Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
Senwei Wng ,Chengo Du ,Xin Shen,c ,Xiong Wu ,Sihui Ouyng,b,* ,Jun Tn,b,* ,Ji She,b,*,Aito Tng,b,Xinhu Chen,b,Fusheng Pn,b
a College of Materials Science and Engineering, Chongqing University, Chongqing, China
b National Engineering Research Center for Mg Alloys, Chongqing University, Chongqing, China
c Beijing Amsinomed Medical Co, Ltd, Beijing, China
Abstract Biodegradable magnesium (Mg) alloys are expected to be promising materials for cardiovascular stents (CVS),which can avoid the longterm clinical problems of current CVS,such as in-stent restenosis,late stent thrombosis,etc.Mg alloy stents exhibit superior biocompatibility and tunable biodegradability,compared with conventional permanent metallic stents.However,the poor formability and non-uniform corrosion of Mg alloy stents hinder their clinical application of CVS.This review focuses on the development of Mg alloys for CVS in recent years.According to the results of bibliometric analysis,we analyzed different biodegradable Mg alloy systems.Moreover,the structural design strategies for Mg alloy stents that can reduce the stress concentration,as well as the surface modification methods to control the corrosion behavior and biological performance of Mg alloy stents are also highlighted.At last,this review systematically discussed the potential directions and challenges of biodegradable magnesium stents (BMgS) in cardiovascular fields.
Keywords: Mg alloy micro-tube;Biodegradable Mg stent;Alloying design;Structural design;Functional properties.
1.Introduction
Coronary artery disease (CAD) is the most common cardiovascular disease and remains the leading cause of death worldwide [1–3].The most effective clinical treatment for CAD is the placement of a cardiovascular stent (CVS) in the coronary artery to expand the narrowed or blocked vessel[4,5].
Based on the expansion principles,CVS can be classified into self-expandable stents and balloon-expandable stents.The first-generation CVS was fabricated from inert metals with good mechanical integrity and corrosion resistance[6].Although first-generation metal stents,including balloonexpandable cobalt-chromium alloy,stainless steel stents,and self-expandable nickel-titanium alloy stents can provide sustainable radial support,they remain in the body for a long time,resulting in repeated vessel inflammation,recurrent atherosclerosis and other complications [7–10].Compared to self-expandable stents,balloon-expandable stents are rigid,provide higher radial outward force,and allow for more precise placement.The implantation of balloon-expandable stents for the treatment of CAD,commonly referred to clinically as percutaneous coronary intervention,is illustrated in Fig.1.The angiographic guidewire travels from the radial artery to the vessel and then locates the vascular plaque by X-ray [11].After locating the plaque,the retracted balloon-expandable stent is inflated by an air pump to support the vessel and promote blood flow [12,13].CVS requires both high strength and ductility as stents go through the process of compressing and expanding,which remains a major challenge for metallic stents.

Fig.1.(a) Schematic illustration of the expansion process of a CVS during percutaneous coronary intervention,and (b) Serial angiographies of a patient implanted received percutaneous coronary intervention at pre-procedure,post-procedure,and 8-month follow-up [14].
The development of biodegradable Mg alloy stents(BMgS)marks a breakthrough in bare metal stents for CVS.Compared with traditional metallic stents,BMgS have advantages in terms of sufficient radial strength,biocompatibility,and Mg2+eV generated by degradation is one of the essential macro-elements for the human body [15,16].Therefore,BMgS are expected to be the next generation of CVS.
Table 1 summarizes the list of typical clinical applications of BMgS.The first reports of BMgS prototypes tested in animal models were reported in 2003[17].The AE21 alloy stents were implanted in the coronary arteries of domestic pigs for 1 month,causing low inflammatory responses.
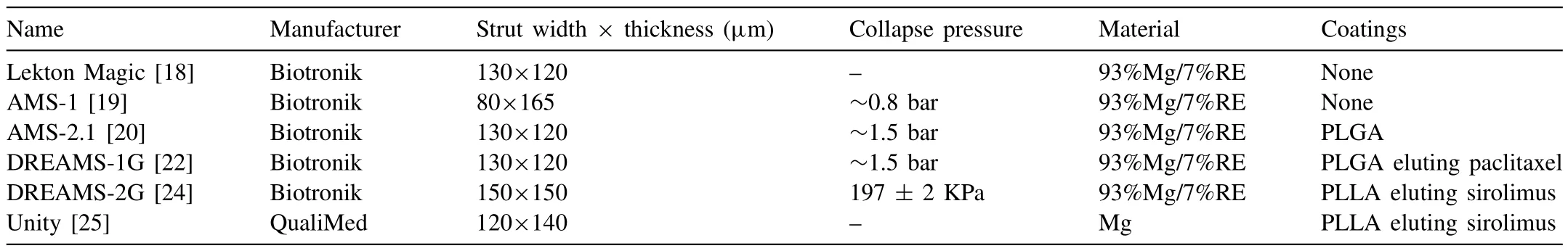
Table 1The list of clinical use of biodegradable BMgS.
The first generation of clinical BMgS is the Lekton Magic coronary stent,which is laser formed from a WE43 Mg alloy tube.The stent was first tested in pigs and then in human peripheral arteries,but the degradation rate remained rapid (<3 months) [16,18].The Lekton Magic stent also become known as the AMS-1 (Biotronik,Germany) designed for CVS [19].The stents are designed without coatings,using a 4-crown 4-link design with 80 × 165 μm struts width and thickness.The results of 63 patients who received AMS-1 stents at 4 months exhibited a major adverse cardiovascular events rate of 23.8%.However,the patency rate decreased significantly compared with the initial implantation.This is due to the absence of anti-proliferative drugs in the stent,resulting in neointimal proliferation and early radial strength loss.
To improve the radical strength,AMS-2.1 (Biotronik,Germany) is developed with a novel strut cross-sectional design into a square-shaped configuration of 130×120 μm to maintain mechanical integrity in a biological environment [20,21].To inhibit the over-proliferation,AMS-2.1 is coated with poly lactic-co-glycolic acid (PLGA) loaded with paclitaxel to form AMS-3.In a porcine model,the optimal PLGA composition(lactide-co-glycolide ratio) was the 85:15 ratio [22],and paclitaxel with a molecular weight of 0.07 μg/mm [23].The final version of AMS-3 is named Drug-Eluting Absorbable Metal Scaffold 1st-generation (DREAMS-1G).
The DREAMS-2G (Biotronik,Germany),also known as Magmaris,is designed to take full advantage of the mechanical properties of Mg alloys to maintain longer mechanical integrity and achieve sufficient radial strength.The stents utilize a geometric 6-crown,2-link geometric design to increase radial strength for better bending flexibility and more uniform vessel coverage.The PLGA coating is replaced by PLLA loaded with 1.4 μg/mm [23].Compared with DREAMS-1G,the degradation time of Magmaris is almost doubled,and the service life is extended to 12 months.In June 2016,Magmaris received the CE mark as the first bioresorbable drug-eluting metal stent and is currently in clinical use in more than 50 countries [24].
To assess the clinical efficacy and safety of the BMgS,the BIOSOLVE clinical program Ⅰ~Ⅳwas established with more than 1500 patients.BIOSOLVE I was a prospective,first-inman,multicenter trial to evaluate the safety and performance of DREAMS-1G The stents have demonstrated excellent clinical results with a 100% procedural and device success rate and a low target lesion failure (TLF) rate of 6.6% [26].However,15% of patients developed moderate to severe calcification after a 3-year follow-up [27].The clinical data of Magmaris from BIOSOLVE II and BIOSOLVE II were pooled and published together.At 3-year follow-up,one of 117 patients had TLF,but no patients had cardiac death or target vessel-myocardial infarction beyond 24 months [28].Encouragingly,consistent with BIOSOLVE I,no definite/probable stent thrombosis was reported at 3 years [29].
To further evaluate the safety and performance of Magmaris,data continues to be collected in the global BIOSOLVE IV registry[30].It is anticipated that 2054 patients will be enrolled to measure the primary endpoints of TLF at 12 months,with 1-year outcomes from the first 1075 enrolled patients enrolled already published [31].The success rates of the device(97.3%) and procedure (98.9%) were high,rates of cardiac death (0.2%) and myocardial infarction (1.1%) were low,and there were only 5 cases of definite/probable stent thrombosis(0.5%).Despite the good clinical outcomes of Magmaris,the total number of patient models is limited.Three key issues must be addressed to achieve widespread use of BMgS:
(1) BMgS provide temporary radial support to the vessel wall for 3 months and are resorbed within 1 year [24].
