Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
Arsh Ftth-lhosseini ,Rzieh Chhrmhli ,Sjd Alizd ,Mosb Kseem
a Department of Materials Engineering, Bu-Ali Sina University, Hamedan 65178-38695, Iran
b Department of Nanotechnology and Advanced Materials Engineering, Sejong University, Seoul 05006, South Korea
Abstract Mg and its alloys have been introduced as promising biodegradable materials for biomedical implant applications due to their excellent biocompatibility,mechanical behavior,and biodegradability.However,their susceptibility to rapid corrosion within the body poses a significant challenge and restricts their applications.To overcome this issue,various surface modification techniques have been developed to enhance the corrosion resistance and bioactivity of Mg-based implants.PEO is a potent technique for producing an oxide film on a surface that significantly minimizes the tendency to corrode.However,the inevitable defects due to discharges and poor biological activity during the coating process remain a concern.Therefore,adding suitable particles during the coating process is a suitable solution.Hydroxyapatite (HAp)has attracted much attention in the development of biomedical applications in the scientific community.HAp shows excellent biocompatibility due to its similarity in chemical composition to the mineral portion of bone.Therefore,its combination with Mg-based implants through PEO has shown significant improvements in their corrosion resistance and bioactivity.This review paper provides a comprehensive overview of the recent advances in the preparation,characterization,corrosion behavior and bioactivity applications of HAp particles on Mg-based implants by PEO.
Keywords: Mg and its alloys;Hydroxyapatite;Corrosion behavior;Composite coatings;Plasma electrolytic oxidation (peo).
1.Introduction
In recent years,Mg alloys have become a research hotspot as a third-generation medicinal material due to their outstanding bioactivity,biocompatibility,and biodegradability [1–5].When compared to other metallic implant materials and synthetic hydroxyapatite(HAp),Mg and its alloys are lightweight metals with a density of 1.74 g/cm3,which is significantly closer to that of natural bone [6,7].Furthermore,when compared to other currently utilized metallic implant biomaterials,the elastic modulus of Mg and its alloys is remarkably near to that of natural bone [8,9].Mg is being considered as a biodegradable implant material due to its remarkable ability to biodegrade in the physiological environment [10–12].When exposed to aqueous media,Mg undergoes oxidation and generates Mg(OH)2.Under the conditions of near neutral pH and in the presence of chloride,the protective coating of Mg(OH)2dissolves and converts into soluble MgCl2[4,13,14].The biocompatibility of a material can be affected by the presence of corrosion products,including OH-ions and H2gas.Accumulation of H2gas in the neighboring tissue can result in the formation of air holes,leading to the separation of tissue layers.Presence of OH-ions can cause surface alkalization,which may potentially harm cells.The aforementioned factors can contribute to a decrease in the structural integrity of the implant during its use [15–18].Regulating the corrosion rate of Mg in bodily fluids is a crucial step towards broadening the scope of applications for biodegradable Mg-based implants [19,20].
Surface coatings can significantly reduce or at least delay the localized degradation of Mg-based materials [21–23].Sol-gel[24],electrophoretic deposition(EPD)[25,26],plasma electrolytic oxidation (PEO) [27–29]and chemical vapor deposition(CVD)[30]are some of the techniques used to obtain protective coatings [31,32].Recent advances in applying the PEO approach for creating coatings on Mg and related alloys has revealed that it is a cost-effective,easy,and ecologically benign process [33–36].The presence of pores and deep cavities resulting from the PEO process can limit the quality and corrosion resistance of the coating [37,38].To address this issue,modifying the electrolyte conditions based on the presence of particles is a useful approach for decreasing coating porosity and enhancing its final properties [39,40].Coating implants with bioactive compounds is a good way to increase their biological characteristics [41].
