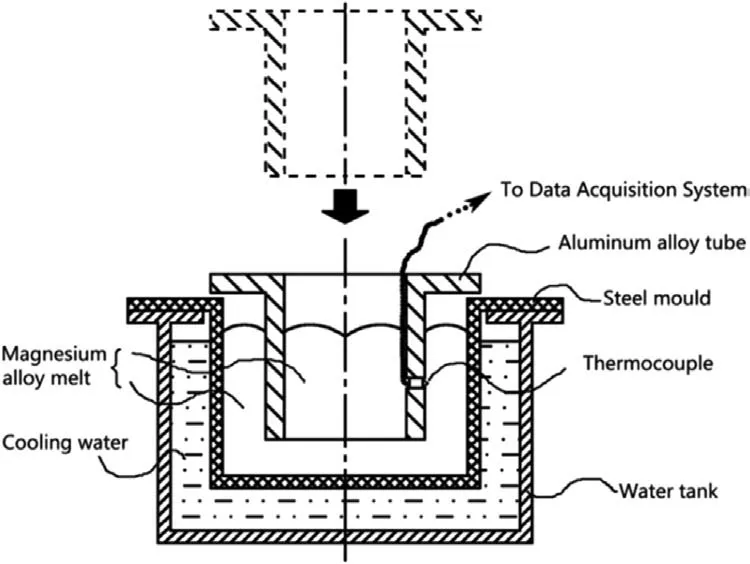
Fig.8.Schematic diagram of the insert molding casting [60].
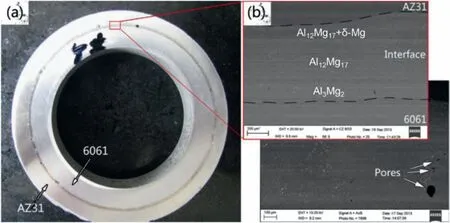
Fig.9.Micromorphology and microstructure of the Al 6001/AZ31 bimetal obtained by the insert molding casting: (a) Micromorphology;(b) SEM image in the interface region [60].
2.4. Graphite casting
Graphite casting refers to the casting method using graphite as a model.Ren et al.[43]put the solid Zl105 into a graphite mold and then poured the AZ91D liquid to prepare the Mg/Al bimetal,and the preparation principle is held up in Fig.10.The research showed that when the casting temperature was 660–680 °C,the Mg/Al bimetal could obtain a good interface bonding.The interface layer was composed of three reaction layers: Mg+Mg17Al12layer near AZ91D,Al3Mg2+Mg2Si+α-Al layer near Zl105 and Al12Mg17+Mg2Si layer in the middle,as exhibited in Fig.11.It can be found that,different from the above bimetal,there are many Mg2Si particles in the Al3Mg2and Al12Mg17regions of the interface layer,which may be caused by the Si element contained in the ZL105 alloy.Other results also manifest that Mg2Si phase will appear in the interfacial layer when the aluminum alloy contains Si element [61–63].

Fig.10.Schematic diagram of the Mg/Al bimetal prepared by graphite mold casting [43].
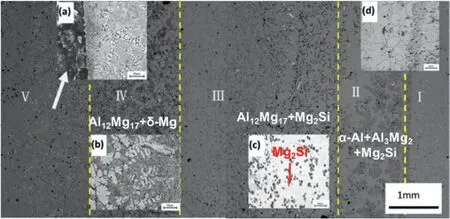
Fig.11.Microstructure of the ZL105/AZ91D bimetal prepared by graphite mold casting [43].
2.5. Centrifugal casting
Centrifugal casting is the technology of injecting liquid metal into the high-speed rotating casting,so that the liquid metal does centrifugal motion to fill the casting.This method is suitable for preparing cylindrical bimetallic castings with cavity structure.Sarvari et al.[44,45]used the centrifugal casting to produce the Mg/Al bimetallic castings,and the principle is displayed in Fig.12.Firstly,the solid pure Mg tube and pure Al tube are obtained by machining.The dimensions of the pure Mg tube are 35 mm height with the inner diameter and outer diameter of 74 and 84 mm respectively,and the dimensions of the pure Al tube are 50 mm height with the inner diameter and outer diameter of 72 and 104 mm respectively.After the solid metal tube is put into the mold,the centrifugal casting equipment is turned on,and the liquid metal is poured into the mold from the position 1 in Fig.12.When the temperature reaches 150 °C,the centrifugal casting equipment is stopped and the casting is taken out,and the bimetal is obtained.It is found that when the liquid Mg is poured into the Al tube,large cracks are produced,and the metallurgical bonding is realized in only part of the positions.However,when the Al melt is poured into the solid Mg tube,the cracks are significantly reduced,and the metallurgical bonding is formed in most of the positions,as exhibited in Fig.13.

Fig.12.Preparation schematic of the Mg/Al bimetal by centrifugal casting[44].

Fig.13.Macro morphology of the Mg/Al bimetal by centrifugal casting: (a)Casting the liquid Mg into the solid Al tube and (b) casting the liquid Al into the solid Mg tube [44].
2.6. Die casting
Die casting is a precision casting method that uses high pressure to force molten metal into a complex metal mold,which is also used to prepare the Mg/Al bimetal.Zhu et al.[46]used high pressure die casting (HPDC) to prepare the Mg/Al bimetal,and the principle is indicated in Fig.14.First,the 6061 aluminum tubes with height of 99 mm,thickness of 3.3 mm as well as diameter of 38.1 and 50.8 mm,respectively,were put into the metal mold and were preheated,as displayed in Fig.14(a).Then,the AM50 magnesium alloy melt was poured into the injection chamber and vacuumed to form a lower pressure than the atmosphere in the cavity,as exhibited in Fig.14(b).Under this pressure,the magnesium alloy liquid was filled,and the Mg/Al bimetallic casting was obtained after solidification,as held up in Fig.15(a)and(b).According to the SEM image of the cross section of the casting,it can be found that there is no metallurgical bonding between Al 6061 and AM50 in the as-cast sample,and the metallurgical reaction layer can be obtained only after subsequent annealing treatment of the as-cast sample,as demonstrated in Fig.15(c)and (d).
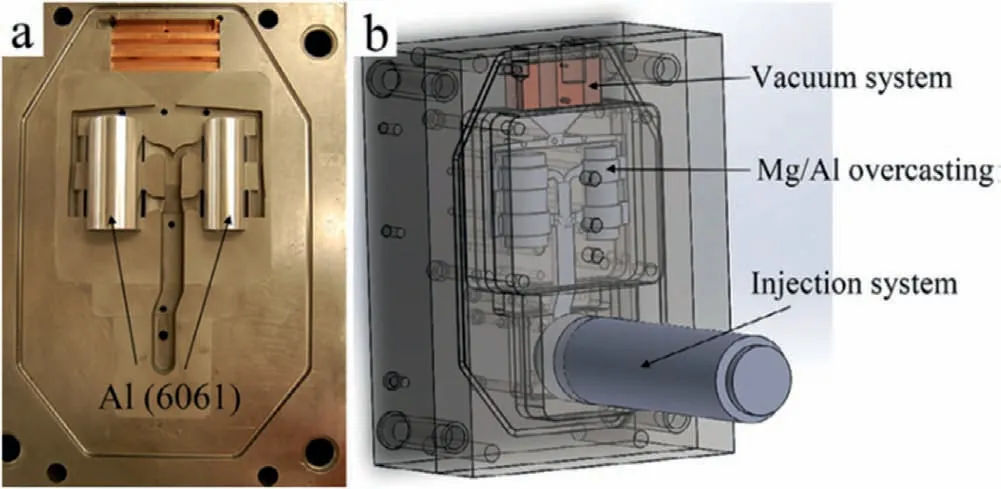
Fig.14.Schematic diagram of the high pressure die casting (HPDC) to prepare the Mg/Al bimetal: (a) Mold of die casting and Al 6061 tubes;(b) die casting equipment after assembly [46].