(2) BMgS are designed to lower the risk of thrombosis by reducing thrombogenicity of the structure,rapid degradation time,less disturbance of laminar blood flow leading to less platelet activation and aggregation,and by improving endothelialization [28].
(3) Clinical data must show promising results with excellent clinical outcomes and a good safety profile to support the use of BMgS in coronary artery disease [24].
Research studies on biodegradable BMgS are ongoing worldwide,and related animal experiments and clinical trials are also in progress.The published BMgS-related papers in 2022 were searched in the Web of Science Core Collection database on January 12,2023.Fig.2 shows the network visualization among different keywords.The size of circle represents the number of papers and the width of the link lines among different keywords indicates the frequency intensity.Intensive frequency are seen among degradability,biocompatibility,mechanical properties and surface modification.

Fig.2.Network visualization among different keywords in “biodegradable magnesium stents” related papers.
According to the keywords of the bibliometric analysis results,the research field of “Biodegradable magnesium stents”can be roughly divided into four categories: (1) mainly focus on the degradability and biocompatibility of Mg alloyin vivo/vitroenvironment,(2)corrosion behavior of Mg alloy,(3)microstructure and mechanical properties of Mg alloy,and(4)research related to implants in vivo.This review will summarize the recent progress of BMgS and present our views in the National Engineering Research Center for Magnesium Alloy Materials (Chongqing,China) on the research of BMgS.
2.Alloying design strategy for Mg alloy stents
Mg is the most abundant element in the human body (30 g for a 70 kg person),with extremely low toxicity and rapid excretion,and it can be degraded in vivo without secondary surgery [17,32].However,Mg has a hexagonal close-packed(HCP) structure,and few slip systems can be activated,resulting in weak plastic formability.Alloying elements cannot only refine the grains of Mg alloys but also impede dislocations through forming a solid solution and precipitating second phases,improving the plastic formability of Mg alloys.The corrosion potential of the second phase is more positive than that of the Mg matrix,leading to galvanic corrosion and rapid failure of Mg alloys.High-purity Mg (Mg≥99.99 wt.%) has a corrosion rate of 0.3~0.5 mm/y [33],but the corrosion rates of most Mg alloys are higher than that of high-purity Mg.The alloying design of biomedical Mg is the addition of nontoxic or micro-toxic elements to pure Mg to transform the metal (under different process conditions) into an alloy with desired properties [34].A wide range of elements ranging from alkaline earths to rare earths(RE) such as zinc (Zn) [35,36],copper (Cu) [37],manganese(Mn) [38],scandium (Sc) [39],yttrium (Y) [40],lanthanum(La) [41],cerium (Ce) [42],samarium (Sm) [43],gadolinium (Gd) [44],neodymium (Nd) [45],have been considered as alloy candidates in BMgS application.Furthermore,the addition of alloying elements plays an important role in the biological properties and corrosion resistance of Mg (Fig.3).

Fig.3.Alloying elements for improving the performance of BMgS.
With the development of Mg alloys in biomedical clinics,it is necessary to understand whether the corrosion resistance of Mg-based biomaterials,the mechanical integrity for a period of time,and the ability to avoid chronic inflammation caused by cell-biomaterial interactions meet the ISO 25539–2:2020(E) standard.Detailed requirements for the BMgS are shown in Fig.4.Therefore,it is important to select alloying elements with good mechanical properties to avoid chronic inflammation and the alloy content should be adjusted within an acceptable dosage range.

Fig.4.The diagram showing the requirements of Mg-based alloys as CVS.
2.1. Mg-Al based alloys
Mg-Al alloy system has generally been used for machining precision parts due to their excellent plastic formability.Al is also the most commonly used alloying element for Mg alloys with a maximum solubility of 12.7 wt.% [32].However,Al is a known neurotoxin that can cause neuronal damage and memory impairment [46,47].Mg-Al-Zn alloy is a commercial Mg alloy known for its favorable mechanical properties and high corrosion resistance,making it a promising candidate for biomedical implants.Over the past few years,there has been extensive research on the degradation behavior and biocompatibility of Mg-Al-Zn alloys,bothin vitroandin vivo.
Increasing the Al content results in an overall improvement in corrosion resistance of Mg alloys.Kim et al.[48]prepared AZ61 alloys with an ultra-fine grain size of 1.1~1.5 μm by using high-ratio differential speed rolling,and the alloy precipitated evenly distributed nanoscaledβ-Mg17Al12phases(70~140 nm).As a result,the corrosion potential ofα-Mg increased to-1.47 V in 5% NaCl solution.Furthermore,the refinement of theβ-Mg17Al12phases effectively reduced the susceptibility to micro-galvanic corrosion.In addition,studies on the in vitro degradation of AZ91 alloys have found that with the enrichment of Al in the grains,a continuous network ofβ-Mg17Al12phases formed,stabilizing the grain boundaries and improving the corrosion resistance of as-cast AZ91 alloys [49,50].However,when the discontinuousβ-Mg17Al12phases were unevenly distributed,they instead acted as cathodes,accelerating the corrosion of the Mg matrix.Furthermore,both in vivo and in vitro degradation experiments have discovered the formation of a protective film composed of calcium phosphate on the surface of AZ31 and AZ91 alloys,which effectively slows down the subsequent corrosion rate [51,52].Kandala et al.[46]fabricated AZ31 stents by photochemical etching and then implanted them in the peripheral arteries of domestic pigs to investigate the feasibility of AZ31 alloys as vascular stents.Although the in vivo corrosion rate of the stents was 0.75 mm/y,cases of fractures,overlaps,degradation,and fragmentation were observed in the struts of the stents,due to the influence of stress corrosion.In general,Mg-Al alloy system is not a recommended material for BMgS.
2.2. Mg-Zn based alloys
Zn can play an important role in cell-mediated immunity and have an anti-inflammatory effect on the human body.As an alloying element,Zn can exert a solid solutionstrengthening effect and contribute to the mechanical properties of Mg alloys [35,36,53].Moreover,the corrosion resistance of Mg-Zn alloys increased dramatically with increasing Zn content up to 4 wt.%,and the EL and UTS reached the maximum at the content of 4 wt.% [54,55,56].Cipriano et al.[57]investigated the effect of the degradation behavior of Mg-Zn alloy system on the cytocompatibility and early inflammatory response of human umbilical vein endothelial cells (HUVECs).Neither high Mg2+concentration(~27.6 mM) nor high alkaline pH (~9) induced detectable adverse effects on HUVECs.However,non-cytotoxic Zn2+concentration induced an increase in vascular cellular adhesion molecule-1 (VCAM-1) levels,indicating an early inflammatory response.Therefore,the synergistic behavior of Mg2+and Zn2+released by the corrosion of Mg alloys on the cellular behavior of vascular endothelial cells needs to be further studied.
Mg-Zn based alloys were further alloyed by adding other elements,including Cu,Gd,Mn,and Y.The introduction of Cu and Y refined the microstructure to improve the mechanical properties [37,40].Lotfpour et al.[37]found that the microstructures of as-extruded Mg alloys were refined by the introduction of Cu,and the corrosion resistance of the alloys was improved after adding 0.1 wt.% Cu.Yin et al.[40]focused on the effects of dynamic recrystallization (DRX) of Mg-Zn-Y alloys by increasing the LPSO volume fraction and obtained a large proportion of DRXed grains with the increase of LPSO volume fraction.The addition of Mn improves the corrosion resistance of Mg-Zn alloys.This can be attributed to the formation of the Mn oxide films,which act as barriers against chloride ions penetration,and the formation of intermetallics with harmful impurities of the cathode [38].Miao et al.[58]found that the as-extruded Mg-2Zn-1Gd alloy exhibited a low in vivo degradation rate(0.31 mm/y) after 30 days implantation in Dawley rats,low cytotoxicity (grade 0–1) to MC3T3-E1 cells and excellent mechanical performance (TYS: 284 MPa,UTS: 338 MPa and EL: 24%).It indicated that the great promise of Mg-Zn-Gd alloy as a biodegradable implant material.
2.3. Mg-RE based alloys
The addition of rare earth elements(REs) enhances mechanical performance and corrosion resistance of Mg alloys[32].REs refer to the group Ⅲmetals scandium (Sc) and yttrium (Y),along with 15 lanthanides: cerium (Ce),lanthanum(La),dysprosium(Dy),and so on[59].Fig.5 shows the phase diagram of the Mg-RE alloy systems,which can divide the Mg-RE alloys into high and low solid solubilities systems.Moreover,according to the solid solubility in Mg,the eutectic system can be further divided into two types,namely high solid solubility and low solid solubility.REs with high solid solubility in Mg alloys have the following superior advantages: (1) the scavenger effect-REs could combine with the impurities and then purify the Mg melt;(2) the formation of protective oxide layers-improve the stability and integrity of the oxide layers.Therefore,REs significantly enhance the corrosion resistance of Mg alloys.