HAp shows excellent biocompatibility due to their similarity in chemical composition to the mineral part of bone[42,43].HAp is a suitable material for implant coating due to its similarity in chemical composition and characteristics to the primary inorganic components present in human teeth and bones.Applying a HAp-containing coating to the surface of Mg alloys offers several advantages,including enhancing their biological activity and reducing the rate of degradation.Additionally,the HAp-containing coating exhibits superior biological activity and bone conductivity [44–47].In the presence of suitable electrolytes,the PEO process produces oxides and HAp particles on the surface of Mg-based implants.Between the PEO process and anodization,there are some distinct differences.Compared to anodization,which typically employs a modest voltage (5–50 V),the PEO method utilizes much higher voltages (100–600 V).In the PEO technique,a high voltage spark and electrochemical oxidation are applied in an aqueous solution containing dissolved compounds for coating[48,49].Furthermore,this approach permits P and Ca ions to be substituted on the surface of Mg alloys [50].When a specific voltage is applied during the PEO process,a plasma discharge is generated on the substrate surface.This discharge can generate bonded oxide coatings on metals that exhibit high performance [51–53].At room temperature,on metallic biomaterials,the PEO method can be used to create coatings with a variety of morphologies [54,55].Composite coatings containing HAp particles created by the PEO process have various advantages over coatings developed by other chemical conversion processes,including notably improved surface characteristics of Mg and related alloys and significantly superior corrosion performance [56,57].
This review comprehensively summarizes the current progress in research on the corrosion behavior of PEO coatings containing HAp particles.Much research has been done on the surface properties of various coating methods containing HAp particles on Mg alloy,but to our knowledge,there has been no comprehensive review on improving the corrosion behavior of PEO coatings in recent years,while the main goal of this text is to cover all aspects of this approach.In this review,the effect of different surface factors such as particle entry method,thickness and phases formed during the coating process are discussed and then the corrosion behavior of the coatings and the challenges in this path will be examined.
2.Plasma electrolytic oxidation (PEO)
2.1. PEO process fundamentals
PEO has emerged as a popular coating method in recent years due to its reliability,cost-effectiveness,and ecofriendliness,and is widely employed to enhance the properties of Mg-based materials [58].The use of PEO as a coating method is driven by the fact that it eliminates the need for expensive equipment that is typically required for vacuumbased plasma processes.Moreover,the PEO treatment offers a method for creating thick,uniform,and durable coatings on metallic objects with intricate shapes,such as medical devices and implants,without requiring the entire substrate to be subjected to high temperatures or the use of complex equipment.The coatings produced by PEO treatment are also fully adherent to the substrate [59].
According to Hussein et al.[60],the growth mechanism of an oxide coating during a typical PEO process occurs in multiple stages (as illustrated in Fig.1) [61].In the initial stage of the process,a passive oxide film rapidly forms around the substrate within the first few seconds (Fig.1a).As the voltage increases,the growth of an insulation layer with a columnar structure perpendicular to the metal substrate starts(Fig.1b).The growth of the oxide layer takes place at both the interface of metal/oxide and oxide/electrolyte,with O2-and cations counter-migrating across the layer.This dielectric breakdown,as shown in Fig.1c,takes place on relatively thinner parts of the oxide layer and leads to the formation of numerous white and fine sparks on the surface of the anode.When the strength of the breakdown field is surpassed,an electron avalanche occurs across the oxide film,resulting in the formation of a discharge channel.The ions present in the discharge channel under these conditions lead to the formation of plasma,which is responsible for plasma-chemical reactions (as depicted in Fig.1d).Anionic species from the electrolyte and substrate alloying elements are attracted to the discharge channel for oxidation by the intense electric field between the anode and cathode.The liquid products resulting from plasma-chemical reactions condense into solid form upon cooling,which solidifies quickly due to the nearby electrolyte’s rapid cooling,leading to the formation of a relatively dense plug mainly composed of crystalline oxides.Finally,the release of the gasses produced during the process out of the discharge channels leads to the formation of a volcanolike structure (as shown in Fig.1e and f).The PEO process can create a rough,thick,and porous crystalline coating that has biofunctional properties,making it suitable for promoting implant-bone anchorage and stimulating bone formation and mineralization [61].