Fig.15.Mg/Al bimetallic castings produced via HPDC: (a) Macro morphology of the Mg/Al bimetal;(b) cross-section of the Mg/Al bimetal;(c)microstructure of the as-cast sample;(d) microstructure of the sample after annealed at 430 °C for 4 h [46].
2.7. Lost foam casting
The lost foam casting (LFC) is also used to prepare the Mg/Al bimetal,and the preparation principle is exhibited in Fig.16.Li et al.[51]fabricated the A356/AZ91D bimetallic composite via the LFC,and the specific process is as follows.First,the foam model and the A356 solid inlay were made,and the two were combined to obtain the composite model,which was brushed with paint and dried.Then,the dried composite model was put into the sand box,and the vibration was exerted on the sandbox after the dry sand without binder filled into the sandbox.Next,the top of the sandbox was covered with a plastic film,and a pouring cup was put on the top of the sprue after the sandbox was vacuumed to reach 0.03 MPa.Finally,the AZ91D melt with a temperature of 730 °C was poured into the model from the pouring cup.The foam was decomposed under the action of high temperature metal and discharged through the coating,so the liquid metal occupied the position of the original foam model and contacted with the A356 inlay.
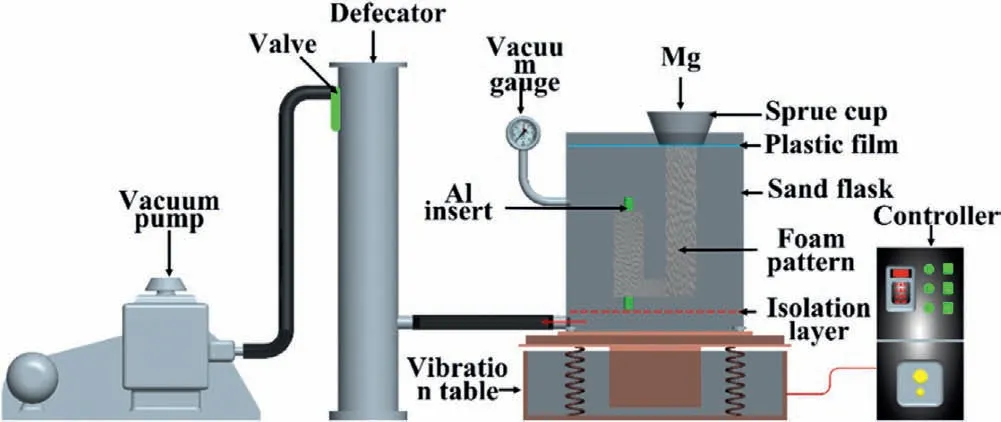
Fig.16.Schematic diagram of the lost foam casting [51].
As can be seen from Fig.17(a) that a good metallurgical bonding is achieved between A356 and AZ91D with an interface layer of 1500 μm thickness.The interface layer is uneven,and it is composed ofδ-Mg+Al12Mg17eutectic structure,Al12Mg17+Mg2Si and Al3Mg2+Mg2Si from AZ91D side to A356 side in sequence,as displayed in Fig.17(b)–(e).
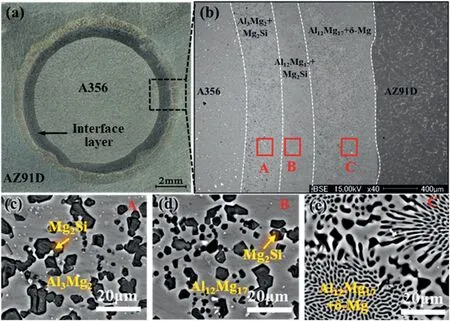
Fig.17.Interfacial morphology and microstructure of the Mg/Al bimetal produced via LFC: (a) Morphology;(b) interfacial microstructure;(c),(d),(e)corresponds to A,B,C region in Fig.17(b),respectively [51].
2.8. Vacuum overcasting
The principle of preparing Mg/Al bimetal by the vacuum overcasting is shown in Fig.18[47].First,the A390 substrate and the AM60 ingot were loaded into the device and vacuumed.Then,the AM60 ingot was heated to melt and the A390 substrate was preheated.Next,the preheated A390 substrate and molten AM60 were placed in the designated position,and the liquid AM60 were dropped on the solid A390 substrate.Finally,the Mg/Al bimetal was obtained after holding for a period of time,and the interfacial morphology was exhibited in Fig.19.It can be seen that a metallurgical connection with a thin interface layer is achieved by this technology.
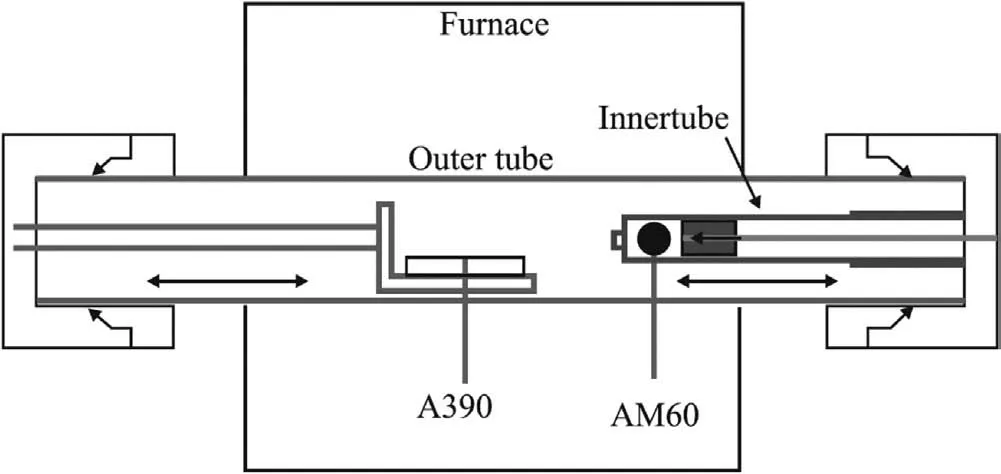
Fig.18.Schematic diagram of the vacuum overcasting [47].

Fig.19.Interfacial morphology of the Mg/Al bimetal obtained by vacuum overcasting: (a) Optical micrograph;(b) SEM images;(c) EDS line scans [47].
2.9. Solid-liquid cast rolling
Yang et al.[52]prepared the Al 6061/AZ31B bimetal by the solid-liquid cast rolling process,and the principle is demonstrated in Fig.20.First,the Al 6061 alloy was melted into a liquid state and a solid AZ31B alloy substrate is placed on the mill with a roll gap of 7 mm at room temperature.Then the liquid Al 6061 was poured on the solid AZ31B substrate,and the rolling mill was started at the same time.The rolling speed was 42 mm/s and the maximum rolling force was 170 kN.The two alloys are discharged together between the two rollers,namely,the solid-liquid cast rolling process was carried out.After cooling,the Mg/Al bimetal was obtained.The effect of the pouring temperature (640 °C,660 °C,680 °C,700 °C) on the microstructure of the Mg/Al bimetal was investigated.As can be seen that the thickness of the interface layer increases with the increasing of the pouring temperature,as displayed in Fig.21.
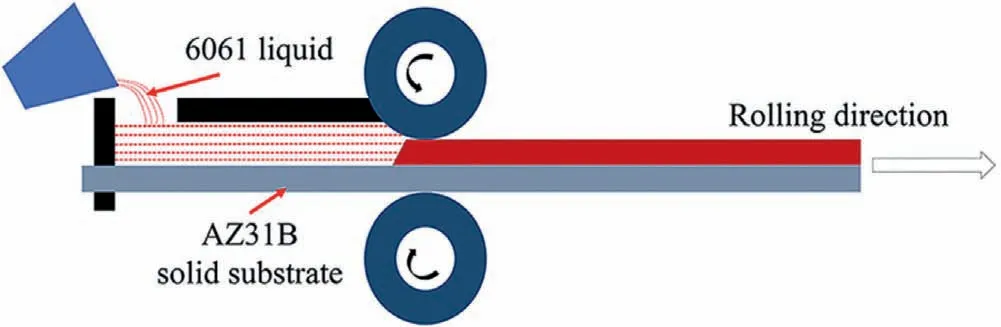
Fig.20.Schematic diagram of the solid-liquid cast rolling [52].

Fig.21.Interfacial microstructure of the Mg/Al bimetal at different pouring temperature: (a) 640 °C;(b) 660 °C;(c) 680 °C;(d) 700 °C [52].
3.Difficulties in the preparation of the Mg/Al bimetal
No matter what method is used to connect the aluminum alloy and magnesium alloy,there are several difficulties in the connection process,including differences in the physical properties,oxide film and Al-Mg IMCs,and specific introduction is as follows.
3.1. Differences in the physical properties
Table 2 lists the common physical properties of the aluminum and magnesium.As can be seen that there are several differences in the physical properties between the aluminum and magnesium,such as the melting points,linear expansion coefficient,specific heat capacity,thermal conductivity,electromagnetic differences,etc.[64–66],which increases the difficulty of connection due to the performance mismatches[67].Generally,the more significant the difference between the melting point,linear expansion coefficient,specific heat capacity and thermal conductivity of the two metals,the more difficult it is to weld.For example,in the research of Sarvari et al.[44,45],the cracks appeared at the interfacial zone,as exhibited in Fig.14.This can be attributed to the shrinkage forces’effects derived from significant difference in linear expansion coefficient (CTE) between the Mg and Al,and the principle is indicated in Fig.22.As can be seen that the extent of the cracks depends on the pouring sequence.Only partial cracks present when the liquid Al is poured on the solid Mg,while most positions have cracks when the liquid Mg is poured on the solid Al,and this phenomenon had been verified by other investigation [39,68].
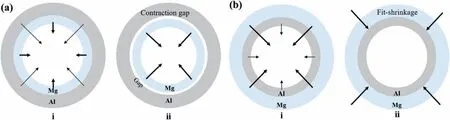
Fig.22.Schematic of shrinkage forces effect in the interface: (a) Pouring Mg liquid on Al solid;(b) pouring Al liquid on Mg solid [44].

Table 2Physical properties of magnesium and aluminum.
3.2. Oxide film
Aluminum alloys and magnesium alloys are both highly oxidized metals.Thus,there is a layer of oxide film on the surface of the aluminum and magnesium in the air.This oxide film is stable and difficult to remove.Even if removed by mechanical or chemical methods,it will re-oxidize very quickly if no other measures are taken to protect it.Moreover,the oxide film on the surface of the magnesium alloy is extremely compact and easy to absorb water [69].
On the one hand,the existence of the oxide film causes the fresh surfaces of the two metals to not be in direct contact during the connection process,which makes poor wettability between the two metals.For example,Xu et al.[47]compared the wettability between the A390 solid without surface treatment and that with “zincate+galvanizing” treatment (without oxidation film) with AM60 liquid,and it was found that the wettability between liquid Mg and solid Al without oxide film was better,as held up in Fig.23.

Fig.23.Wettability test between solid A390 and liquid AM60: (a) Without surface treatment;(b) with “zincate+galvanizing” treatment [47].
On the other hand,the oxide film will lead to the generation of metal oxides during the bonding process and cause oxidized inclusion defects,as displayed in Fig.24.According to the research of Hajjari et al.[70],the formation mechanisms of the oxidized inclusion defect may be summarized into two kinds.One is due to the oxidation of the magnesium melt during the casting process,and these oxides are sucked into the molten metal to produce a double oxide film(Fig.25),which is dispersed throughout the interfacial region to form oxidation defects.The other is attributed to the reaction between the Mg melt and the oxidation film on the surface of the Al insert.According to the Eqs.(3–1),(3–2)and (3–3) [39]:

Fig.24.Interfacial microstructure of the Al 413/Mg bimetal: (a) Microstructures with low magnification;(b) map scanning of O element;(c) and (d):microstructures with high magnification [70].