Fig.5.Schematic phase diagrams of Mg-RE based alloys: Eutectic systems with (a) high solid solubility in Mg,(b) low solid solubility in Mg,and (c)peritectic systems [60].
Sc exhibits a high solubility of 24.5 wt.% in Mg,so it is difficult to trigger micro-galvanic corrosion with Mg matrix[39].Notably,Sc has no adverse effect on the proliferation of human vascular smooth muscle cells (HVSMCs) compared to all other REs [42,61].In addition,the mechanical properties and corrosion resistance of Mg alloys can be improved by the alloying element Sc.Ogawa et al.[62]reported that Mg-19.6Sc with weak texture showed high EL of>30% and moderately high UTS of 244 MPa.Sc also has the potential to transform the Mg matrix fromαphase with hexagonal closed-packed structure intoβphase with body-cubic centered structure [63].Liu et al.[64]found that Mg-30Sc alloy with a singleβphase could be obtained by adjusting the heat treatment process.This alloy exhibited excellent mechanical properties with ultimate compressive strength of 603 MPa,acceptable degradation rate in vivo (0.06 mm/y),no cytotoxicity on the MC3T3 cell model,and good mechanical integrity (maintained up to 24 weeks in the rat femur bone).
Y has a relatively high solubility (12.4 wt.%) in Mg-RE alloys eutectic system.With a great difference between the atomic radius of Y and Mg,Y results in an effective solid solution hardening,so it is a commonly used alloying element for BMgS.WE series alloys contain one or more REs alloyed with Y,and have relatively poor corrosion resistance.Among them,WE43 alloys have entered clinical trials and received CE marking approval.Öcal et al.[65]reported that the formation of intermetallic phase in WE43 alloys intensified the corrosion rate (17.61 mm/y) in SBF.Besides,WE54 alloy after T4 treatment exhibited a slower corrosion rate than that after T6 treatment,which indicated that the precipitation of intermetallic phase during the aging treatment has a great influence on the corrosion rates of WE series alloys [66].One of the most important criteria for Mg alloy vascular stents is the acceptable range of released metallic ions.Hirano et al.[67]studied the metabolic pathway of Y3+by injecting different doses of YCl3into the vein of rats and found that Y3+exhibited high affinity for the spleen and liver,with the Y3+displacing Ca2+eV in organs.Therefore,Mg alloys with low Y content can be used as implants for BMgS.Chou et al.[68]reported that WX41 alloys showed excellent corrosion resistance (0.61 mm/y),in vitro cytocompatibility,and in vivo host response compared with AZ31 and pure Mg.According to some reports,solid solution treatment of Mg-Y alloys reduces the residual casting stresses and the eutectic phases,thus obtaining a homogeneous distribution of elements in the Mg matrix [69].These changes are beneficial for improving corrosion resistance.Vlček et al.[70]found that the solid solution treatment (525°C,3 h) for Mg-4Y-1Ag alloys dissolved coarse Mg24Y5particles and decreased the corrosion rate by one-half compared to as-cast alloys.
Mg-Gd system alloy has been extensively studied,because of its preeminence in improving mechanical performance [66,71].However,when Mg alloys with high Gd content come into contact with abraded skin,severe and chronic ulceration (extending to muscle tissue) occurs,as has been reported for the alloying element Sm.Compared to other RE ions,Gd3+was less toxic in vitro and intravenous ingestion of GdCl3into rats did not increase liver lipid content[72,73].In addition,the solubility of Gd in Mg is~23.5 wt.% and minor addition of Gd can evolve RE texture to improve mechanical properties [44,74].The introduction of 0.3 wt.% Gd into Mg-Zn based alloys can dramatically enhance the ductility (~35%) by large strain rolling [75].Zhao et al.[76]further explored the effect of minor Gd additions on DRX and texture.The precipitation of Gd solutes along grain boundaries affected the recrystallization behavior and promoted the shear bands,leading to the formation of different textures.The non-basal slips(prismatic slip and pyramidal slip) can be activated easily with increasing Gd content,and grain sizes decrease due to a more intense solute drag effect.These effects can enhance strength without reducing ductility.Kubásek et al.[77]also found that the addition of Gd improved the corrosion resistance of Mg(OH)2layer,and Gd3+interacted with Cl-,hindering the penetration of Cl-from the Mg(OH)2layer to the Mg substrate.Due to the high solubility of Gd in Mg (23.5 wt.%),it contributes to solid solution strengthening and the electrode potential of the Mg matrix can be increased to improve corrosion resistance.Cai et al.[78]reported that the corrosion resistance improved with the increase of Gd content.When the Gd content increased to 20 wt.%,the corrosion depth of Mg-20Gd was reduced by 83%.In addition,after adding Zn to Mg-Gd based alloy,a lamellar LPSO phase whose electrode potential is closer to that of theα-Mg matrix can be precipitated by adjusting the Zn/Gd ratio.And a uniform oxide film with rapid repair ability is easily formed during the corrosion process,further reducing the corrosion rate of Mg-Gd-Zn alloys [79,80].
In physiological solution,Nd mainly exists stably in the trivalent oxidation state.Kim et al.[81]investigated the inhalation toxicity of nano-sized Nd2O3particles in Sprague-Dawley rats.After 28 days of repeated exposure to aerosols containing Nd2O3particles,the particles accumulated in the lungs,resulting in increased lung weight,pulmonary alveolar proteinosis,and infiltration of inflammatory cells (>2.5 mg/m3dose).The use of Nd as an alloying element for biomedical Mg-based alloys has not yet been systematically investigated in vitro and in vivo [59].The maximum solid solution of Nd in Mg is only 3.6 wt.% [60].When the Nd content increases above 4.0 wt.%,the volume fraction of Mg12Nd phase increases,leading to a significant increase in the corrosion rate [77,82].Zhang et al.[45]investigated the effect of intermetallic compound content on the degradation of as-extruded Mg-Nd alloys with different Nd contents in DMEM+10% FBS.When the Nd content is less than 2 wt.%,the formation of Mg41Nd5phases enhances the microgalvanic corrosion to accelerate the degradation process.However,with the further increase of Nd content,Mg41Nd5phases form a continuous and compact corrosion layer to reduce the corrosion rate.The Mg-Nd-Zn based alloys (JDBM) developed in Shanghai Jiao Tong University with moderate strength(UTS and YS of 180–280 MPa) and high ductility (elongation of 20%–32%) for BMgS [83,84].Nd has been shown to improve the mechanical properties and corrosion resistance of Mg alloys while having acceptable toxicity at low doses.Therefore,it is acceptable to add a small amount of Nd in the alloy design of BMgS.
Sm has the highest solid solubility (5.7 wt.%) in Mg among the low solid solubility eutectic systems,and it can optimize the mechanical properties of Mg alloys through solid solution/precipitation strengthening [85,86].Lyu et al.[43]developed Mg-Zn based alloys by Y/Sm alloying,hot extrusion,and aging treatment.UTS,YS,and EL of the Mg alloy reached 465 MPa,413 MPa,and 6.5%,respectively.Therefore,Sm is a suitable RE for the design of low-cost,high-strength Mg-RE alloys.A large number of researchers have worked on the development of Mg alloys with Sm addition,but few reports have been made on the biological properties of Mg-Sm alloys [87–89].Sm is of average toxicity compared to other REs.The average toxicity of its chloride or nitrate via various modes of injection exposure is not high,but it is noteworthy that the oral toxicity of SmCl3could not be assessed because it exceeds the solubility limit of the compound [90,91].Significant adverse effects were observed after spraying solid SmCl3on abraded skin of rabbits,resulting in significant perforating ulcers in the muscle tissue that did not heal within two weeks before death [90].Therefore,its influence rule on biological organisms is not clear in different Mg alloy systems and needs to be further explored.
As for other REs,they exhibit different mechanical performances,biological properties,and corrosion resistance.The TYS and UTS of Mg-xDy (x=5~15 wt.%) alloys decreased after T4 treatment but the corrosion rate slowed down(7.9 mm/y to 0.5 mm/y) [92].Mg-xCe alloy had shown adverse effects with increasing doses of Ce (up to 10 wt.%)in vitro tests using HVSMCs [42].Chu et al.[41]indicated that Ce/La element was uniformly dispersed in the precipitation phase particles and improved the corrosion resistance of Mg-Zn-Ca alloys.In general,REs as low concentration (≤10 wt.%) additives in Mg alloys have no significant adverse effects on the proliferation of HVSMCs and high concentrations of REs additives can lead to severe inflammatory responses in the surrounding tissues [17,42,59].The corrosion rate,mechanical and biological properties of typical Mg alloy systems are listed in Table 2.