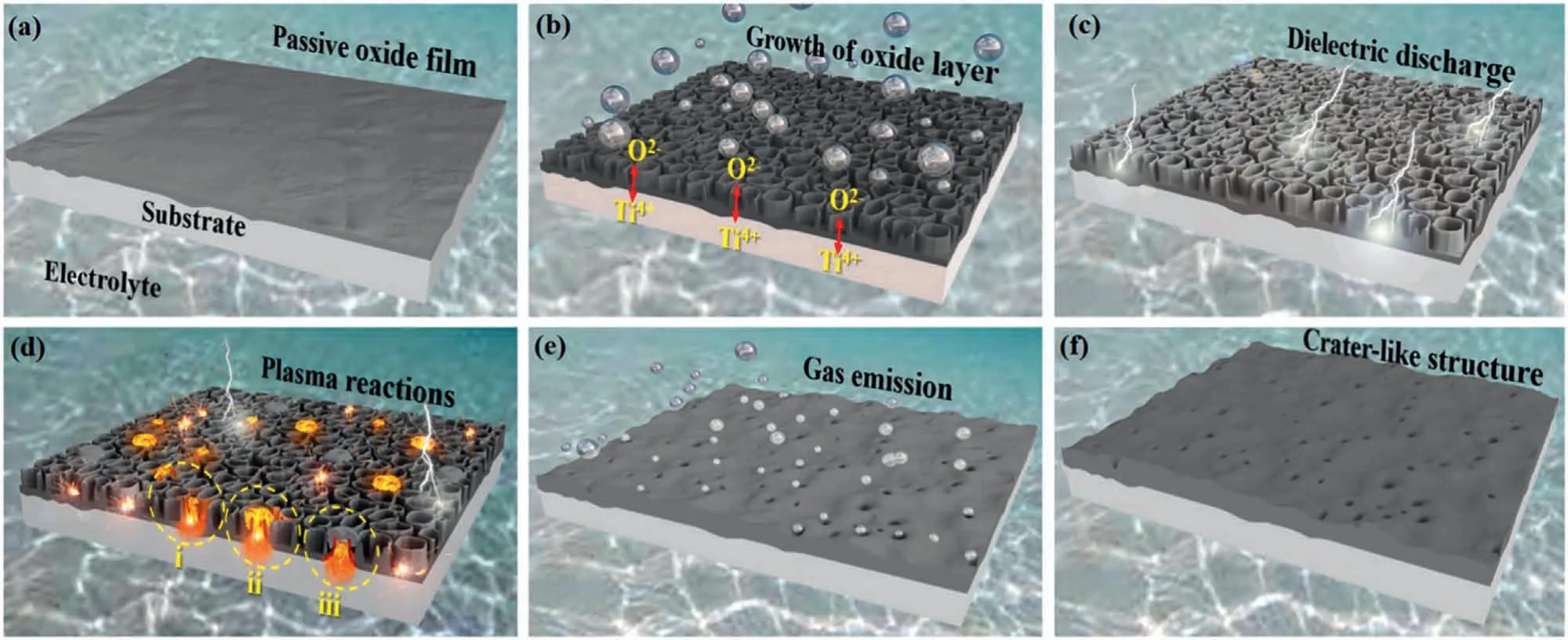
Fig.1.A schematic representation of PEO process coating [61].(With permission from Ref.[61];License Number: 5,572,860,148,707,License date: Jun 19,2023).
Several factors can influence the quality,properties,and morphology of the oxide films created through the PEO process,including the process temperature,chemical composition of the electrolyte used,oxidation time,electrical parameters,and the type of substrate employed [62–64].The systematic modification of the surface topography resulting from the coating parameters enables the acquisition of distinct coating characteristics.
One of the limitations of the PEO process is the presence of pores and deep cavities on the surface,which affect the corrosion performance of coatings [65–67].In fact,it can be said that adding particles is one of the most effective ways to reduce the porosity of the coating and improve its final qualities [33,68–70].Changing the electrolyte conditions in response to particle presence and the uniform dispersion of particles in the electrolyte is a very important issue in the coating process.
2.2. Mechanism incorporation of HAp NPs
When particles are introduced into the electrolyte and an electric current passes through it,the surface of the particles acquires an electric charge [71,72].This generates specific effects on the surface of the particles due to the application of an external electric field.The motion of a charged particle in a fluid under the action of an external electric field is called the electrophoretic effect [73].It is one of the most promising factors that brings the particles near the anode.The behavior of charging at the solid-liquid interface is largely influenced by the zeta potential (ζ),which determines the electrophoretic force.During successive discharges,nanoparticles are adsorbed and integrated into the PEO coating via different transport pathways,including microcracks,micropores,and short-circuit channels.The incorporation of nanoparticles into the PEO coating occurs due to a combination of two factors: the mechanical mixing caused by the fluctuating molten oxide and electrophoretic force [74–76].
In this regard,Seyfi et al.[77],obtained the amount ofζfor HAp NPs at various values of pHs.In the PEO alkaline electrolyte (pH>7),the zeta potential of HAp NPs is negative (as shown in Fig.2a),resulting in the negatively charged surface of the nanoparticles as soon as they come into contact with the electrolyte.The strong electric field present in the system drives the nanoparticles towards the positive pole(i.e.,the substrate) (as depicted in Fig.2b).The speed of mechanical mixing plays a critical role in determining how particles are distributed within the interface between the substrate and coating.According to Asgari et al.[78],low rates of mechanical mixing do not provide sufficient stirring power to facilitate the incorporation of particles into the coating.On the other hand,high rates of electrolyte stirring can lead to particle movement away from the substrate surface due to excessive turbulence.