Fig.25.Formation mechanisms due to the oxidation of the magnesium melt during the casting process [70].
the Mg melt can react easily with the oxidation film consisted of Al2O3or SiO2existed in the Al alloys containing Si element owing to the negative standard Gibbs free energy.These oxidation products remain in the interface to form defects.Therefore,the bimetallic connection performance is weakened.
3.3. Al-Mg IMCs
According to the Al-Mg binary diagram and experimental result,Al and Mg is easy to react to generate Al-Mg IMCs such as Al12Mg17,Al3Mg2and Al30Mg23with big grain size[71–73],as displayed in Fig.26.In addition,these Al-Mg IMCs also have preferred orientation and stress concentration[51],as indicated in Fig.27.
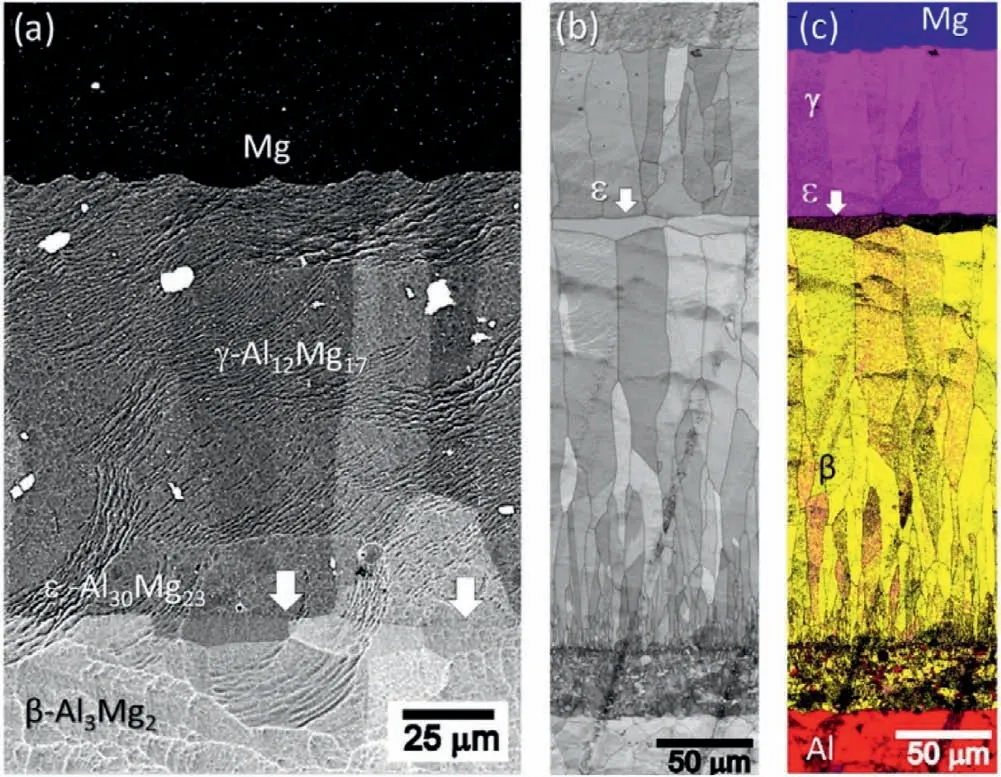
Fig.26.Interfacial microstructure and EBSD results of the Mg/Al bimetal:(a) BSE image;(b) band contrast image;(c) phase maps [71].

Fig.27.EBSD results of the A356/AZ91D bimetal: (a) SE image;(b) phase mapping;(c) stress distribution;(d) IPF profile [51].
The hardness of these Al-Mg IMCs is 250–370 HV,which is much higher than that of the Al matrix and Mg matrix(60–70 HV) [51,74],as listed in Table 5.Moreover,these IMCs are brittle and easy to crack under the action of external forces.Their ability to resist external forces can be expressed by the fracture toughness by the Eq.(3–4) [74]:
where,Kc means the fracture toughness,α is a constant(0.016±0.004)calculated by Anstis et al.[75],E denotes the Young’s modulus,H represents the Vickers hardness,F signifies the peak load applied during micro-indentation (0.5 N),and c represents the length of a radial crack extending from the corner of an indenter impression.
In the investigations of Wang et al.[74],the c value was measured by the SEM image of the Vickers indentation,as held up in Fig.28.As can be seen that the cracks trend to propagate preferentially along the direction parallel to the interface,so only the crack length c in the horizontal direction is chosen to calculate the fracture toughness.The value of E and H can be found in Table 3.Thus,the fracture toughness ofγ-Al12Mg17andβ-Al3Mg2are 0.105 MPam1/2and 0.062 MPam1/2,respectively,whose values are even lower than that of the Ti or Fe aluminides,as listed in Table 3.These results represent that the Al-Mg IMCs have low fracture toughness.

Fig.28.SEM image of the Vickers indentation: (a) γ-Al12Mg17;(b) β-Al3Mg2 [74].
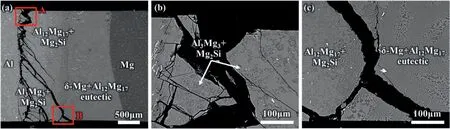
Fig.29.SEM images of the cross-sections vertical to the surfaces of the bimetals: (a) Image with low magnification;(b),(c) corresponds to the image of high magnification in region A,B,respectively [89].

Fig.30.SEM image of the fracture: (a) Al side;(b) Mg side [89].

Table 3Mechanical properties of the Al-Mg IMCs measured in Wang et al.’s and other researcher’s work as well as Ti and Fe aluminides compiled from the literature.
Therefore,the fracture occurs at the interface layer due to the difference of the fracture toughness between the matrix and the Al-Mg IMCs and shows a typical brittle fracture morphology [89],as shown in Figs.29 and 30.
4.Interfacial regulation and strengthening
The practical performance of the bimetal is mainly related to the connection strength of bimetal.The test methods of the bimetallic strength have nine kinds,namely,tensile test[9,10],whole tensile test [52],compression-shear test [20],tensile-shear test[19],push-out shear test[39],whole bending test [90],bending test [43],compression test [16]and rollerdrum peel test [91],and the principles are demonstrated in Fig.31.The results of these strength tests be summed up as the shear strength,tensile strength and bending strength peel strength.The push-out shear test is the most widely used test method for the bimetallic strength in the compound casting.Therefore,unless otherwise specified,the shear strength below refers to the value measured by the push-out shear test.
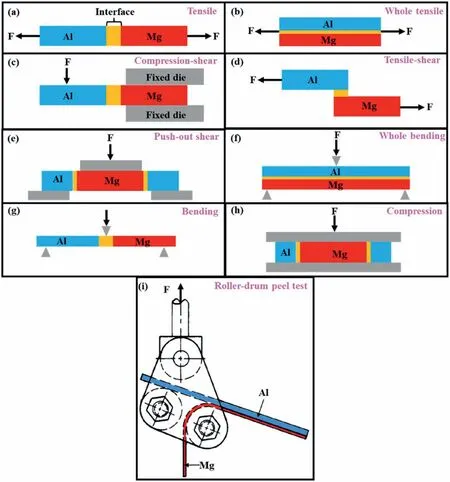
Fig.31.Principle of the strength test: (a) Tensile test [9–10];(b) whole tensile test [52];(c) compression-shear test [20];(d) tensile-shear test [19];(e) push-out shear test [39];(f) whole bending test [90];(g) bending test [43];(h) compression test [16];(i) roller-drum peel test [91].
Due to the difficulties described in Section 3,the performance of the Mg/Al bimetal is low,which has greatly influenced the popularization and application of the bimetal.According to our research,the bimetallic performances are mainly related to the interfacial microstructure and performance,including defects (inclusion,crack,pores,etc.),thickness of interface layer,phase constitution,composition uniformity and grain size of the interface layer,orientation distribution and stress distribution in the interface zone,the hardness and fracture toughness of the phases,and the effective weight of different factors is exhibited in Fig.32.Therefore,it is necessary to regulate the interfacial microstructure and performance to realize the strengthening of the bimetal.This paper summarizes the methods of the interface regulation and strengthening of the Mg/Al bimetals including adding interlayer,alloying treatment,applying vibration field etc.,as illustrated in Fig.33.These methods will be introduced in detail below.