Table 2The corrosion rate,mechanical and biological properties of Mg alloy systems.
3.Fabrication process of Mg alloy tubes for stent application
For most micro-tubes,the first step is to prepare Mg ingots by casting and heat treatment[119–121].The second step is to fabricate the required micro-tubes by using methods including hot extrusion and cold drawing.Then,stents are usually machined from micro-tubes by laser cutting and annealing treatment.Laser cutting treatment is the most effective method to make micro-tubes into mesh-like stents because it is localized,non-contact,and precise [119,122].The annealing treatment is used to improve the mechanical performance of the stent,promote the recrystallization of deformed grains and release the residual stress during laser cutting [121].The final step is acid pickling and electropolishing to remove slags and burrs generated during the laser cutting and drawing process[123].
In the hot extrusion process,the Mg ingot must be reduced in cross-section to obtain a small hollow profile,and then the heated ingot passes through the die under pressure to obtain the final micro-tube in a single step (Fig.6).However,Mg ingots have coarse grains,and the wall thickness of the desired micro-tubes is less than 200 μm,which leads to severe hot cracking during extrusion.In addition,it is difficult to control the dimensions of the micro-tubes at high temperatures by using extrusion alone.As a result,the hot extrusion process is not used to directly produce thin-walled micro-tubes and is used to produce intermediate shapes,and some other processes such as cold drawing and annealing treatment are followed to precisely control the dimension of thin-walled micro-tubes [124].Vedani et al.[125]prepared biodegradable ZM21 and AZ31B alloy intermediate tubes by using hot extrusion at 410°C.The results showed that recrystallization during hot extrusion can promote the formation of homogeneous microstructures in both alloys,while the TYS of both alloys is always significantly higher than the CYS,with an obvious tension-compression asymmetry.For BMgS,reducing the tension-compression asymmetry is more important than achieving the maximum TYS,thus facilitating the design of the stent expansion.Ge et al.[126]investigated the extrusion process parameters and hot compression behavior of ZM21 and AZ31B alloy tubes.When the strain rate was 2.78×10-3s-1and the extrusion temperature was 410°C,the tension-compression asymmetry of Mg tubes was improved and thin-walled tubes (0.5 mm of thickness) with homogeneous microstructures could be obtained.Moreover,Guo et al.[127]combined hot extrusion and rapid cooling to fabricate homogeneous Mg-Zn-Y-Nd alloy micro-tubes with excellent corrosion resistance and mechanical performance.The micro-tubes had an outer diameter of 3.0 mm and a wall thickness of 0.35 mm,but the wall thickness of micro-tubes still needs to be further thinned before they are applied for BMgS.
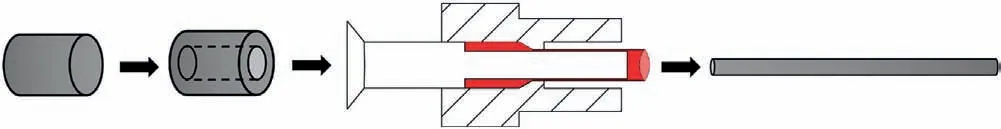
Fig.6.Schematic preparation process of the semi-finished tubes.
The cold drawing of extruded thin-walled tubes is subjected to strong plastic deformation at room temperature by means of the fixed mandrel method (Fig.7).Through the multi-pass drawing process,the tensile stress is applied to control the wall thickness of micro-tubes and the mandrel can be used to keep the inner diameter from changing,which can improve the dimensional accuracy of the micro-tubes.Especially,a multi-pass drawing process triggers a reduction in ductility and an increase in hardness,resulting in brittle fracture of the tubes during the process.Due to poor ductility and formability,the manufacture of high-precision Mg alloy micro-tubes and the separation of a mandrel from a microtube are still challenges [128].Wang et al.[129]developed a composite process involving hot extrusion and fourth-pass drawing to fabricate ZM21 alloy micro-tubes with an outer diameter of 2.9 mm and a wall thickness of 0.2 mm.Although this process is feasible to fabricate Mg alloy micro-tubes,the significant formation of twins is limited to further drawing during the multi-pass drawing.To solve this problem,Furushima et al.[130]proposed a die-less drawing with wire mandrels to mass-produce Mg alloy micro-tubes by drawing passes and intermediate annealing.The diameters of microtubes were controlled by changing the drawing speed and die size,but this method was not accurate and the mandrel was too tightly attached to be separated.National Engineering Research Center for Mg Alloys developed a novel method including multi-pass drawing and separation of a mandrel from a tube to obtain high-precision micro-tubes [131].The advantages and disadvantages of different fabrication processes of Mg alloy micro-tubes are listed in Table 3.

Table 3The fabrication process of Mg alloy micro-tubes.
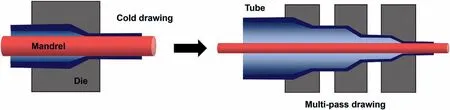
Fig.7.Schematic diagram of the cold drawing by fixed mandrel method.
The laser-cutting process facilitates the rapid production of various geometries on micro-tubes with high precision and low cost,illustrated in Fig.8.It is essential to construct a computer-aided design of the stent with an appropriate structure before cutting.After analyzing the data with the finite element method,the data is transferred to the laser processing system,and the laser-cutting process is performed.There are two laser cutting methods for producing BMgS:the first is the direct method,in which the laser is directly focused on the tube;the second is the masked projection method,in which the laser passes through a mask before being focused on the tube.The cutting quality is closely related to the laser cutting parameters,such as cutting speed,laser power,and beam size[132].Sealy et al.[133]investigated the relationship between laser cutting parameters and the quality of BMgS.It was shown that the use of low laser power (300 W and 600 W)and cutting speed(<700 mm/min),the stent exhibited a small taper angle (<1°),homogeneous microstructure (no columnar stripe damage),and a reduction of surface roughness.The laser-cutting process inevitably results in slag deposits,burrs on the cutting surface,and oxidation along the cutting edge.Therefore,the final step in the fabrication of stents is to remove these defects through acid-pickling or electropolishing.

Fig.8.Schematic diagram of the laser cutting system for metallic stents[132].
4.Structural design of Mg alloy stents
Proper structural design is one of the most important conditions for BMgS to achieve excellent comprehensive performance.In addition to the composition of BMgS,the geometry,and structure of BMgS also affect the angiographic restenosis and clinical trial success rates [134].The geometry of BMgS is based on the desired design and the composition of Mg alloys [135].Following the development of Mg alloys and several types of stents,the selection of stent combinations is very important.
4.1. Forms of metallic stents
As shown in Fig.9,traditional metallic stents can be classified into 4 major forms,including sheet,wire,ribbon,and tube [136].Foils and sheets can be cut or rolled to various dimensions for geometry design [137].However,sheets are not as common as tubes,and it is difficult to draw tubes to thin wall thicknesses (<200 μm) [138].The ribbon is fabricated in the form of continuous strips from hot-rolled wires.As reported by Tesař [139],thin Mg (99.92%) wires with a diameter of 250 μm were prepared by direct extrusion with a reduction ratio of 1: 576.Li et al.[140]fabricated Mg-2Zn-0.46Y-0.5Nd alloy micro-tubes with a wall thickness of 0.14 mm and an outer diameter of Ф2.46 mm using the hollow billet direct extrusion technique.The results exhibited excellent mechanical properties (TYS: 240.1 MPa,UTS: 319.9 MPa,EL: 22.2%) and slowed down the hydrogen evolution rate (0.37 mg/cm2× h) of the tubes in SBF (simulated body fluid).

Fig.9.Four forms of stents: (a) sheet,(b) ribbon,(c) wire,and (d) tube[22,46,141-143].
Wires and tubes are currently the most widely used types for balloon-expandable and self-expandable stents.Stents can be formed from wires in a variety of ways,including coiling,braiding,and knitting [144].Stents also can be fabricated by laser cutting tubes into structures with desired patterns.The tubes are then modified (deburring,slag removing) and shaped using progressively larger mandrels to meet the size of required stents [145].Fabrication from sheet metal consists of patterning followed by rolling the photochemically etched sheet metal into tubular structures.Photochemical etching as an effective method can cut many parts in a single step and meet the requirements of stents.Ribbons are used to form stents only by coiling.Ribbon stents are more common in non-vascular applications due to their recyclability after implantation and good flexibility [143].However,sheet and ribbon stents have relatively low radial strength and limited expansion ratio.The flexibility of tube stents is limited compared to others,but the selection type of stents prefers tubes due to their high radial strength and low cost [146].