Fig.2.(a) The zeta potential of HAp NPs at different pH levels and (b) a schematic illustration of the mechanism for the penetration of HAp NPs into the coating [77].
3.Characterization of PEO coating
PEO coatings have porous microstructures that are beneficial for tissue growth.PEO coatings have two types of pores: the first type are the pores that are on the surface and are open,and the second type are the pores that are closed[58,79,80].There are open pores in the outermost layer of the PEO coating and they come in direct contact with the corrosive solution.These pores are located throughout the coating and provide suitable pathways for the electrolyte to penetrate into the coating,allowing the substrate to be in direct contact with the electrolyte.It is very important to control the amount of porosity at the coating surface [81,82].
In general,various results have been obtained in studies conducted by researchers to investigate the role of nanoparticles in electrolytes in relation to surface morphology.This diversity is related to the distribution of particles in the coating and can generally be classified in four main groups.The first group,NPs due to melting in discharge channels,lead to the closure of the pores on the surface of the coating[83],the second group,NPs are placed inside the micropores (Entrapment) [84].In the third group,NPs are seen both in the holes and on the coating surface [85],and in the fourth group,NPs are seen on the surface of the coating and there is no significant change in the porosity of the surface [86].
Yang et al.[19]investigated various concentrations of HAp NPs on the morphology of the coating.The surface morphology of both PEO and PEO–HAp coating specimens is shown by SEM images in Fig.3a.They observed that the surface of all coatings had small cavities and cracks on the surface.The results also revealed that the size and number of pores on the surface decrease with an increasing amount of HAp NPs and the surface becomes denser [87](first group).

Fig.3.The process of incorporation HAp NPs in the coating: (a) the closure of coating pores due to melting of HAp NPs [19],(b) the entrapment of HAp NPs into pores [90]and (c) the presence of HAp NPs in pores and surface of coating [96].(With permission from Ref.[19]&[90]&[96];License Number:5,572,830,736,694,License date: Jun 19,2023).
In their study,Ma et al.[88]examined how the addition of HAp particles to the electrolyte in the PEO coating process affected the morphology of the coating on an Mg alloy.The surface of PEO coating has a lot of porosity while the surface of PEO–HAp has less porosity with white spots on the surface.The addition of HAp in the coating process causes partial pores to form on the surface and some pores to be filled by NPs.NPs enter the discharge channels under a strong electric field,and this may cause a lot of blockage of the discharge channels,thus reducing the percentage of porosity on the surface in the presence of NPs.
Similar results were also reported by Chen et al.[89]and Lin et al.[41]upon adding HAp NPs into the supporting electrolyte.The SEM micrographs of the surface morphology of the coatings,as shown in Fig.3b,reveal the entrapment of nanoparticles within the grown film in contrast to the pure PEO sample [90].
NPs can be efficiently entrapped in the growing ceramic layer by erupting and perturbing molten oxide in every spark that occurs at the sample’s surface during the PEO process(second group).NPs are first absorbed from the middle of the discharge channels,and their parts are then flung out of the inner oxide films by molten oxide eruption [91–93].Simultaneously,the fluctuation of molten oxide attracts the other parts of the nanopowders in the solution.The NPs are thought to be trapped beneath a coating of solidified oxide.As seen in Fig.3b,NPs are replenished more frequently in the affinity and pores than in other locations.Near and within the pores adjacent to single NPs,there are instances of agglomerated NPs.The fluctuation of molten oxide present at the surface of the Mg alloy is a possible explanation for this phenomenon.The rapid solidification of molten oxide within each spark can cause the repositioning of the absorbed NPs.Comparing and analyzing the surface structures of the nanocomposite and pure PEO layers revealed that the incorporation of NPs during the growth process leads to a decrease in surface porosity.It is due to the intensity of the anode surface discharge [94,95].Another study conducted by Liu et al.[96]demonstrated that the addition of HAp NPs into the electrolyte led to a surface morphology that was completely distinct from the one observed in the absence of nanoparticles (as illustrated in Fig.3c).A large number of pimples effectively sealed most of the microcracks and micropores on the outer surface.HAp NPs were integrated into the molten oxide without the use of any additional additives.Despite this,HAp had a tendency to aggregate within the micropores present on the coating surface,leading to the formation of clusters that filled these pores and decreased the coating’s porosity.A greater accumulation of HAp NPs was observed inside the coating defects compared to other areas(belonging to the third group).So far,no research has been conducted on whether HAp NPs can only be located on the surface of the coating and have no effect on the percentage of porosity.