Fig.32.The weight of different factors affecting bimetallic properties.
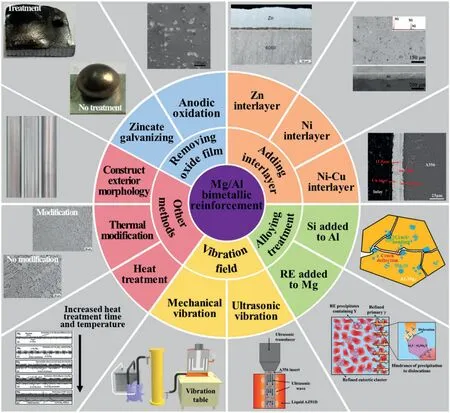
Fig.33.Classification of Mg/Al bimetallic strengthening methods.
4.1. Removing oxide film
Due to the oxide film on the surface of the alloy,the bonding performance of the bimetal is affected.Therefore,if the oxide film can be removed,the performance of the bimetal will be effectively improved.Relevant studies are as follows.
4.1.1.Zincate galvanizing
Xu et al.[47]developed a “zincate+galvanizing” technology to remove the oxide film on the surface of aluminum alloy,and the process consists of the following steps: (a) Degrease;(b) Alkali etching;(c) First picking;(d) First zincate immersion;(e) Second picking;(f) Second zincate immersion;(g) Zinc galvanizing.Solution formulation and process parameters for each step are listed in Table 4.The reactions from step (a) to step (f) are as follows:
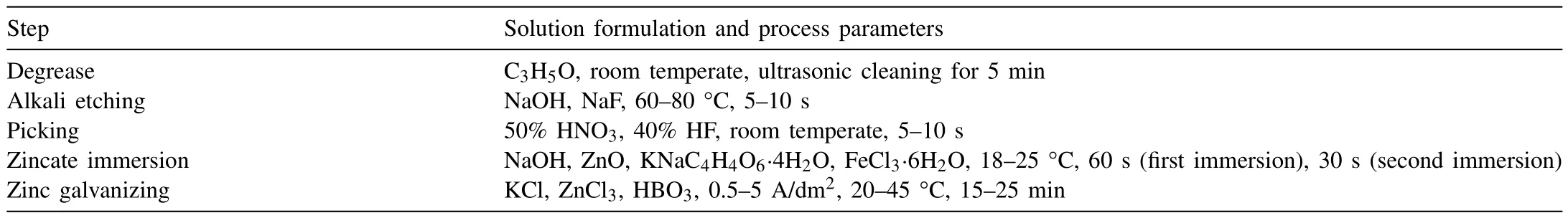
Table 4Solution formulation and process parameters for “zincate+galvanizing” technology.
(i) Dissolution of the Al2O3film:
(ii) Dissolution of Al:
(iii) Reduction of H+,Zn2+and Fe2+:
(iv) Zinc deposition:
Thus,the Zn film is formed on the surface of A390,and its thickness increases with the immersion time.
The XPS results of the aluminum alloy without and with“zincate+galvanizing” treatment are displayed in Fig.34.It can be seen from Fig.34(a) and (b) that the surface of untreated aluminum alloy mainly contains O,Al,Cl and Na elements.Among these elements,Cl and Na are pollutants adsorbed on the surface,the peak value of O is the highest,and the peak value of Al is at 74.7 eV,which is consistent with the binding energy Al3+,indicating that the main component of the surface is Al2O3.It can be seen from Fig.34(c)and (d) that the surface of the treated aluminum alloy mainly contains Zn,Al,Cl,Na,O elements.Thereinto,the peak value of Zn element is the highest,and the peak values of Al element is 72.7 eV,which is the binding energy of metallic Al,indicating that the main component of the surface is Zn.These results prove that “zincate+galvanizing” treatment can effectively remove the oxide film and prevent reoxidation.It is obvious that the wettability of the “zincate+galvanizing”treated aluminum alloy is better than that without treatment,as exhibited in Fig.35 [92].The better wettability is conducive to the connection between the Al and Mg,therefore achieving the strengthening of bimetallic properties.
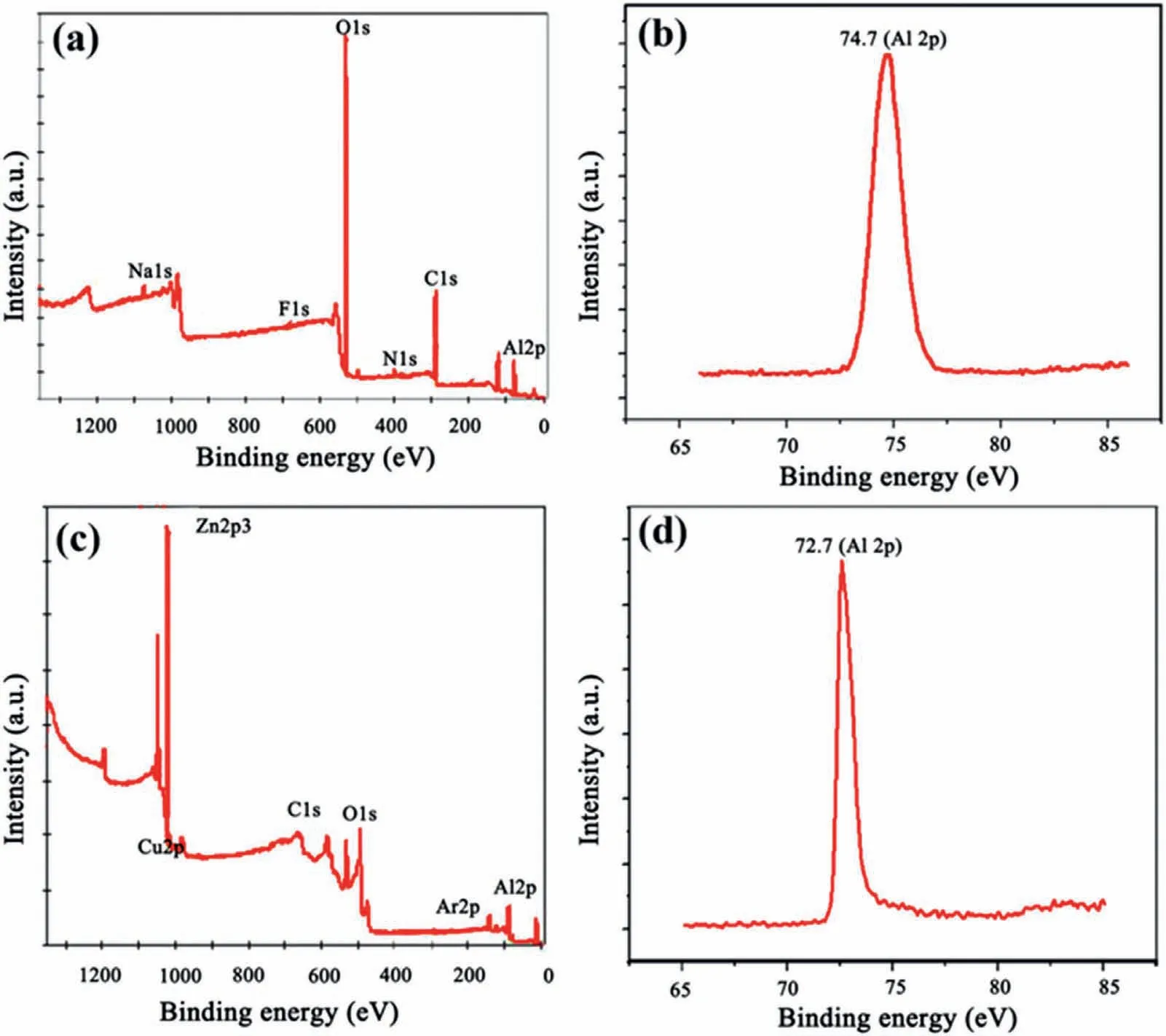
Fig.34.XPS results of A390: (a,b) Without treatment;(c,d) with “zincate+galvanizing” treatment [47].
4.1.2.Anodic oxidation
Zhang et al.[93]used an “electrolytic polishing+anodic oxidation” technology to treat the pure aluminum and Al-0.08 wt.%Ga alloy.The specific process is as follows.Firstly,the Al plates were grinded,polished and cleaned to remove the contaminant.Then,the Al plates were electropolished in an electrolyte consisted of 80 vol.% perchloric acid/ethanol at 16.5 V and 298 K for 300 s.Finally,these specimens were anodized in 0.01 M ammonium pentaborate electrolyte at 50 V and 293 K for 600 s.The SEM images of the surface of Al plates are displayed in Fig.36 after “electrolytic polishing+anodic oxidation” treatment.As can be seen from Fig.36 that the pure aluminum’s surface is smooth,while many pits appear on the surface of Al-0.08 wt.% Ga alloys,demonstrating the “electrolytic polishing+anodic oxidation”treatment is effective to break the oxide film on the surface of Al-0.08 wt.% Ga alloys but invalid to the pure Al.And the principle can be explained with Fig.37.Thus,the wettability of the Al-0.08 wt.% Ga alloys with “electrolytic polishing+anodic oxidation” treatment is better than that pure Al with “electrolytic polishing+anodic oxidation” treatment and Al-0.08 wt.% Ga alloys without any treatment.This technique also works for the Al-1 wt.% Sn alloy,as reported in another research of Zhang et al.[94].
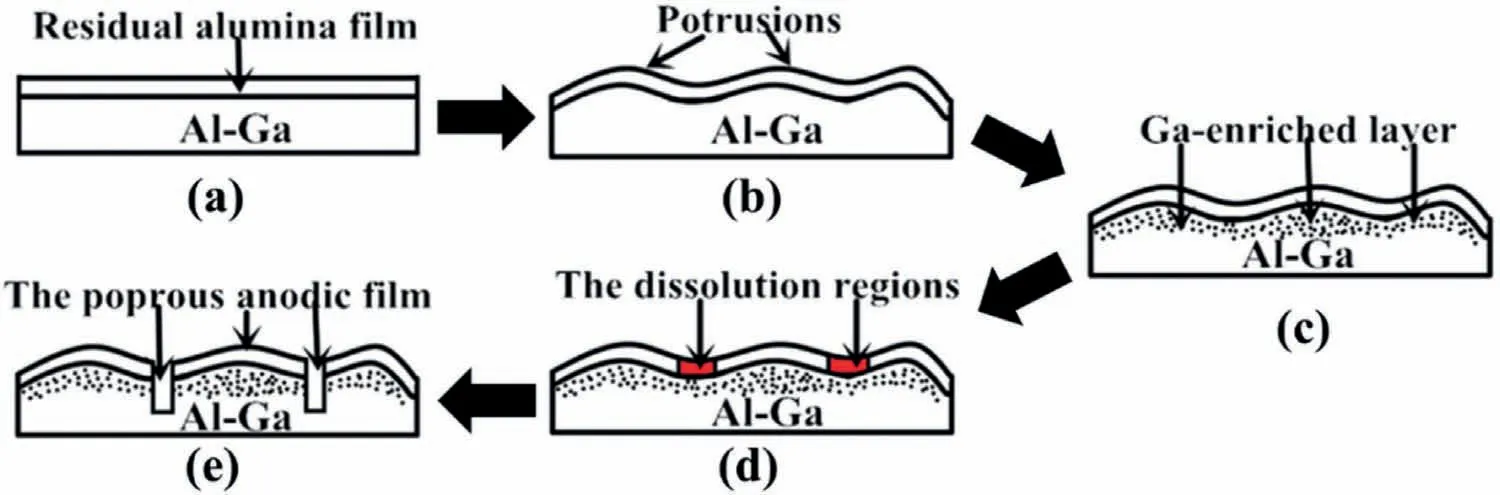
Fig.37.Crushing process of the oxidation film by “electrolytic polishing+anodic oxidation”: (a) Al plate with natural oxide layer;(b) preferential oxidation of aluminum;(c) Ga-enriched layer,(d) field-assisted dissolution of the thinner regions;(e) protrusions film [94].
The strengthening mechanism of these methods is to increase the wettability by removing or crushing the oxide film.The increase of wettability is conducive to metallurgical bonding,thus improving the properties of the bimetal.
4.2. Adding interlayer
The hardness of the Al-Mg IMCs is high with a value of 240–300 HV,which exhibits a high brittleness.These brittle and hard Al-Mg IMCs are prone to fracture under the action of force,becoming the source of fracture.Therefore,many Al-Mg IMCs are not conducive to the interface connection.A good solution is placing an interlayer different from the substrate between the aluminum and magnesium,and the metal coating can reduce the Al-Mg IMCs or generate phases different from the Al-Mg IMCs with low brittleness,thus improving the connection performance.
According to the research of Shah et al.[95],the general design guideline for mitigating the Al-Mg IMCs through adding the interlayer can be concluded in three steps,as displayed in Fig.38,and the specific process is as follows.Firstly,to evaluate whether the element’s enthalpy of formationΔHand the Gibbs energy of compoundΔGof the chosen interlayer are lower than those of the Al-Mg system.If so,the next step is to proceed.The enthalpies of formation of different elements with the Al or Mg can be evaluated by the Miedema model [96],and the results are displayed in Fig.39.It is found that Ni,Cu and rare earth elements have the lower formation enthalpies with the Al and Mg at the same time.Then,to evaluate whether the brittleness of the compounds expected to form is lower than that of the Al-Mg IMCs and consider other factors such as melting point,cost and accessibility.The third step is to conduct the trial verification and match the appropriate process parameters.
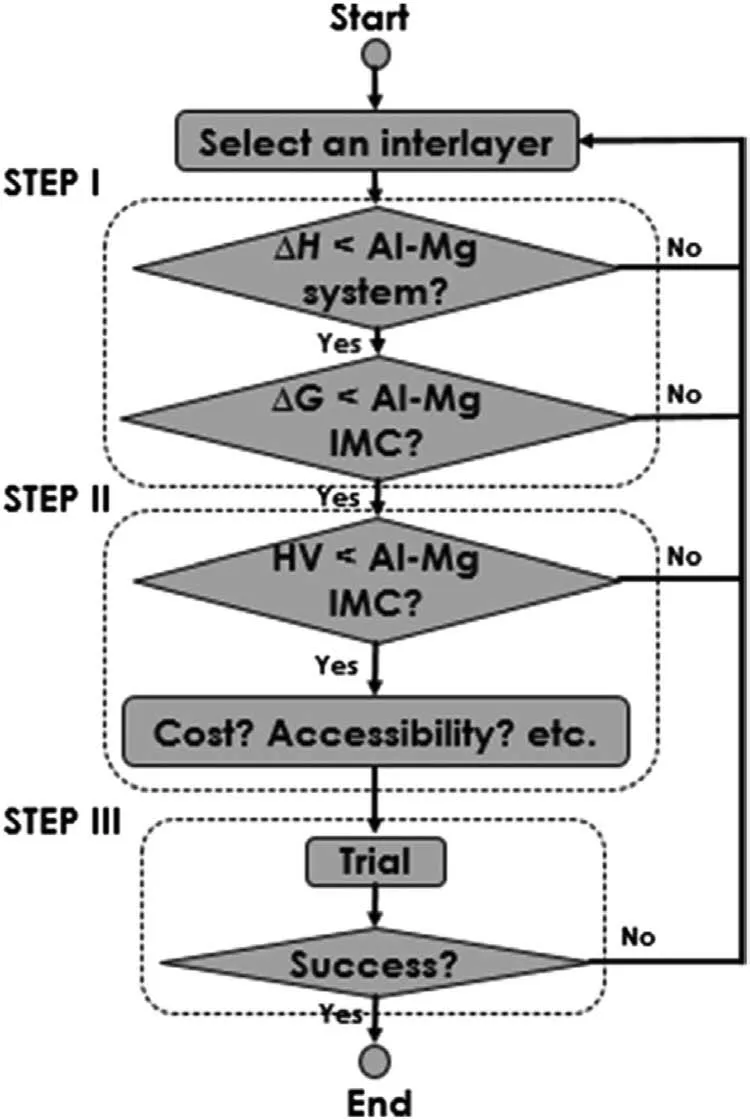
Fig.38.General design guideline for mitigating the Al-Mg IMCs through adding the interlayer [95].