4.2. Geometry and pattern of stent strut
Recently,clinical studies on BMgS have gradually discovered that strut geometry is related to the long-term implantation effect [147].To enhance the radial strength of BMgS and ensure sufficient support to the vessel,increasing the thickness or width of the strut is a viable option.However,it should be noted that greater strut thickness is associated with a higher incidence of myocardial infarction [148,149],and a greater likelihood of late thrombotic stenosis [150–152].Especially when the strut thickness is greater than 100 μm,the risk of late-stent thrombosis significantly increases [149,153,154].To avoid restenosis and thrombosis,the metallic stent typically has a thickness in the range of 0.07~0.14 mm [143].To ensure the mechanical integrity of BMgS remains for 6 months after implantation,it has been proved feasible to appropriately increase the strut thickness to 150 μm.As BMgS design advances,the finite element method is emerging as an effective method for optimizing structures.For example,the collapse pressure of the DREAMS-1G stent can be increased to 1.5 bar with the optimized structural design,compared to 0.8 bar for the AMS-1 stent [155,156].Li et al.[157]reported that a thin-walled BMgS with a novel support ring structure was obtained by using finite element analysis.The radial strength of the optimized thin-walled (100 μm) BMgS was the same as that of 150 μm BMgS,and it exhibited a significant reduction in the maximum principal strain (0.207 vs 0.283).To minimize the plastic strain,Chen et al.[158]validated the simulation results by in vivo and in vitro evaluations and promoted a uniform distribution of residual stresses using a shape optimization strategy.Different types of vascular stents require different geometries and strut patterns,including bare metal stents (BMS),drug-eluting stents (DES),bioresorbable stents (BDS),and drug-eluting fully-resorbable stents (DFS)[159].In 1986,BMS was first implanted in the human body as a CVS and demonstrated high patency rates in angioplasty[160,161].However,BMS causes adverse effects,including neoplastic hyperplasia which is an inflammatory response to the stent,and excessive proliferation of smooth muscle cells which can lead to in-stent restenosis.Therefore,many studies on the delivery of antineoplastic drugs that can inhibit over-proliferation and inflammatory responses have concentrated on addressing the adverse effects of BMS [162].Following numerous clinical trials,the second-generation stent-DES was approved by the US Food and Drug Administration (FDA) in 2003 [163–165].Both TAXUS eluting paclitaxel and Cypher eluting sirolimus demonstrated relatively low restenosis rates for 6 months after implantation [166].Unfortunately,DES has been associated with fatal complications and late-stent thrombosis,with a mortality rate up to 30% [167].The third-generation stent-BDS,based on incompletely resorbable materials,is an ideal candidate for replacing BMS and DES [168].BDS can be absorbed and eliminated by the body after a certain period,resulting in a low incidence of adverse effects such as in-stent restenosis and late-stent thrombosis [169].The strut patterns and geometry of commercial stents are concluded by Im [14],as shown in Fig.10.As for the fourth generation stent-DFS,three factors need to be taken into consideration: material,structural design,and coating.BMgS has shown potential as a strong candidate for the fourth-generation stent due to its in vivo degradability and biocompatibility.However,it needs to overcome certain challenges such as non-uniform corrosion and rapid corrosion rates [24,34,167].
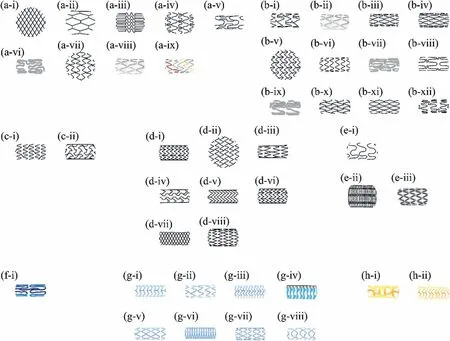
Fig.10.The strut patterns and geometries of commercial stents [14].(a-i) Wallstent,(a-ii) Palmaz-Schatz,(a-iii) Cypher,(a-iv) TAXUS Express,(a-v)Supralimus,(a-vi) Yukon Choice PC,(a-vii) BioMatrix Flex,(a-viii) Nobori,(a-ix) Combo,(b-i) Xience-V,(b-ii) Nevo,(b-iii) Endeavor,(b-iv) Resolute Onyx,(b-v) ELUNI,(b-vi) DESyne,(b-vii) ORSIRO,(b-viii) SupraFLEX,(b-ix) ULTIMASTER,(b-x) INSPIRON,(b-xi) BIOMIME,(b-xii) MIStent,(c-i)PROMUS Element,(c-ii) TAXUS Liberte,(d-i) ZILVER,(d-ii) S.M.A.R.T.,(d-iii) Axxess,(d-iv) SPARROW,(d-v) Acculink,(d-vi) Eluvia,(d-vii) Supera,(d-viii) EverFlex,(e-i) Wiktor Stent,(e-ii) Strecker Stent,(e-iii) Drug-Filled Stent,(f-i) Magmaris,(g-i) Absorb BVS 1.1,(g-ii) Igaki-Tamai Stent,(g-iii)DESolve,(g-iv) Ideal BioStent,(g-v) ART BRS,(g-vi) Mirage,(g-vii) XINSORB,(g-viii) MERES 100,(h-i) ReZolve,and (h-ii) Fantom.
The specific strut patterns and geometries are shown in Fig.11.The application of the geometric design needs to be considered in terms of width,strut pattern,and other factors[136].Slotted tubes (Fig.11a) and coiled stents (Fig.11b)are used as conventional stents.Most of the stents available on the market are generally slotted tube stents prepared by laser cutting.Although slotted tube stents are easy to prepare and have excellent radial strength,they exhibit low flexibility and deliverability [170,171].Coiled stents are made of metal strips or metal wires that are wound into a circular coil shape,and all of them on the market can be used for balloon expansion [172].Braided stents (Fig.11c) can be fabricated from one or more metallic wires and exhibit excellent mechanical support when applied to the artery via braided wires covering the surface area.Braided structures are common in self-expandable stents,but plaque compression after stent expansion may not be sufficient to cause restenosis [136,173].Individual ring dissection stents (Fig.11d) are typically used to support grafts.These individual ring structures are usually not served as BMgS alone but can be attached to graft materials during the manufacturing process [173,174].Helical spiral stents (Fig.11e) have good flexibility but lack internal junctions,resulting in radical or longitudinal recoil that fails to support the vessel [73].Sequential ring stents (Fig.11f)consist of expandable Z-shaped structural elements,hinges,and connection points.The majority of commercially available balloon-expandable and self-expandable BMgS have used the geometry category of sequential rings because they can provide the appropriate flexibility,strength,and small diameter for the stents [175,176].
5.Surface modification of Mg alloy stents
As a temporary implant,BMgS avoids the risks associated with permanent implants,such as persistent inflammation and late-stent thrombosis.While the rapid corrosion rate of Mg alloys under physiological conditions hinders their widespread use as implant materials [183].Surface modification is not only an efficacious tactic to ameliorate the corrosion resistance of BMgS but also can endow biological functionality,such as anti-inflammatory [184,185].This paper mainly categorizes the surface modification methods for BMgS into three main types:chemical conversion,micro-arc oxidation(MAO),and electrophoretic deposition (EPD) [186].
5.1. Chemical conversion coatings
The Mg substrate surface is converted by an electrochemical or chemical process into the conversion coating.Conversion coatings are considered a layer of oxide or other compounds forming through the interaction of Mg dissolution and precipitation in solutions [187].Therefore,the formed layer improves the corrosion resistance of the substrate.The quality and thickness of coatings depend on solution composition,alloys,and experimental parameters [188,189].The chemical conversion technique is used for metal hydroxides,inorganic non-metals,and polymer deposition on Mg alloys [190–193].
MgF2coatings can be obtained by surface treatment of Mg alloys with fluoride solution.Quan et al.[190]indicated that HF treatment was an effective way to improve the biofunctional properties of Mg-Nd-Y-Zn-Zr alloys.The formation of the MgF2coating on Mg alloys had decreased the corrosion rate effectively (0.874 mm/y vs 0.094 mm/y).Mao et al.[191]developed a simple and feasible method of preparing the nanoscale MgF2(nanoscale flake feature: 200~300 nm,thickness:~800 nm) on Mg-Nd-Zn-Zr alloys in 0.l M KF solution.It was noted that the degradation rate in artificial plasma was reduced by 20%from 0.337 mm/y to 0.269 mm/y and the results of implantation of the nano-coating stent to rabbit abdominal aorta had no rejections.