The addition of HAp NPs can also lead to changes in the thickness of the PEO coating.Electrical conditions and electrolyte composition determine the bonding state of HAp particles as well as the composition of the coating.The thickness of the coating in the presence of HAp particles depends on the participation rate of the particles,their electrical parameters.In terms of cross-sectional morphology,all coatings made can be generally divided into three different areas,the outer layer,the middle layer,and the inner compact layer.In general,defects are caused by short-term sparks and immediate freezing by the electrolyte [60,97].
Liu et al.[96]conducted a study to investigate the impact of HAp NPs on the surface microstructure of PEO coatings on AZ31B.The PEO/HAp coating showed a higher level of compactness than the PEO coating,and the addition of HAp NPs into the electrolyte resulted in a slight increase in the thickness of the PEO coating.Additionally,the authors demonstrated that the barrier layer of the PEO/HAp coating did not contain calcium,suggesting that the inward transport of HAp in the PEO coating did not readily reach the barrier layer.The distribution of calcium throughout the porous outer layer of the PEO/HAp coating suggested that HAp NPs were not only attached to the outermost surface but were also extensively distributed throughout the porous layer.Similar results were reported by Chaharmahali et al.[98].
The influence of particles on the microstructure and thickness of coatings indicates that the particles are inert or active during the coating process.Liu et al.[96]conducted a study on the impact of incorporating HAp NPs into the PEO coating.Upon the addition of HAp,distinct diffraction peaks corresponding to HAp were observed without the formation of any new phase.This observation indicated that the HAp NPs remained inert during the microarc discharge process and did not react with MgO (as depicted in Fig.4a).
In against,Some researchers have shown that HAp NPs actively incorporate into the PEO coating [88].The size of particles and their melting temperature are critical factors that strongly influence their incorporation into the PEO coating.Most HAp NPs can penetrate the coating through the discharge channels due to their small size and deposit in the pore band of the coating.Once the oxide coating is broken down by the spark discharge,it creates conditions of extreme high temperature and pressure.It is plausible to assume that the melting of HAp particles,along with their small size,could result in the formation of a novel composite phase,Na2CaMg7(PO4)6(Fig.4b) [19].
4.Properties of coating
4.1. Corrosion behavior of HAp-containing coating
Many researchers have looked into the protective properties of PEO coatings.It has been proven that using PEO coating increases corrosion resistance and thus improves corrosion protection performance [99].The corrosion performance of orthopedic implants are influenced by the surface morphology of coatings.The morphology of the coating and the characteristics of the PEO coating depend to a large extent on the electrolyte [100,101].In the structure of PEO ceramic coating,microcracks and micro-cavities are unavoidable.The pores generated by the electrical discharges allow corrosive ions to infiltrate,causing damage to the protective layer (Fig.5) [102].The corrosion performance of PEO coatings in corrosive environments is determined by a number of parameters,including the coating’s composition,defects,and thickness.Corrosive ions can penetrate the oxide film through irregular cracks and pores,reducing the protective characteristics of PEO coatings against corrosive agents.
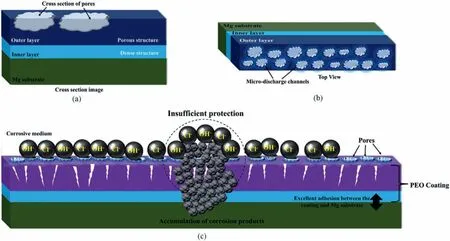
Fig.5.(a) A cross-sectional view and (b) a top view of the PEO coating;and (c) the penetration of corrosive species through pores and the coating degradation[102].(With permission from Ref.[102];License Number: 5,572,840,476,916,License date: Jun 19,2023).