Fig.39.The enthalpies of formation of different elements with Al or Mg: (a) Al and other elements;(b) Mg and other elements;(c) Al and rare earth elements;(c) Mg and rare earth elements [96].
At present,many kinds of interlayers are applied in the Mg/Al bimetal to improve the performance,such as Zn,Sn,Al,Ni,Cu,Fe,Ti,Ag,Ag-Cu-Zn,SiC,Au,Zr and Ce and so on,and the corresponding interlayer types,preparation methods and shear strength are listed in Table 5.As can be seen that these interlayers can be roughly divided into three categories.The first category is pure metal with a low melting point,such as Zn,Sn and Al,etc.These interlayers generally play a role in increasing the wettability of aluminum and magnesium.Melting occurs during the connection process,and aluminum and magnesium are connected mainly through melting and diffusion.The other is pure metal with a high melting point,such as Ni,Cu,Fe,Ti,Ag,Ce,Mn and Zr,etc.These interlayers will not melt in the connection process,and aluminum and magnesium are mainly connected by element diffusion.The third category is composite metals,such as Al-Mg eutectic alloy,Al/Ni,Ag-Cu-Zn,CuNi/Ag/CuNi,Cu/Ni,Sn-coated steel and Zn-coated steel and so on.These metals partially melt and partially do not melt during the connection process,and the aluminum and magnesium are joined by melting and diffusion.These interlayers,on the one hand,avoid the oxidation of the alloy surface,on the other hand,they reduce or eliminate the Mg/Al intermetallic compounds,to achieve the enhancement of the Mg/Al bimetal.

Table 5Different types of interlayers applied in the Mg/Al bimetal.
These interlayers are widely used in the welding field,while the application in the field of the compound casting is less,mainly including Zn,Ni,Ni-Cu and Mn.Below,the effects of these interlayers on the microstructure and properties of the Mg/Al bimetal prepared by the compound casting are described in detail.
4.2.1.Zn interlayer
The effect of Zn coating depends on its thickness.When the thickness of the Zn coating is thin,the Zn coating only plays a role in increasing wettability.The Zn coating can reduce the Al-Mg IMCs only when it reaches a certain thickness.
Xu et al.[47]and Chen et al.[92]used the“zincate+galvanizing” technology to obtain compact Zn coating with a thickness of 5–6 μm the on the surface of A390.However,there are not components containing Zn element found in the interface layer.This because the Zn coating of low melting point(419°C)will evaporate during the high preheating (500–580 °C) and pouring (700–730 °C).The function of Zn coating is mainly to prevent the reoxidation of aluminum alloy and increase the wettability,which is conducive to the metallurgical combination between aluminum and magnesium,thus improving the performance of the bimetal.
Mola et al.[106]prepared a Zn coating on the surface of Al 6060 by diffusion connection.By comparing the microstructure and XRD results,it can be found that the interface layer is replaced by Al-Mg IMCs (Al3Mg2,Al12Mg17,Mg+Al12Mg17eutectic) with Al-Mg-Zn IMCs (Mg5Al2Zn2+Mg eutectic,Mg32(Al,Zn)49,Mg32(Al,Zn)49+Mg eutectic) because of the existence of Zn coating,as held up in Fig.40.The indentation results of the hardness test show that these Al-Mg-Zn IMCs have lower hardness than that of the Al-Mg IMCs,showing a more excellent resistance to plastic deformation,as indicated in Fig.40(a) and (b).Therefore,the shear strength is increased from 5.5–11.3 MPa to 39.8–46.6 MPa,which significantly improves the performance of the bimetal.

Fig.40.Microstructure and XRD results of the Al 6060/Z31 bimetal with Zn coating: (a) SEM images of interfacial microstructure and indentation without Zn coating;(b) SEM images of interfacial microstructure and indentation with Zn coating;(c) XRD results of the interfacial zone without Zn coating;(d)XRD results of the interfacial zone with Zn coating [106].
4.2.2.Ni interlayer
The presence of Ni coatings can reduce or even eliminate the Al-Mg IMCs,but the effect of Ni coatings prepared by different methods is different.
Liu et al.[41]used the plasma spraying to prepare a pure Ni layer with a thickness of about 96 μm on the surface of pure Al,as exhibited in Fig.41(a).Then the Mg/Al bimetal was prepared by pouring pure Mg liquid at different pouring temperatures (680 °C,700 °C,720 °C).The effects of the pouring temperature and Ni coating on the microstructure and properties of the bimetal were studied.It was found that the bimetals could achieve metallurgical bonding only at the pouring temperatures of 700 and 720 °C,as displayed in Fig.41(b).The microstructure results show that the presence of Ni coating can effectively hinder the formation of Al-Mg IMCs,and the interface layer is mainly composed of Mg-Ni and Al-Ni IMCs,as indicated in Fig.41(c) and (d).These Al-Ni and Mg-Ni IMCs have lower hardness than that of Al-Mg IMCs,as held up in Fig.41(e).As a result,the shear strength is significantly improved and reaches a maximum of 25.4 MPa at the pouring temperature of 700 °C,which is a 46.8% improvement compared with that of the bimetal without Ni coating (17.3 MPa),as demonstrated in Fig.41(f).

Fig.41.Microstructure and properties of the Mg/Al bimetal with plasma sprayed Ni coating: (a) Ni coating fabricated by plasma spraying;(b) morphology of the Mg/Al bimetal at different pouring temperatures;(c) microstructure of the Mg/Al bimetal at 700 °C;(d) microstructure of the Mg/Al bimetal at 720 °C;(e) hardness;(f) shear strength [41].
Zhang et al.[126]produced a dense Ni coating with a thickness of 5–6 μm on Al 6061 surface by’zincate+nickelling’surface treatment,and the presence of Ni coating significantly increased the wettability between magnesium and aluminum,as shown in Fig.42(a) and (b).The microstructure and properties of bimetals at different preheating temperatures (540–620 °C) were studied,and the results are shown in Fig.42(c) and (d).It can be found that the Ni coating still exists when the preheating temperature is 560°C,and there is no Al-Mg IMCs in the interface layer which is mainly composed of Ni,Ni (Mg) solid solution,Mg2Ni and Mg (Ni) solid solution,as exhibited in Fig.42(c).When the preheating temperature is increased to 600 °C,the Ni coating completely disappears,and the interface layer consists of Al3Ni+Al3Mg2andα-Mg+Al12Mg17,as displayed in Fig.42(d).This indicates that high preheating temperature is not conducive to hinder the formation of Al-Mg IMCs.At the preheating temperature of 560 °C,the Vickers hardness of the interface layer is lower than 180 HV,which is significantly lower than that of Al-Mg IMCs.The shear test results show that the shear strength first increases and then decreases with the increase of preheating temperature and reaches the highest value of 47.5 MPa at 560 °C.
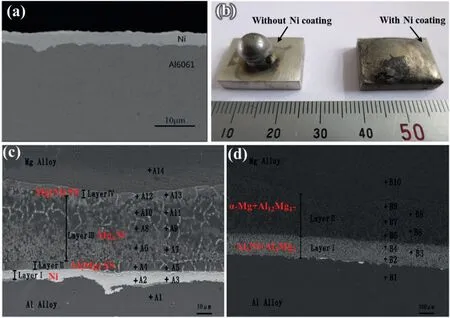
Fig.42.Microstructure of the Mg/Al bimetals with Ni coating fabricated by ‘zincate+nickelling’: (a) Ni coating prepared by ’zincate+nickelling’;(b)wettability test results;(c) microstructure of the Mg/Al bimetal at preheating temperature of 560 °C;(d) microstructure of the Mg/Al bimetal at preheating temperature of 600 °C [126].
Li et al.[119]compared the effects of the Ni coatings prepared by different methods,namely,electronickelling(EN),electroless nickel plating (ENP),plasma sprayed nickel(PSN),on the microstructure and properties of the Mg/Al bimetal.The surface morphology and roughness as well as the microstructure and compositions of the cross section of the Ni coatings prepared by different methods are exhibited in Fig.38.It can be seen from Fig.43(a)–(c) that the surface of Al alloy fabricated by PSN is coarser than that prepared by EN and ENP.The surface roughness (Ra) of the Al matrix with EN,ENP and PSN are 3.208,5.303 and 57.055 nm,respectively,as indicated in Fig.43(d)–(f).The microstructure of the cross section shows that the thickness of the Ni coating for PSN is uneven,but is uniform for EN and ENP,as displayed in Fig.43(g)–(i).