Phytic acid (PA) surface treatment has been developed as an effective and simple technique method for BMgS and PA (C6H18O24P6) is a nontoxic organic molecular compound that can be extracted from most legumes [194–196].Moreover,PA exhibits the ability to stimulate genes to promote cell differentiation and anti-cancer.Ye et al.[197]reported in vitro degradability and biocompatibility of a PA-coated WE43 alloy.It was found that when the pH value of the PA-modified solutions was 5,the PA-coated Mg alloy had the lowest degradation rate and the optimum hemocompatibility.Meng et al.[198]compared the biocompatibility of chemical conversion coatings on Mg alloys by different treatments,including NaOH,H3PO4,HF,and PA treatment.While the corrosion resistance of the Mg-PA coating was relatively poor,endothelial cell growth behaviors and hemolysis rate were much better.Moreover,Song et al.[199]prepared the polyhydric coating on Mg using ultrasonic micro-arc oxidation,PA conversion coating,and electroless copper plating.Introducing 0.24 wt.%Cu content into the coating can significantly promote the adhesion,proliferation,and differentiation of MC3T3-E1 cells as well as show excellent anti-bacterial rates in 24 h (E.coli: 94.6%,S.aureus: 81.3%).
In addition,it is reported that silane coatings exhibited good biocompatibility and low degradation rate.Silanization of Mg alloys is generally composed of two steps: the first step is to treat the NaOH-activated alloys with bistriethoxysilylethane (BTSE) and the second step is treating alloys with 3-aminopropyltrimethoxysilane (γ-APS) [200].BTSE (bissilane) andγ-APS (mono-silane) promote further attachment of bioactive molecules to enhance the interfacial interaction of Mg implants with surrounding tissues and cells [201].Liu et al.[202]developed a two-step method to prepare the bio-functionalized anti-corrosive (BTSE-γ-APS) coating on AZ31.Mg-B-A-heparin had better corrosion resistance and reduced platelet adhesion compared to bare AZ31.
Recently,Liu et al.[203]investigated a one-step process to introduce the cross-linked 3-amino-propyltrimethoxysilane(APTES) silane physical barrier layer on Mg-Zn-Y-Nd alloys and then electrostatic spray with rapamycin-eluting poly-(lactic-co-glycolic acid)(PLGA)layer.The APTES pretreated Mg-Zn-Y-Nd stent not only exhibited excellent in vitro mechanical properties but also demonstrated benign tissue compatibility and re-endothelialization without stent thrombosis or in-stent restenosis after 6 months of implantation in porcine coronary arteries.
5.2. Micro-arc oxidation coatings
Micro-arc oxidation (MAO) is considered to be an ecofriendly surface treatment derived from conventional anode oxidation [204].In the MAO process,Mg alloys are used as the anode and a container made of steel serves as the cathode.At first,an oxide film is formed on the Mg alloys accompanied by strong gas evolution at high temperatures[205].Then the voltage continues to increase but the rate decreases,which results in the fracture at weak points of films and an increase of the thickness [206].Anions derived from the electrolyte react with Mg2+from the anode,and the coating with good wear resistance and corrosion resistance can be fabricated [207,208].
The MAO coating on the Mg alloy serves as a physical barrier that can effectively separate Mg substrates from the solution to obtain a low corrosion rate compared with other coatings [209,210].However,the external layer of the MAO coating has pores that lead to poor corrosion resistance,and its internal layer has excellent corrosion and wear resistance[211].Sun et al.[212]compared the degradation behavior of Mg-Zn-Y-Nd alloy intestinal stents coated with MAO and MAO/poly(l-lactide)/paclitaxel in rabbits and their biocompatibility with intestinal tissues.The results showed that the cracks between the pores of the ceramic oxide film became larger.The micro-pores of the MAO layer are detrimental to the long-term stability of the implant,thus the single MAO coating is not suitable for implants.
In order to improve corrosion resistance and biocompatibility,various polymers and nanoparticles (NPs) have been developed to fill the pores of MAO coatings [213].While natural and synthetic polymers can be used as coatings for BMgS [214],synthetic polymers are more competitive due to their controlled degradation rate and flexibility [215].Rebelo et al.[216]prepared silver-based nano-coating coronary stents through direct current magnetron sputtering and found that aged Ag nano-coating exhibited a mild bacterial effect,with tolerable cytotoxicity towards fibroblasts.However,it is known that high Ag content will cause toxicity in tissue.Xu et al.[217]prepared a composite coating that can effectively control the bio-corrosion rate and drug release on the surface MAO layer of WE42 alloys.The composite coatings were fabricated by mixing different degrees of cross-linked gelatin with paclitaxel-loaded poly (DL-lactide co-glycolic acid)(PLGA)NPs.Ghafarzadeh et al.[218]found that bilayer copper-chitosan NPs (Cu-Ch NPs) incorporated polyglycerol sebacate (PGS)/MAO (MAO-PGS/Cu-Ch NPs) coating for BMgS.By adding 5 wt.% Cu-Ch NPs,the coating exhibited excellent corrosion resistance (Icorr=20 nA/cm2,Ecorr=-1.22 V)in PBS,hemocompatibility(Decrease in platelet adhesion),and cytocompatibility (Improved proliferation and attachment of HUVECs).
5.3. Electrophoretic deposition coatings
Electrophoretic deposition (EPD) coatings is an electrochemical deposition of charged particles on a surface of opposite charges after applying the electric field.The method is widely used to improve the corrosion resistance of Mg alloys[219–221].As shown in Fig.12,EPD is mainly composed of four parts: power supply,electrolyte,cathode,and anode.The main advantage of EPD is the capability to provide a homogeneous coating on complex substrate shapes with heterogeneous morphology and the ability to easily adjust the thickness by altering the deposition time and applied voltage[222,223].

Fig.12.Schematic diagram of the EPD process.
Polymers coatings are applied to commercial drug-eluting BMgS due to effective improvement of corrosion resistance[224,225].As reported by Wang [224],poly-L-lactic acid(PLLA) loaded paclitaxel was covered on the MAO coating of Mg-Zn-Y-Nd alloy stents.When stents were implanted into the intestine of rabbits for 8 to 12 days,the structure of the stent collapsed and discharged from the body without intestinal tissue proliferation.Kang et al.[225]introduce PEI(polyethyleneimine) and poly(lactic-co-glycolic acid) (PLGA)as surface coating polymers on WE43 Mg alloy stents for testing corrosion resistance in artificial blood plasma.The Icorrof the bare WE43 was substantially reduced by applying surface coating layers (62.24 vs 3.00 μA/cm2)(Fig.13a) and the superior corrosion resistance of the PLGA/PEI coating was also shown in the pH variation measured at a predetermined time during 7 days(Fig.13b).While some portions of the two ends of coated stents are lost after 14 days (Fig.13c),they still have well-preserved original stent geometry and possess almost 85% of their original volume.As shown in Fig.13d,average radial stiffness gradually decreased to 0.12,0.08,and 0.03 N/mm after 3,7,and 14 d of corrosion,respectively.

Fig.13.Degradation performance assessment of bare and coated WE43 samples immersed in artificial blood plasma at 37 °C: (a) Potentiodynamic polarization curves,(b) pH evolution,(c) 3D images of stents at different time intervals,and (d) remaining volumes and radial stiffness of stents.
However,polymers are prone to be ruptured and exfoliated after long-term immersion in physiological media due to their unsatisfactory adhesion and bulk-eroding properties[226,227].To solve these questions,many reports have used naturally occurring bioactive molecules (such as proteins,enzymes,and biopolymers) to prepare biocompatible coatings on BMgS through the EPD method [228–231].Wang et al.[230]found a facile and green EPD method to fabricate the bioactive hydrogel coating by combining copper ions,chitosan,and catechol groups on the CVS surfaces.The results demonstrated that the biopolymer hydrogel coating with cooper ion into the CVS may generate NO to reduce clinical complications and the coating exhibits excellent cytocompatibility that can promote the proliferation and adhesion of cells.Pan et al.[232]found the preparation of the biodegradable aliphatic polycarbonate (APC) coating by EPD and then photo-crosslinking treatment to obtain BMgS with excellent surface biocompatibility and corrosion resistance.APCs on Mg substrates decreased hemolysis rate,enhanced endothelial cell adhesion and proliferation,and reduced tissue inflammation.The constant erosion rates of the coating were low at 1.9 μm per monthin vitroand 1.4 μm per monthin vivo,respectively.