In this regard,Seyfoori et al.[90],studied the corrosion properties of HAp-containing PEO-coatings.Electrochemical impedance spectroscopy (EIS) and potentiodynamic curves of AZ31 substrate with and without HAp particles in SBF are shown in Fig.6a and b.According to the potentiodynamic curves,the current density of AZ31 rapidly increased at the beginning of the anodic branch.The fast corrosion rate resulted in the observation of diffusion-controlled anodic current behavior towards the end of the curves.The SBF was found to cause severe damage to the Mg alloy substrate.Once an HAp-containing PEO-coating was applied on AZ31,the anodic current density gradually increased from the free corrosion potential.Mg alloy can be protected from corrosion using the HAp-containing coating.This means that the Mg alloys can degrade better in SBF when they have a HAp-containing PEO-coating [25,88].
According to EIS plots,the addition of HAp particle causes a noticeable shift.At high and low frequencies,the Mg alloy substrate showed two capacitance loops,respectively.The electric double layer’s characteristics were characterized by the high-frequency capacitance loop.The low-frequency loop indicated the adsorption of corrosion products on the surface of AZ31.Conversely,the HAp-containing coating plot exhibited only one capacitance loop,indicating that the coating was not degraded.Moreover,the capacitance loop diameter of the HAp-containing coating was larger than that of the Mg alloy substrate.Consequently,the HAp-containing coating can effectively decrease the rate of biodegradation of the Mg alloy in the SBF.
These findings shed light on the mechanism through which the addition of HAp NPs influences the corrosion behavior of the PEO coating.Fig.6c and d present schematic diagrams that illustrate the corrosion mechanism of both PEO and PEO/HAp coatings.The ability of PEO coatings to resist corrosion is dependent on various coating characteristics,such as porosity,thickness,and chemical stability.As discussed in Section 3,the PEO coating primarily consisted of MgO and contained numerous micropores and microcracks throughout its structure [103].Upon exposure to a corrosive solution,the structural defects present within the PEO coating created pathways that allowed the corrosive medium to quickly infiltrate the interior of the coating (as shown in Fig.6c).Additionally,in simulated body fluid,MgO exhibits low chemical stability and can be readily transformed into Mg(OH)2through the process of hydration.As a consequence of the hydration process of MgO,the internal stress within the coating increased,resulting in the formation of numerous widened microcracks due to the large molar volume ratio of Mg(OH)2to MgO[104,105].The combination of these factors significantly limited the ability of the PEO coating to protect against corrosion.Nevertheless,the incorporation of HAp NPs led to a significant reduction in the structural defects of the PEO coating by effectively sealing microcracks and micropores,which served to slow down the infiltration process of the corrosive solution (as shown in Fig.6d).Furthermore,through the inert incorporation of HAp NPs,the phase composition of the PEO coating was optimized,leading to an improvement in its chemical stability.As a result,the PEO/HAp coating exhibited superior corrosion resistance compared to the PEO coating.
As observed,the presence of HAp particles increases the corrosion resistance of the substrate.In addition,different concentrations of these NPs affect the corrosion properties of coatings.In this regard,the influence of varying concentrations of HAp (5,10,and 15 g/l) on the corrosion properties of coatings on Mg alloy was examined by Chaharmahali et al.[98].The Nyquist and polarization curves for coated samples at various HAp concentrations are shown in Fig.7.Due to the formation of a porous oxide film on the surface of Mg alloys when exposed to the environment,the Nyquist diagram (Fig.7a) displays an inductive behavior for the uncoated sample.When subjected to a corrosive solution,the oxide film is penetrated,enabling the corrosive solution to reach the substrate,resulting in an inductive behavior due to the low corrosion resistance.The Nyquist plot shows that coatings prepared using various HAp concentrations exhibit similar behavior.The presence of two capacitive loops and inductive behavior in the coatings indicates the existence of three processes.The loop created at high frequencies corresponds to the outer porous layer and at medium frequencies represents the dense inner layer,and also at low frequencies,an inductive behavior of the corrosion process is revealed.The presence of cavitation corrosion in the samples is indicated by induction behavior.The diameter of the Nyquist loop grew as the amount of HAp NPs was raised from 5 to 15 g/l,indicating an increase in the coatings’ corrosion performance.

Fig.7.(a) Nyquist and (b) polarization curves of samples coated at various concentrations of HAp [98].
