Protective and water-repellent properties of alkylcarboxylic and alkylphosphonic acid films on technically pure magnesium
Viktoriia A.Luchkina,Michael S.Min’kin,Andrei Yu Luchkin,Yurii I.Kuznetsov
Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Leninskii Pr. 31, Moscow 119071, Russia
Abstract The formation of superhydrophobic coatings using low–toxicity corrosion inhibitors is a promising method for corrosion protection of metals and alloys.In this study,the effects of surface roughness and the of the adsorbed substance structure on wettability and corrosion resistance of commercially pure magnesium were investigated.Surface roughness was created by three different methods: paper grinding,etching in nitric acid solution and laser treatment.Oleic,stearic and octadecylphosphonic acids were investigated as potential surface modifiers for the formation of corrosion resistant superhydrophobic coatings.It has been shown that the protective and hydrophobic properties of acid films on magnesium,as well as their stability,are determined by both the initial surface morphology and the nature of the inhibitors.Experimentally,the laser treatment was found to be preferable to mechanical and chemical surface preparation and the best hydrophobic agent among the studied acids is phosphonic acid.The most stable films with excellent superhydrophobic and protective properties in atmospheres of high humidity and salt spray clamber are formed in a solution of 0.001 M octadecylphosphonic acid on the surface of magnesium with high roughness.In addition,the effect of vinyltrimethoxysilane on the protective and hydrophobic properties of stearic acid and octadecylphosphonic acid films was investigated.The results of direct corrosion tests and wetting contact angle degradation kinetics studies showed that the protective and hydrophobic properties of stearic acid can be enhanced by its layer-by-layer adsorption with silane.They practically reach the parameters of octadecylphosphonic acid.
Keywords: Magnesium;Corrosion inhibitors;Superhydrophobic coatings;Alkylcarboxylic acids;Alkylphosphonic acid.
1.Introduction
The range of applications for magnesium and its alloys as a structural material is being actively expanded.This is due to the increasing requirements for products in the aviation,aerospace,electronic and other industries,which,in turn,lead to the need to search for modern technological solutions and the use of environmentally friendly materials with high strength properties.From this point of view,magnesium alloys are worthy competitors to aluminum alloys [1,2].
Magnesium and its alloys hardly corrode in dry atmospheres.Atmospheric corrosion depends on the presence of water on the metal surface in the form of a thin continuous layer or individual drops.A distinctive feature of atmospheric corrosion from the dissolution of magnesium in solution is the significant contribution of oxygen reduction [2,3].
The protection of metals in humid atmospheres and various aqueous solutions can be achieved by the formation of thin barrier coatings on their surfaces due to the adsorption and more complex chemical interaction of organic corrosion inhibitors (CIs) with the protected metal [4].Among the CIs,alkylcarboxylic and alkylphosphonic acids and their salts should be pointed out.They have a number of advantages over other organic compounds: low toxicity,the ability to stabilize the passive state of metals and alloys in nearneutral environments.
A comparison of alkylphosphonic acids with alkylcarboxylic acids with the same number of methylene groups in the alkyl showed that phosphonates are able to form more stable self-assembled self-asembled monolayers (SAM) on the Mg alloy than carboxylates [5,6].This is associated with the fact that phosphonic acids,when interacting with the magnesium oxide-hydroxide layer,are both mono-and bidentate bound to the surface [7].In agreement with the voltametric measurements described in [8]the best inhibiting properties were exhibited by octadecylphosphonic acid (ODPA) adsorbtion layers,which reduced the corrosion current density by 30% [8,9].Even when applied from the vapor phase with the same alkyl length,ODPA protected Mg better than stearic(SA) or isostearic acids.The presence of fluorine atoms in the molecule contributed to the denser packing of SAM 2–(perfluorohexyl)ethylphosphonic acid on the AZ31 alloy.According to the electrochemical impedance spectroscopy results,the inhibitory efficacy of the F–containing phosphonate was the highest among the CIs investigated in [8].For carboxylic and phosphonic acids there is a tendency for the protective properties of the adsorption layers to improve with increasing hydrocarbon radical length [5–7].