In the EPD synthesis process,polymers are oxidized and they need negative charged particles to provide charge balance.The drug can be incorporated into the polymer structure as charged particles in this process.For example,Polytetrafluoroethylene (PTFE)exhibits a negative charge in ethanol[233],and a homogeneous PTFE-polymers coating also can be obtained by altering EPD parameters [234,235].PTFE has been a popular synthetic material used for vascular bypass occlusion and is also used in various biomedical fields such as hernia membranes,surgical sutures,and hemodialysis [236–238].However,studies about the application of PTFE coatings on BMgS are relatively poor.Wang et al.[239]found that an expandable PTFE membrane covered Mg stents used in the common carotid artery(CCA).The diameter of the covered stented CCA was significantly larger at 6 and 12 months than immediately after stent placement,and the mean lumen area of the covered stent was significantly larger than that of the Willis stent at the same time points.However,PTFE is not the ideal coating for endothelial cell adhesion,and further improvement of the biocompatibility of PTFE coatings is necessary [237,240].
6.Functionalized composite coatings on Mg alloy stents
6.1. Drug-eluting coatings
It suggests that the loading of drugs in the coating of BMgS can be optimized to reduce both early thrombogenicity and late neointimal formation.This can be achieved by having a properly designed drug release coating that allows the drugs to be released through diffusion mechanisms or during polymer breakdown,but without posing a significant risk of systemic toxicity.Overall,the goal is to improve the performance of the stent in terms of its safety and efficacy,particularly in limiting the risks of complications associated with stent implantation.There are three types of commercial drugs: sirolimus,everolimus,and paclitaxel.Early-DES releases sirolimus or paclitaxel to prevent over-proliferation,while new-DES loaded with everolimus exhibit better biocompatibility [185].
Paclitaxel(Fig.14a)is extracted from the bark of the slowgrowing western yew.While paclitaxel has excellent antiproliferative properties[241],its high lipophilicity and insolubility in water indicate a low therapeutic index for stent loading alone [242,243].Considering the anti-proliferative properties of paclitaxel [241,244],polymer coatings loaded with paclitaxel can be used to improve its hydrophilicity and control drug release [225].Wang et al.[224]designed a drugrelease coating consisting of MAO and PLLA coating containing paclitaxel on Mg-Zn-Y-Nd alloy stents.The PLLA coating completely plugged the pores on the MAO layer.In addition,MAO/PLLA/paclitaxel-coated stents were not significantly toxic to vital organs,and there were no significant differences in serologic parameters at different stages of degradation.Lu et al.[245]developed the MAO/PLLA/PLGA coating,which was loaded with paclitaxel and had good hemocompatibility,including less platelet adhesion,activation,and aggregation.
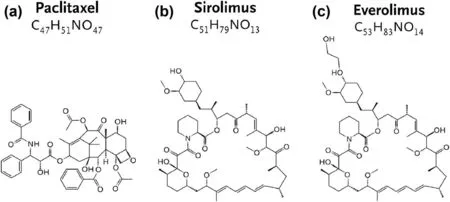
Fig.14.The molecular formulas and structures of commercial drugs: (a) Paclitaxel,(b) Sirolimus,(c) Everolimus.
Sirolimus (Fig.14b) is an anti-proliferative drug that forms a complex with FK-binding proteins and then interacts with mammalian rapamycin target proteins,which are related to cell proliferation and growth [246].Moreover,sirolimus has a broader therapeutic index compared to other anti-proliferative drugs and does not induce cell death even at high concentrations [247].Therefore,early-DES inhibits cell over-proliferation and reduces in-stent restenosis by loading sirolimus.Li et al.[248]implanted BMgS and sirolimuseluting (140±40 mg/cm2) BMgS in the infrarenal abdominal aorta of 20 New Zealand White rabbits.The sirolimuseluting BMgS further improved the luminal area and reduced endothelial proliferation compared to uncoated BMgS,while delaying vessel healing and endothelialization.In addition,Magmaris (sirolimus-eluting BMgS) is the only CE-marked biodegradable stent and is available for clinical use [24].The effectiveness of Magmaris in treating CAD has been demonstrated to be remarkably safe,evidenced by its low rates of ischemia-driven target lesion revascularization and TLF,and only one case of stent thrombosis was reported in the BIOSOLVE study.
Everolimus (Fig.14c) is a potent anti-proliferative agent which forms a complex with the cytoplasmic protein-FKBP12 to inhibit the proliferation of HVSMCs [249,250].It also has a powerful immunosuppressive effect that was well tolerated in clinical trials for the prevention of transplant rejection[251,252].Serruys et al.[253]have investigated the safety and efficacy of the everolimus-eluting BMgS in treating a single coronary artery lesion for 2 years.The result showed promising outcomes as the stent was absorbed,vasomotion was restored,and in-stent restenosis was prevented.However,it should be noted that the study had some limitations such as small sample size and the use of non-validated imaging methods.Therefore,further research with larger sample sizes and more rigorous methods is imperative to confirm the findings and evaluate the long-term safety and efficacy of the BMgS stent.
6.2. Multilayer coatings
To control degradation kinetics and improve interactions between implant and host,multilayer coatings of BMgS with different functional characteristics have attracted attention in recent years.Combining different surface modification methods to construct multilayer coatings on BMgS has been proven that could inhibit the over-proliferation of smooth muscle cells and benefit the phenotypic modulation of endothelial cells because the topological structure of the surface can regulate cell behavior [251].
Multilayer coatings on BMgS exhibited better biological performance compared with single-layer BMgS.Polymer coatings are a favorable choice among surface functionalized treatment strategies due to their high efficiency,versatility in terms of available functional groups,and relatively simple preparation methods.Gallic acid is a phenolic acid with attractive biological functions,including antiinflammation,promotion of endothelial cell proliferation,and inhibition of smooth muscle cell growth [254–256].Poly(lactide-co-glycolic) acid (PLGA) is used as an anti-corrosive layer to control the degradation rate and drug release rate.Their synergistic effect is expected to improve the biological properties and corrosion resistance of BMgS.Liu et al.[202]prepared three-layer polymer coatings on Mg alloys.The coating exhibited both improved corrosion resistance and reduced platelet adhesion,but effects on endothelial cells and smooth muscle cells were not evaluated.The development of a surface functionalization strategy that can simultaneously control the corrosion resistance of Mg alloys with good biocompatibility is important for BMgS.Lin et al.[105]developed a sandwich-like coating in which a GA layer was sandwiched between two layers of PLGA layers on ZK60 alloys for coronary artery stents.The electrochemical results revealed that the PLGA/GA/PLGA coating significantly improved the corrosion resistance of ZK60 alloys,and the degradation rate was approximately 2000 times lower than that of uncoated ZK60 alloys.A recent in vivo study in porcine coronary arteries suggested that triple-layered PLGA-APTESMgZnYNd stents confirmed superior tissue compatibility and re-endothelialization capacity with no major signs of injury,thrombosis,or restenosis for up to 6 months [203].As shown in Fig.15(a),the dimensions (length of 15.00±0.08 mm and optical density of 2.00±0.03 mm) met the need of application as the coronary artery and the surfaces of the stent struts were smooth without any detected peeling after in vitro expanding at a nominal pressure of 6 bar Moreover,the functionalized coating (Fig.15b) can be covered over the stent uniformly,showing outstanding fastness and stability.For the cytoskeleton and cell morphology shown in Fig.15(c),the more stained filaments implied the more favorable cytoskeletal morphology of adherent HUVECs,with no significant reduction in the number of HUVECs.There were 105±17 cells/mm2(n=5) and 116±8 cells/mm2(n=5) adhered to the surface of 2% and 4% PLGA-APTES-MgZnYNd samples,respectively.No coronary artery occlusion,thrombosis,or calcification was observed in any of the samples during the 6-month observation,as judged by Fig.15(d).
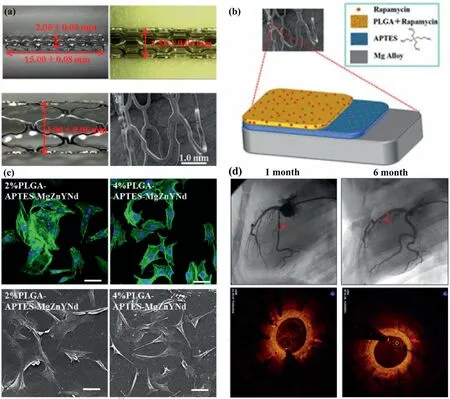
Fig.15.(a) Macroscope and microscope images of MgZnYNd stents,(b) schematic diagram of biocompatible composite coatings,(c) confocal microscopy and SEM images of human umbilical vein endothelial cells cultured on 2% and 4% PLGA-APTES-MgZnYNd samples,(d) coronary angiography.