After immersion in an SBF solution for 30 min,the polarization curves are illustrated in Fig.7b.The potentiodynamic polarization plots of all coatings on AZ31B are shifted towards a more negative potential and lower corrosion density relative to the substrate,following the application of ceramic coatings.The application of ceramic coatings enhances the thermodynamic tendency for corrosion to occur,while reducing the corrosion kinetics.The study’s findings revealed that the porosity level decreases from 80.50% to 39.64% as the HAp concentration increases from 5 to 15 g/l.Coating porosity is a critical flaw because corrosive solutions can penetrate the coating through these pores,ultimately destroying the coating and reaching the substrate.As a result,porosity plays a vital role in the coating’s different qualities,particularly its corrosion resistance.The results show that the corrosion resistance increases with increasing concentration of NPs.The goal is to make a coating that is denser and has a reduced porosity percentage.
Adjusting the appropriate parameters may significantly reduce the pores of the coating and increase the thickness of the inner layer.The influence of voltage on PEO/HAp coatings was examined by Tang et al.[106].Different voltages of 250,300,350,400,450,and 500 V were selected to perform the coating process.They found that as the voltage increased,the thickness and roughness of the coating increased.Also,with increasing voltage,the number and size of surface porosities decreased and increased,respectively.Corrosion behavior of coated samples using potentiodynamic polarization test revealed that the corrosion performance of coatings increased with increasing voltage up to 400 V and then decreased by further increasing the voltage to 450 and 500 V due to large porosity on the surface.The impact of adding HAp NPs on the corrosion properties of PEO coatings can be found in Table 1 [41,88–90,96,98,107].

Table 1Influence of particle HAp addition on corrosion properties.
4.2. Bioactivity behavior of HAp-containing coating
In the case of PEO coatings on biodegradable Mg alloys,long-term protection is of utmost importance.However,attention has gradually shifted to bioactive coatings,which can promote the healing process with minimal side effects while providing adequate corrosion protection.Based on this need,significant research efforts have been made to produce PEO coatings containing biologically compatible compounds[41,108].The long-term protective capability of the coating in a physiological environment is affected by three crucial factors: the initial structure of the coating (including its compactness and thickness),the ability of the coating to facilitate apatite formation,and the chemical stability of the coating material [109–111].
According to Lin et al.[41],the HAp-containing coating has been demonstrated to be denser.Surface structure modification leads to better apatite-forming strength and long-term corrosion properties of PEO/HAp coatings as compared to HAp-free coatings.HAp particles are used not only to modify corrosion behavior but also to enable the ability to produce apatite for PEO coatings applied to Mg alloys.
After immersing the specimens in r-SBF solution,their apatite-forming ability was assessed.As shown in Fig.8a,the nanocomposite coating displayed a larger amount of apatite formation on its surface after 3 days of immersion compared to the pure PEO film.Two distinct factors may account for this phenomenon.The bioactive properties of HAp within the oxide film structure can facilitate the nucleation of bone-like apatite via the hydroxyl and charged groups on its surface[112].The amount of apatite deposition can also be influenced by the surface roughness of the nanocomposite coating,which represents the second factor.The addition of HAp NPs to the oxide film can promote the formation of bone-like apatite and establish a strong chemical bond between the implant and host tissue through a Ca-P rich layer.As shown in Fig.8b,the pH value of the SBF solution varied significantly less over time for the nanocomposite coating than for the pure PEO coating,indicating a lower degradation rate of the nanocomposite film compared to the pure oxide layer.
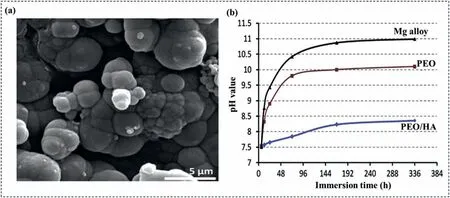
Fig.8.(a) The apatite forming ability of the nanocomposite coating after being immersed in SBF for 3 days and (b) the changes in pH value of the SBF solution with respect to immersion time [90].(With permission from Ref.[90];License Number: 5,572,850,100,778,License date: Jun 19,2023).
Yang et al.[19]used HAp particles to increase corrosion resistance and improve the biocompatibility of Mg-based implants.The degradation and corrosion process of coatings was determined using EIS in SBF solution for up to 72 h (Fig.9a and b).Initially,coatings exhibit a two-loop pattern in the Nyquist diagram after one-hour fixation in the SBF solution,where the loop formed at high frequencies corresponds to the outer porous layer and the one at low frequencies represents the dense inner layer.They discovered that adding HAp to coating boosted their corrosion performance substantially when compared to coatings without it.The presence of NPs in higher percentages (20 g/l) led to the filling of cavities,reduced porosity,and thus reduced defects.However,this effect intensified during immersion.The coating formed without HAp particles showed little corrosion resistance over long periods of time,but when the HAp particles entered the coating,the coatings showed greater corrosion resistance after 72 h of immersion [113].