Fig.43.Microstructure and roughness of different Ni coatings: (a),(b),(c) corresponds to surficial microstructure of the coating prepared by EN,ENP and PSN,respectively;(d),(e),(f) corresponds to surface roughness of the coating prepared by EN,ENP and PSN,respectively [119].
The microstructure of the Mg/Al bimetals with different Ni coatings is shown in Fig.44.It can be found that there is metallurgical bonding between Ni coating and Al matrix but no metallurgical bonding between Ni coating and Mg matrix at the bimetallic interface of EN and ENP,and the interfacial layers are mainly composed of Ni solid solution (Ni SS) and Al3Ni,as exhibited in Fig.44(a) and (b).For the bimetal containing PSN coating,metallurgical reaction layers occur between the Ni coating with Al substrate and Mg substrate,and the interfacial layer is mainly composed of Mg2Ni,Ni SS and Al3Ni,because the rough coating is more conducive to the metallurgical bonding,as displayed in Fig.44(c).EBSD results verify the existence of these phases,and these phases have small grain size and random orientation,as exhibited in Fig.44(d) and (e).The shear test results show that the shear strength of the bimetal with EN and ENP coatings was lower than that of the bimetal without coatings.Only the shear strength of the bimetal with PSN coating is improved,with a shear strength of 69.8 MPa,which is 69% higher than that of the bimetal without coatings.
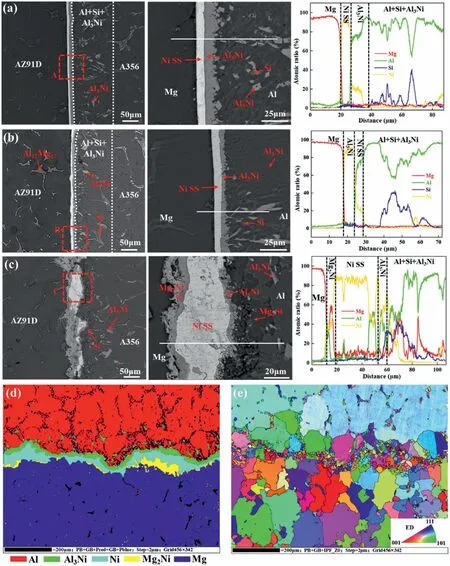
Fig.44.Microstructure and EBSD results of the bimetal with different Ni coatings: (a) EN;(b) ENP;(c) PSN;(d) phase distribution of PSN;(e) IPF mapping of PSN [119].
Li et al.[118]also studied the influence of high velocity oxygen fuel sprayed (HVOF) Ni coating and its thickness on the microstructure and properties of the Mg/Al bimetal.It was found that the number of Al-Mg IMCs decreased with the increase of Ni coating thickness,and Al-Mg IMCs and the original Ni coating almost disappeared only when the Ni coating thickness was 45 μm,as exhibited in Fig.45(a)–(c).The interface layer of bimetal with 45 μm Ni coating is composed of Mg SS,Mg+Al12Mg17eutectic,Ni2Mg3Al,Mg+Mg2Ni eutectic,Ni SS,Mg+Mg2Ni eutectic,Ni SS,Al3Ni2closed to Mg matrix and dispersed Al+Al3Ni eutectic particles near Al matrix,as indicated in Fig.45(d)–(n).The shear strength of the bimetal without Ni coating is lower than that with Ni coating of 10 and 45 μm but higher than that with Ni coating of 190 μm,and the maximum shear strength is 61 MPa when the thickness of Ni coating is 45 μm.This signifies the ideal thickness of Ni coating should eliminate Al-Mg IMCs without remaining the original Ni coating.

Fig.45.SEM images and TEM results of the Mg/Al bimetal with different thickness’s HVOF Ni coatings: (a) 15 μm;(b) 45 μm;(c) 190 μm;(d) and (i)SEM images corresponding to I and II region in Fig.45(b),respectively;(e-g) SEM images corresponding to A,B and C region in Fig.45(d),respectively;(h) SEM image corresponding to D region in Fig.45(g);(j) FIB sampling place;(k) TEM bright field image;(l-n) SAED patterns corresponding to E,F and G region in Fig.45(k) [118].
4.2.3.Ni-Cu interlayer
The above studies indicate that the Ni coatings of EN and ENP could not form metallurgical bonding with the Mg matrix,so the shear strength is reduced.The research of Shang et al.[119]demonstrates that metallurgical connections tend to occur between Cu coatings and Mg substrates.Therefore,in order to solve the problem,Li et al.[134]electroplated a Cu coating on the surface of the Al matrix with an electroless Ni layer to obtain the Ni-Cu composited coating,as held up in Fig.46(a).The coating can realize metallurgical bonding between the coating with both the Al and Mg substrate,and the interface layer is composed of Al7Cu3Mg6,Mg2Cu,Cu solid solution (Cu SS),Ni SS,Al3Ni and Al+Al3Ni eutectic,as exhibited in Fig.46(b).According to the Gibbs energies of the formation (ΔG) which is displayed in Fig.46(c),the formation sequence of these phases is Al3Ni,Al7Cu3Mg6,Mg2Cu in succession.Thus,the formation mechanism of the interface layer can be summarized with Fig.46(d),which is attributed to the element diffusion mainly.
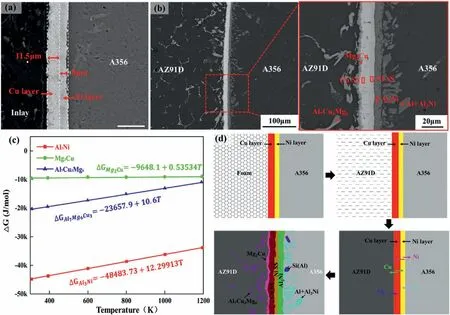
Fig.46.Microstructure of Ni-Cu composited coating and bimetal: (a) SEM image of Ni-Cu composited coating;(b) SEM image of the bimetal;(c) Gibbs energies of different IMCs;(d) interfacial formation mechanism [119].
Nanoindentation test results show that most phases in the interface layer for the bimetal containing Ni-Cu composited coating have lower hardness and higher elastic modulus than Al-Mg IMCs,as held up in Fig.47(a) and (b).As a result,the shear strength is 20.3% higher than that of the uncoated bimetal.The effects of Ni-Cu composite coatings on bimetallic corrosion properties were also studied for the first time.It was found that the existence of Ni-Cu composited coating slightly reduced the corrosion resistance of the bimetal,as indicated in Fig.47(c) and Table 6.
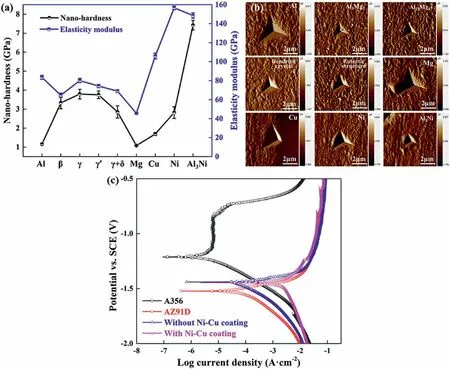
Fig.47.Nanoindentation test results and polarization curves: (a) Nano-hardness and elasticity modulus of different IMCs;(b) AFM images of the nanoindentations;(c) polarization curves of the A356,AZ91D,A356/AZ91D bimetals with and without Ni-Cu composite interlayers [119].
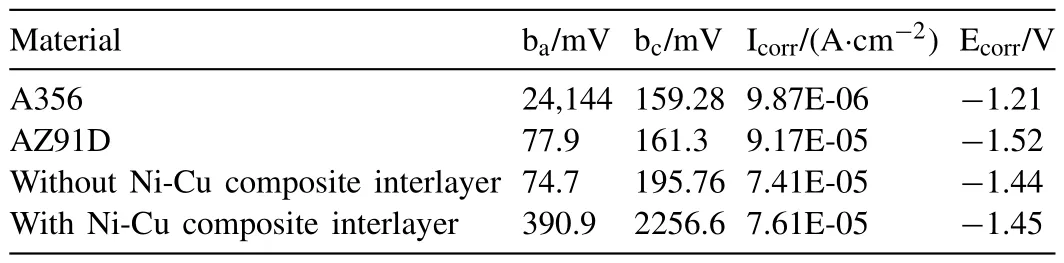
Table 6Corrosion current densities and corrosion potentials of different materials.
4.3. Alloying treatment
It is well known that if the bimetallic matrix is pure magnesium and pure aluminum,the phase composition of the interface layer must be Al-Mg intermetallic compound.However,in Section 3.3,we know that Al-Mg IMCs are brittle and hard phase,accompanied by coarse grains,uneven composition,stress concentration,preferred orientation and other problems.In practice,the matrix alloy usually contains other elements,and the presence of these elements will also affect the interface structure of the bimetal.Therefore,alloying is also an effective method to improve the structure and properties of the interface by adjusting the element type or content of the matrix alloy.
At present,the elements used in the study of alloying in compound casting can be divided into two categories: one is Si element;the other is rare earth alloy,including La,Ce,Nd,Y and Gd,as listed in Table 7.The effects and mechanisms of different alloying elements are as follows.
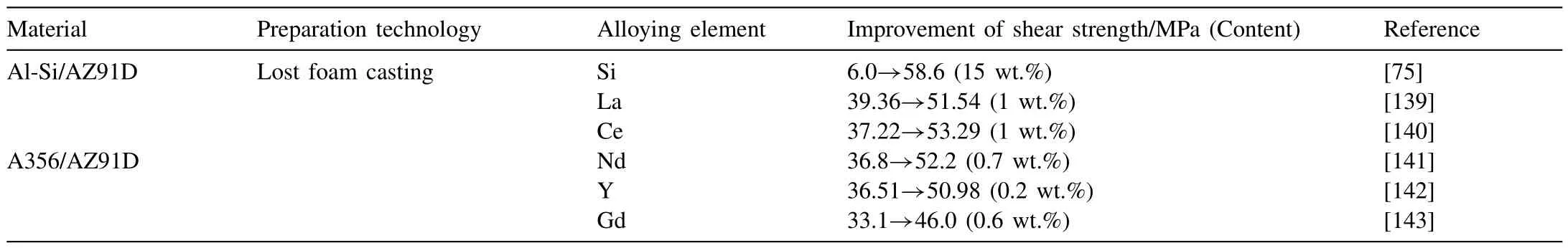
Table 7Alloying element used in compound casting.
4.3.1.Si
Li et al.[75]compared the microstructure and properties of Al-Si/AZ91D bimetals with different Si contents (0–21 wt.%)in Al matrix.It is found that bimetals cannot achieve metallurgical bonding when the matrix is pure Al,but all bimetals containing Si elements can achieve metallurgical bonding.The bimetals can be divided into hypoeutectic bimetals(6–12 wt.% Si) and hypereutectic bimetals (15–21 wt.% Si)according to the difference of Si content in Al matrix.
The metallurgical reaction layers of bimetals with different Si contents can be divided into three regions from Al matrix to Mg matrix,which are intermetallic compound layer (IMC layer),transition layer (T layer) and eutectic layer (E layer).The interfacial phases of the hypoeutectic bimetal from Mg matrix to Al matrix are Al12Mg17+δ-Mg eutectic structure (E layer),Al12Mg17+δ-Mg eutectic structure+Mg2Si+Al12Mg17dendrite (T layer) and Al3Mg2+Al12Mg17+polygonal Mg2Si(IMC layer).In addition to these phases,the hypereutectic bimetals also have Si solid solution and flocculent Mg2Si+Al-Mg IMCs in the IMC layer.With the increase of Si content,the amount of oxidized inclusion decreases and the amount of Mg2Si increases,as exhibited in Fig.48(a) and (b).The shear strength of bimetals firstly increases and then decreases with the increase of Si content,and reaches the highest value of 58.6 MPa when Si content is 15 wt.%,which is almost 10 times that of bimetals without Si.