At present,research on the construction of functionalized polymer coatings is mostly concentrated on polyesters such as poly(e-caprolactone),PLGA,and PLLA [251].However,most polyester coatings are easily delaminated from the surface because their adhesion to Mg substrates is weak and their degradation products have no therapeutic effect or cause extraside effects [212].A polymer that would provide additional adhesion and therapeutic benefits can be a useful functionalized composite coating for BMgS.Many natural bioactive molecules from plants and animals exhibit unique biological activities;these bioactive molecules can promote the development of polymer coatings if they can be successfully produced as biomedical materials with therapeutic benefits.
Many studies have reported that phenolic compounds may prevent cardiovascular disease and reduce the risk of stent thrombosis[254,257,258].Catechol acid and phenolic groups have the potential to suppress the proliferation of HVSMCs to prevent in-stent restenosis,which can be extracted from certain plants by dry distillation [258,259].It is commonly used as a pharmaceutical intermediate,cross-linked with other drugs or polymers for modification of various surfaces[254,257,260].Lih et al.[260]found that Co-Cr stents were coated with dopamine-conjugated hyaluronic acid with different ratios of the CA group to improve re-endothelialization and hemocompatibility.The results demonstrated that there were optimal amounts of CA (100 μmol) in the dopamineconjugated hyaluronic acid existed in terms of properties such as cellular responses and hemocompatibility.Based on the crosslinking of CA and PEI,Zhang et al.[257]developed a CA/PEI conversion coating on MgZnMn alloy.This coating showed excellent corrosion resistance due to the aggregation and strong bonding of the units.After heparin (Hep) grafting,the MgZnMn-CA/PEI-Hep showed significant improvement in hemocompatibility and obvious improvement in HUVECs proliferation,while considerable inhibition in HVSMCs proliferation.In addition,Liu et al.[254]also found that CA groups of MgF2/polydopamine coating created a favorable environment for HUVECs to have a competitive advantage over HVSMCs,which was preferable for re-endothelialization.
Tannic acid (TA),a natural polyphenol containing five pyrogallol and five catechol groups,has been shown to have various biological properties,including platelet adhesion [261],antioxidant [262],and anti-inflammatory [262,263]effects.Yang et al.[261]demonstrated that the coordination-driven polyphenol coatings could effectively prevent platelet adhesion and the platelet-repellent effect was much better in a long term compared with the most commonly used poly (ethylene glycol).Wang et al.[262]found that the TA coating had a promising strong antioxidant activity as it showed a radical scavenging activity of over 80% in the long term and no significant inflammatory responses were observed in the rat subcutaneous implantation test.Moreover,Qiu et al.[263]developed a plant-inspired phenolic-amine chemistry coating to combine the biological functions of a plant polyphenol,TA,and the thrombin inhibitor bivalirudin (BVLD) for surface modification of CVS.The abundant phenolic hydroxyl groups of TA imparted the stent with the ability to suppress inflammation and the combined functions of the TA and BVLD facilitated the rapid stent re-endothelialization to reduce intimal hyperplasiain vivo.
α(DL)-thioctic acid (α-TA) is a natural antioxidant synthesized in the human body.It can significantly increase the synthesis of NO to enhance endothelium-dependent NO-mediated vasodilation [264,265].α-TA can be self-crosslinked to form clear yellow solid polymers (linear poly TA),but the polymers exhibit metastable properties because they may suffer from reverse ring closure depolymerization,rendering polymers rigid and opaque [266,267].Zhang et al.[268]found that the divinylic compound(1,3-di isopropenyl benzene)and Fe3+were used to quench the terminal diradicals by inverse vulcanization and replace partial weak H-bonds,thus strengthening the stability of the network.Zhang et al.[269]fabricated polymeric coatings on the ZE21B alloy stent.The problems of thrombosis and restenosis caused by the implantation of the BMgS were solved by the therapeutic effect of the polymeric coating itself.Fig.16(a) illustrated the fabrication of the polymeric coating on the alkaline-treated ZE21B alloy.TA powder is heated at 130°C to form a transparent and low-viscosity Poly(TA) polymer,then add ZnCl2solution with different Zn2+contents to form a stable homopolymer network,and finally apply it on the alkali-heat-treated ZEB1 by one-step dip-coating method.The molecular dynamics results demonstrated that it was difficult for two Poly(TA)molecules to form a stable Poly(TA) polymer without Zn2+.When appropriate Zn2+(22Zn,44Zn) was added,the center of mass decreased rapidly,forming a stable Poly(TA) polymer (Fig.16b).As shown in Fig.16c and d,it can be found that the Poly(TA-Zn)-REDV sample exhibit higher endothelial cells (ECs) and lower smooth muscle cells (SMCs) viability than other samples throughout the entire cell incubation stage,indicating a substantial promotive effect on the proliferation of endothelial cells and inhibitory effects on the attachment of SMCs (Fig.16f).It is because the TA molecule can effectively inhibit the proliferation of SMCs[270]and the immobilization of the REDV peptide further suppresses the growth of SMCs [271].For the ZE21B alloy stent,the Poly(TA-Zn)-REDV coating also inhibits platelet activation and prevents severe blood clotting (Fig.16e).

Fig.16.(a) Diagrammatic sketch of Poly(TA-Zn) coating on Mg(OH)2 treated ZE21B alloy,(b) molecule’s center of mass coordinate of Poly(TA) in the z direction for different models,(c) cytotoxicity and relative cell viability of ECs after culture for different times intervals,(d) cytotoxicity and relative cell viabilityof SMCs after for 24 h of culture (*p <0.05,**p <0.01),(e) schematic diagram of anticoagulation of Poly(TA-Zn)-REDV samples.(f) Schematic illustration of Poly(TA-Zn)-REDV samples enhancing re-endothelialization.
BMgS is considered as the next-generation CVS due to its excellent flexibility and biodegradability.However,biocompatibility and corrosion resistance are limited in the physiological environment,therefore,it is necessary for functionalized composite coatings to control the degradability and biocompatibility of BMgS.The physical barrier layer is used to improve corrosion resistance,and the bioactive molecule layer or drug layer promotes HUVECs proliferation and inhibits HVSMCs over-proliferation to avoid adverse effects after implantation.Notably,the limitations of each method must be taken into account before surface modification of BMgS can be performed.The MAO,conversion and EPD coating can be considered the cost-effective physical barrier layer.The bioactive molecule layer or drug layer requires consideration of temperature,pH,and implant environment.All these objective factors can affect the release rate or efficacy.Here we mainly focus on reviewing the latest advances in constructing the functionalized composite coatings on BMgS and analyzing the advantages and disadvantages of various surface modification technologies,as shown in Table 4.

Table 4Different types of coatings for Mg alloy micro-tubes.
7.Conclusion and outlook
BMgS is a highly advantageous vascular stent,but it still has a few challenges that need addressing.It is a multidisciplinary project that involves alloy composition design,microtube processing,structural design optimization,and biofunctional coating preparation.Despite the progress made so far,the pressing scientific issue of balancing the mechanical performance,degradation behavior,and biological properties of BMgS remains to be solved.The main conclusions are listed as follows:
(1) The preparation technology of micro-tubes has a major
impact on the radial support strength and final quality of balloon-expandable BMgS.Although multi-pass cold drawing can effectively control the micro-tube diameters,the accumulated strain during the process creates mass twins,leading to the fracture of micro-tubes.Intermediate annealing treatment can promote grain recrystallization and reduce residual stress to improve the ductility of micro-tubes.However,the absence of a method for separating mandrels from micro-tubes necessitates further research.
(2) The properties of stents,including the strut pattern,thickness,and geometry,are directly linked to their overall performance.To further optimize the strut structure of BMgS,it is crucial to utilize numerical simulation to leverage its advantages in analyzing mechanical properties and identifying local stress corrosion factors.
(3) Composite coatings offer a promising solution to address the poor adhesion and deficient bioactivity of Mg-based stents as they can combine the advantageous properties of every single-layer coating.Moreover,the use of drug-loaded multilayer coatings offers a new approach to expedite endothelialization and regulate the corrosion rate of BMgS.Although composite coatings can be highly effective,their synergy mechanism is complex and requires long-term evaluation in future studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Jun Tan,Jia She,Xianhua Chen and Fusheng Pan are an editorial board member/editor-in-chief for Journal of Magnesium and Alloys and were not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgements
This research was funded by the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0993),the Chongqing Academician Special Fund (2022YSZXJCX0014CSTB),National Natural Science Foundation of China (52225101),and the China Postdoctoral Science Foundation (2022M720551).
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
- Achieving high ductility and strength in magnesium alloy through cryogenic-hot forming