Fig.9.(a) Nyquist curves for PEO coatings following various immersion periods and (b) surface morphology of the coatings after 7 days of immersion in an SBF solution [19].(With permission from Ref.[19];License Number: 5,572,830,736,694,License date: Jun 19,2023).
The SEM images of the coated samples following a 7-day immersion in SBF solution are presented in Fig.9c and d.The images reveal that particles of apatite are formed on the surface of the HAp nanoparticle-coated samples when they are immersed in SBF solution.Moreover,it should be noted that the precipitates have a tendency to deposit preferentially along or inside the defects (such as pores and cracks) where nucleation is facilitated compared to smooth regions.The presence of calcium phosphate in both the SBF solution and on the metal surface allows for simultaneous nucleation,which is why the precipitates discovered on the sample surface are believed to be Ca-P compounds.These precipitate products progressively seal the defects present in the coatings,which could partly explain the subsequent rise in the coating impedance.
When HAp-containing coatings are immersed in a simulated fluid,the surface promotes the formation of secondary apatite.Phosphorus atoms on the surface of HAp-containing coatings increase the ionic activity between the surrounding fluid and the surface,forming phosphate ions on the surface.Upon immersion in body temperature,there are positively charged calcium ions in SBF that undergo electrostatic interactions with the surface.The concentration of calcium ions on the PEO surface increases,resulting in a positively charged surface.Negatively charged phosphate ions migrate towards the positively charged PEO surface to balance the charge.The phosphate and calcium ions then react with each other upon immersion,leading to the deposition of secondary apatite at the PEO surface.As more ions enter,apatite structures grow spontaneously and coat the entire surface [114–117].
5.Challenges and future perspectives
PEO coatings containing HAp can increase the corrosion resistance of Mg alloys,but enhancing the corrosion performance of these coatings poses some challenges.These are some of the main challenges:
•The composite coatings with HAp on the Mg alloy substrate need to have good adhesion and integrity for long-term corrosion protection.Otherwise,the coating may develop cracks or delamination that can cause localized corrosion and speed up degradation.
•Corrosive species can penetrate the coating and reach the Mg alloy substrate through the porosity of the PEO coatings.This challenge can be reduced by controlling the porosity and optimizing the coating process parameters.Denser and thicker PEO coatings have been formed at the optimized working parameters,but these coatings still need to be enhanced.
•One challenge is the potential degradation of HAp in corrosive environments for long term,where chloride ions are present.This dissolution can weaken the coating’s integrity and cause corrosion of the Mg alloy substrate.
These challenges need to be addressed in the future and will surely be the focus of biomedical development in the next years.For future research work,it is essential to create anin-vitroenvironment that resembles the human physiological conditions for evaluating the degradation of these advanced coatings.This can better simulate their performance in the physiological environment.Manyin-vitrodegradation experiments on Mg-based materials have been done in the last decade,but most of them used simulate body fluide and room temperature.This is the reality.Providing essential guidelines for the design and development of new high-performance,multi-functional HAp coatings for Mg-based materials is very important.
6.Conclusions
Mg and its alloys possess good biocompatibility,biodegradability,and desirable mechanical properties,making them suitable for biological implants.The widespread use of Mg alloys as biodegradable implant materials is limited due to their rapid degradation rate.To enhance their use as implants,it is crucial to control the amount of corrosion and its destruction in the body environment.HAp-containing PEO-coatings have attracted much attention for biomedical applications over the past decade.PEO is a widely used method for fabricating HAp-containing PEO-coating on Mg-based material biofilms.The application of an HAp-containing coating can help minimize the rate of deterioration of Mg alloys,increase their biological activity,and facilitate bone formation.By applying an HAp-containing coating to the surface of degradable Mg alloys,some of the challenges associated with their use can be reduced.Recent advancements suggest that biodegradable Mg alloys have significant potential as a class of biological materials.In conclusion,it can be observed that HAp-containing coating reduces the rate of corrosion of Mg alloys,allowing for controlled degradation.As a result,HAp-containing PEOcoatings on Mg-based implants by PEO have shown great potential for biomedical applications.The development of HApcoated Mg-based implants with optimized surface properties and improved biocompatibility can open new avenues for the development of next-generation biodegradable implant materials.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
- Achieving high ductility and strength in magnesium alloy through cryogenic-hot forming