Fig.48.Microstructure of the Al-xSi/AZ91D bimetal and schematic diagram of the crack propagation: (a) microstructure of Al-6Si/AZ91D bimetal;(b)microstructure of Al-15Si/AZ91D bimetal;(c) crack propagation in bulk Al3Mg2;(d) crack propagation in bulk Al3Mg2 with Mg2Si particles;(e) crack propagation in the hypoeutectic Al-xSi/AZ91D bimetal;(f) crack propagation in the hypereutectic Al-xSi/AZ91D bimetal [75].
The strengthening of Si element to bimetal is mainly attributed to the following points.On the one hand,Si element can form Mg2Si with Mg element,which is a good reinforced particle with high melting point (1083 °C) and Young’s modulus (120 GPa),low density (1.99 g/cm3) and thermal expansion coefficient (7.5 × 10-6K-1).In the IMC layer of Mg/Al bimetal,the fine and massive Mg2Si particles distributed in the grains of Al3Mg2or Al12Mg17cause the crack to skew or bend in the process of crack growth,thus increasing the crack growth resistance and path length,and the mechanism is indicated in Fig.48(c) and (d).Therefore,a large number of fine and dispersed Mg2Si particles are beneficial to improve the interfacial fracture toughness.However,when the content of Si element is too much to be completely converted into Mg2Si,the residual Si phase is not conducive to the further improvement of bimetallic properties.On the other hand,the presence of Si element reduces the proportion of oxide film on the surface of the aluminum matrix,thus reducing the oxide inclusion defects in the interfacial layer.The reduction of surface oxide inclusion makes the cracks continue to expand towards the E layer after expanding to T layer,which is conducive to improving the shear strength of the bimetal,as displayed in Fig.48(e) and (f).Third,the hardness test results show that the E and T layers have higher toughness than the IMC layers,while the hypereutectic bimetals have thinner interfacial layers and a lower Al-Mg IMC layer ratio,so the shear strength is further improved.Fourth,Al-Mg IMC in the interfacial layer has an obvious preferred orientation.When there are a large number of Mg2Si particles with no preferred orientation and a higher proportion of T layer,the orientation of E layer and T layer of hypereutectic bimetal is inconsistent,which is also conducive to the improvement of bimetal strength,as demonstrated in Fig.49.Therefore,the strength of hypereutectic bimetals is higher than that of hypoeutectic bimetals under the combined action of the above factors.
4.3.2.RE
Z.Zhang et al.and F.Guan et al.regulated the microstructure and properties of A356/AZ91D bimetal by introducing RE elements (La [139],Ce [140],Nd [141],Y [142],Gd[143]) into AZ91D magnesium alloy.It is found that the addition of RE elements can enhance the properties of bimetals,and the strengthening mechanism is the same.Taking La element as an example,the addition of La element mainly affects the structure of eutectic layer.As mentioned in Section 3.3,for A356/AZ91D bimetals,the eutectic layer has oxidized inclusion and coarse primary Al12Mg17dendrites and clumpy eutectic structures.After the addition of La element,some short rod-shaped and clumpy RE precipitated phases were formed in the eutectic layer,as held up in Fig.50.TEM test confirmed that these RE precipitated phases were Al11La3and Al8Mn4La,and their included angles with the crystal axis of Al12Mg17were 122.51° and 60.23°,respectively,as indicated in Fig.51.With the increase of La content,the RE precipitated phase gradually increased and presented an aggregation state,while the coarse primary Al12Mg17dendrites and oxide inclusions gradually decreased,and the eutectic structure was also refined,as exhibited in Fig.50(a)–(c).This is because these RE precipitated phases form preferently during solidification,which plays a role in breaking oxide film,promoting nucleation and inhibiting dendrite growth.When La content is 1.0 wt.%,the shear strength of bimetal reaches the maximum 51.54 MPa.Heavy RE Y can achieve the same strengthening effect as light RE La with a lower addition amount,and the strengthening mechanism is exhibited in Fig.52.According to the results in Table 7,it can be found that among all the RE elements,Ce element has the best strengthening effect,which can reach 53.29 MPa at the highest.

Fig.50.Microstructure of the A356/AZ91D bimetal with different amounts of La: (a) 0.7 wt.%;(b) 1 wt.%;(c) 1.3 wt.% [139].

Fig.51.TEM results of the RE precipitates in the E area with La addition: (a) Bright field image of the phase interface of Al11La3 and γ: region A stands for Al11La3,region B stands for γ,region C stands for the phase interface;(b) bright field image of the phase interface of Al8Mn4La and γ: region D stands for Al8Mn4La,region E stands for γ,region F stands for the phase interface;(c)–(e) SAED results of Al11La3, γ and Al8Mn4La;(f) and (g) SAED result and high resolution image of the Al11La3 and γ phase interface;(h) and (i) SAED result and high resolution image of the Al8Mn4La and γ phase interface[139].
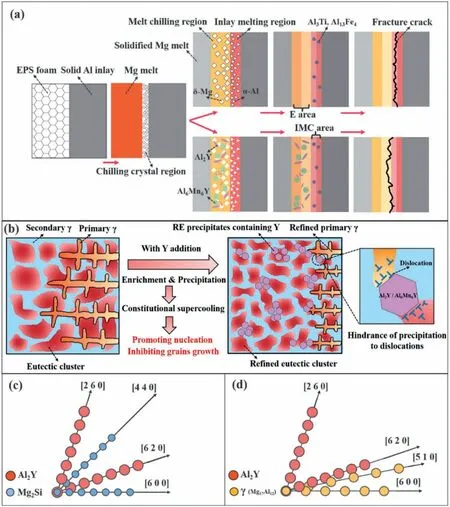
Fig.52.Schematic of the Mg/Al bimetallic reinforcement with RE Y: (a) Schematic diagram of formation process and fracture distribution of the Mg/Al bimetallic interface with Y addition;(b) schematic diagram of microstructure refinement and Mg/Al interface strengthening mechanism;(c) and (d) schematic diagram of the lattice matching between the Al2Y and different matrix structures based on (001) crystal plane [142].
4.4. Applying vibration field
Many research indicated that vibration applied in the solidification process of the metal could refine grain,reduce segregation as well as other effects.Thus,researchers,in our group,speculated that vibration applied in the solidification process of the Mg/Al bimetal might achieve the same effect to strengthen the bimetal properties.Specific research results are as follows.
4.4.1.Mechanical vibration
Li et al.[144]studied the effect of mechanical vibration on the filling and solidification process of the Mg/Al bimetal.It was found that the application of mechanical vibration can increase the filling rate and wettability between AZ91D melt and A356 inlay,and make the temperature distribution of the whole casting more uniform.
Guan et al.[145]studied the effect of mechanical vibration on the microstructure and properties of bimetals.It is found that after vibration,due to forced convection of metal liquid caused by vibration,oxidation inclusion and coarse Al12Mg17dendrites are reduced,Mg2Si and Al3Mg2in the interface are refined,and Mg2Si is distributed more evenly,as held up in Fig.53.TEM analysis showed that the interface between Al3Mg2and Mg2Si phases was non-coherent,indicating that Mg2Si particles could not be used as the base of heterogeneous nucleating during the solidification process,as indicated in Fig.54.Compared with the non-vibrating bimetal,the shear strength of the bimetal after vibration is increased by about 50% from 31.7 MPa to 47.5 MPa.

Fig.54.TEM analysis results of the bimetal with and without vibration: (a) and (b) TEM bright-field images of the layer I without vibration;(c),(f),and(h) SAED patterns of the Mg2Si,Al3Mg2,and Al18Mg3Mn2 phases in (b);(d),(e) and (g) high-resolution TEM images of the region marked with letters A,B,and C in (b);(i) TEM bright-field image of the layer I with vibration [145].
Wang et al.[146]and Guan et al.[147]continued to study the effects of vibration time and acceleration on the microstructure and properties of AZ91D/A356 bimetal.The results show that with the increase of vibration time and acceleration,the solidification speed of the bimetal is accelerated,the thickness of the IMC layer is reduced,and the distribution Mg2Si phase changed from agglomeration to dispersion with a smaller size,therefore improving the shear strength.
4.4.2.Ultrasonic vibration
Guan et al.[148]tried to apply ultrasonic vibration to the aluminum inlay to enhance the Mg/Al bimetallic properties in the solidification process,and the principle was exhibited in Fig.55(a).The results demonstrates that Mg2Si,Al12Mg17and Al12Mg17+δ-Mg eutectic phases at the interface are significantly refined after ultrasonic vibration.Moreover,the Mg2Si phase is uniformly dispersed throughout the interface,continuous oxidation inclusion defects are eliminated,and the interface structure is exhibited in Fig.55(b).Therefore,the shear performance was significantly improved,reaching 69 MPa,which was 86.5% higher than that of nonvibrating bimetal,and the strengthening principle is indicated in Fig.55(c).

Fig.55.Influence of ultrasonic vibration on Mg/Al bimetal: (a) Process schematic diagram;(b) IPF profile without ultrasonic vibration;(c) IPF profile with ultrasonic vibration;(d) reinforcement schematic [148].
4.5. Other methods
In addition to the above strengthening methods,the Mg/Al bimetals can also be strengthened by other ways,such as secondary rolling,thermal modification of the matrix,heat treatment,changing the surface morphology of the inlay,etc.
4.5.1.Secondary rolling
Chen et al.[117]applied multi-pass caliber rolling to the Mg/Al bimetals prepared by compound casting.It is found that the Ni intermediate layer is broken during the rolling process and transformed into dispersed particles at the interface.At the same time,the fresh AZ31 and 6061 base alloys were extruded and combined under the action of rolling force,forming a well-combined interface with no obvious defects.
4.5.2.Thermal modification
Renata et al.obtained a thermally modified AlSi12 matrix by remelting AlSi12 alloy on a steel plate at room temperature,then prepared the AZ91/AlSi12 bimetal by solidliquid compound casting [149].It is found that compared with the unmodified bimetal,the Mg2Si in the interface region of the modified AZ91/AlSi12 bimetal is distributed as fine particles in the IMC layer uniformly.Hardness test results showed that the interface structure after thermal modification was not prone to crack under the action of load,as indicated in Fig.56.This will effectively improve the ability of the bimetal to resist the fracture under the action of external forces.

Fig.56.Microstructure and hardness points of the AZ91/AlSi12 bimetal: (a) Unmodified;(b) thermal modification [149].
4.5.3.Heat treatment
Zhu et al.[46]prepared the AM50/Al 6061 bimetal by vacuum-assisted die casting.There was no metallurgical reaction layer at the bimetallic interface in the as-cast state,but a diffusion layer was generated after annealing.The researchers controlled the thickness and phase composition of the diffusion layer by controlling the annealing temperature and annealing time.When the annealing parameter is 200 °C*3 h,the thickness of the diffusion layer arrived at the largest value and no Al-Mg brittle phases were generated,and the shear strength had a maximum of 8.09 MPa,as exhibited in Fig.57.
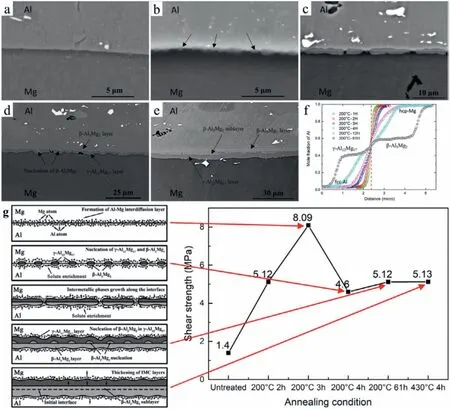
Fig.57.Microstructure of 6061/AM50 bimetal annealed at 200 °C for different times: (a) 3 h;(b) 4 h;(c) 12 h;(d) 61 h;(e) 235 h;(f) mole fraction of Al at the diffusion couple interface annealed at 200 °C for 1–61 h;(g) schematic illustration of the interfacial formation process and shear strength [46].
4.5.4.Constructing exterior 3D morphology
Li et al.[89]studied the effect of surface morphologies of Al insert,including smooth surface (SS),straight knurling (SK) and hatching knurling (HK) on the microstructure and mechanical properties of Mg/Al bimetallic,and the different morphologies of Al insert are exhibited in Fig.58.It was found that the bimetal with an Al insert of SK and HK had fewer inclusion defects and a higher proportion of eutectic layer than that with a SS’s Al insert,which led to a longer crack propagation distance,therefore enhancing the shear strength with a ratio of 34.2 and 50.2%,respectively.

Fig.58.The surface morphologies and specific sizes of the A356 bars: (a)SS’s macro-profile;(b) SK’s macro-profile;(c) HK’s macro-profile;(d) the cross section of SK’s A356 bar;(e) the surface of HK’s A356 bar;(f) part of the cross section for HK’s A356 bar [89].
The shear strength of the Mg/Al bimetal has been significantly improved after many studies on the performance strengthening of the Mg/Al bimetal.This section summarizes the strength of the Mg/Al bimetal under different process and strengthening methods for reference by subsequent researchers,as listed in Table 8.As can be seen that,in the field of the compound casting,the highest shear strength of the Mg/Al bimetals prepared by lost foam casting with Ni interlayer is 69.8 MPa in our current work,which is in a leading position.The growth ratio of shear strengths arrived at a maximum of 747.3% by adding a Zn interlayer.

Table 8Growth ratio of shear strengths by the different enhancement methods.
5.Prospect
The Mg/Al bimetal,as a new material with excellent comprehensive performance and conducive to the further realization of lightweight,has a short development history.Therefore,the research on it remains to be done further,and the future research directions are as follows:
(1) The oxide film on the metallic surface has a great influence on the bonding property of the bimetal,because the existence of the oxide film will form loose oxide impurities during the connection process.The surface of Al and Mg is very easy to produce oxide film,and even if it has been removed but will soon generate again.Current methods are complicated and the removal effect is limited,so it is necessary to develop a method with simple operation and strong removal ability of oxide film.The possible method is to coat the metal surface with a brazing flux which reacts with the oxide film at high temperature under the action of the poured metal liquid,so that the oxide film is stripped from the metal surface and floats to the top with the liquid metal,such as Cesium fluoroaluminate or potassium fluoroaluminate.
(2) Adding intermediate layer is a good way to reduce or even eliminate brittle Al-Mg IMCs.In the field of welding or composite rolling,many types of interlayers have been used.However,in the field of compound casting,the types and studies of adding intermediate layers are still relatively few.This is because welding and composite rolling belong to solid-solid compound,and the connection interface shape is simple,so the interlayer can be directly placed between the two metals in solid,liquid or powder form,and the operation process is simple and easy to achieve.The solid-liquid compound requires the interlayer to be attached on the surface of solid inlay in advance by various processes,such as electroplating,electroless plating,plasma spraying,high velocity oxygen fuel,magnetron sputtering,physical vapor deposition,chemical vapor deposition,etc.These preparation processes are complex,costly,often targeted at specific materials,and some are not suitable for the coating preparation of complex surfaces.Therefore,if a new coating needs to be developed,it is often necessary to re-explore the appropriate preparation process according to the coating type.These difficulties in coating technology have resulted in less research on coating methods in the field of compound casting.Therefore,it is necessary to develop a coating preparation method suitable for compound casting,which needs to be easy to operate,low cost and suitable for different types of coating types.At present,powder paste brushing or aerosol spraying may be a better solution,but the research in this area has not been reported.In addition,some new coatings also need to be further developed,rare earth coating,for example Ni-Cr composite coating,Li coating,Mg-Cu-Y amorphous/nanocrystalline composite coating,etc.
(3) Alloying is a simple and effective method to improve the properties of bimetals.At present,only the alloying effects of Si element and RE elements on bimetals have been studied,and further studies on the effects of other elements,such as Li,Er,Sc,Sr,etc.,are needed.Moreover,it is necessary to ensure that the addition of these alloying elements cannot damage the performance of the matrix.The addition of some alloying elements can not only enhance the interface connection strength,but also improve the performance of the matrix,which is the best element for alloying.
(4) The vibration field has a good effect of homogenizing and refining the interface structure,especially the ultrasonic vibration field.However,at present,the application of ultrasonic field directly acts on the surface of solid inlay,which is difficult to operate and control,and there is no systematic study on the influence of ultrasonic vibration parameters on the bimetals.All these need to be optimized and studied in the future.In addition,in addition to vibration field,we can also consider other external fields such as electromagnetic field and force field,which are potential future research directions.
(5) Heat treatment is also a key strengthening technique.On the one hand,the heat treatment can adjust the interface structure,including the thickness of the interface layer,phase distribution and composition,grain morphology and size.On the other hand,the heat treatment can also improve the microstructure and performance of the matrix.The performance of the bimetal not only depends on the connection strength of the interface,but also the performance of the matrix.However,the current heat treatment process only considers how to improve the interface microstructure and properties,but does not consider whether the matrix is affected in the heat treatment.Because the physical properties of Al and Mg are very different,which leads to the significant difference in the heat treatment process and parameters between them,so it is urgent to develop a heat treatment process that can strengthen the interfacial structure and ensure the properties of the matrix at the same time.
(6) The bonding mechanism of the Mg/Al bimetals is not very clear at present.More advanced analytical methods are needed to study the interface bonding mechanism to lay a theoretical foundation for subsequent research.For example,synchrotron radiation technology can be used to directly observe the dynamic image of the Mg/Al bimetallic composite process.The fracture process of the Mg/Al bimetal under different loads can be observed by TEM or SEM in-situ tensile,shear,bending or compression techniques.Molecular dynamics and first principles can simulate the interfacial bonding process of bimetals.
(7) The application examples and scope of the Mg/Al bimetal are still limited.It is necessary to develop more application scenarios and specific parts of the Mg/Al bimetal,such as the parts related to the new energy vehicle,including the motor,frame,instrument panel,and other possible parts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the supports provided by the National Natural Science Foundation of China (Grant Nos.52271102,52075198 and 52205359),the China Postdoctoral Science Foundation (Grant No.2021M691112),and the Analytical and Testing Center,HUST.
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
- Achieving high ductility and strength in magnesium alloy through cryogenic-hot forming