According to the literature data,heat treatment of adsorption alkylphosphonate layers (dodecylphosphonic and octadecylphosphonic acids) enhances their barrier properties [8–10]by strengthening the chemical bonding of the compounds with the Mg alloy.The chemical composition of the substrate surface also has a strong influence on the barrier properties.For example,according to [11],plasma modification of Zn-Mg-Al magnesium alloy promotes the formation of ordered ODPA monolayers.The most significant inhibition was observed for acid adsorption on the alloy after a two-step plasma treatment first with Ar/H2followed by O2plasma modification.This is due to the removal of carbon impurities and the change in composition and thickness of the oxide layer compared to the original surface.
A special case of the application of organic CIs is their use for the formation of superhydrophobic (SHP) coatings on the surface of metals and alloys Research in this field,including for improving the corrosion resistance of magnesium alloys,has been gaining popularity in recent years [12].It is known that achieving the SHP state is possible if two conditions are met — the presence of a polymodal surface morphology (with micro-and nanoscale hierarchical structures)and the treatment of such a surface with a low surface energy compound.The fulfillment of these two conditions can be achieved simultaneously [13,14]or in stages [15,16].
Among the types of surface pretreatments described in the literature,hydrothermal treatment is the most common way to create surface micro/nanostructures on magnesium alloys.According to the review [17],it is this technology that is most commonly found in publications for the period 2015–2020.Then,in descending order of the number of papers describing such technology,are immersion and soaking in solutions,micro-arc oxidation,anodic oxidation treatment,chemical etching,etc.
Another way of creating surface roughness is laser processing.It has a number of advantages,including environmental friendliness,ease of scaling the process and,most importantly,the reproducibility of the surface topography.By varying the laser parameters,different surface morphology with different properties can be obtained [18,19].With the laser processing,it is possible to achieve both texturing of the Mg surface and its remelting [18].Laser surface melting makes it possible to increase the corrosion resistance of a number of Mg alloys[20].The improvement in corrosion resistance is usually associated with the formation of small dendritic grains in the modified area,evaporation and redistribution of some alloying elements.However,there are exceptions: for example,the corrosion resistance of AZ91D and AM60B alloys decreased after laser treatment [21],which the authors attributed to an increased content of the Mg17Al12intermetallic phase.Laser pre-treatment can be used as a surface preparation prior to coating [22–24](to improve adhesion) and to improve the protective properties of an already formed PEO coating [25].
In most cases,the surface of the metal is super-hydrophilic after exposure to a laser.Hydrophobic or SHP properties can be imparted by adsorption of organic contaminants during storage or furnace annealing [26,27],and chemical modification in solutions or vapours of low surface energy substances[28–31].
Currently,there are only a few papers in the world literature that use such surface preparation of Mg alloys to form SHP coatings.For example,the authors of [28,29]developed methods to form an SHP coating on the magnesium alloy Ma8 by laser texturing followed by the vapor phase deposition of fluoroalkoxysilane.Such compounds are usually expensive and their use is unsafe from an environmental point of view.Recently,Chinese researchers have used a more environmentally friendly octyltrimethoxysilane for this purpose[30].To create a SHP coating with high protective properties,they formed a layered double hydroxide (LDH) coating on laser textured AZ31B alloy.The silane was applied to the prepared surface from an aqueous ethanol solution,having previously allowed it to hydrolyse for 24 h.The authors note that in addition to high protective properties,the formed coating system showed self–healing ability.
Li et al.[31]used the most common and safe hydrophobic agent (HPA),SA,in their study.As in most papers,an additional surface treatment of AZ31 was carried out between laser processing and HPA deposition;in this case,the alloy was etched in an AgNO3solution.
The above–mentioned papers have investigated either the effect of the adsorbate structure alone on the protective properties of the formed coatings,or the effect of different surface morphology on the corrosion resistance of magnesium alloys.The combined effect of these two factors on the magnesium surface has not been considered previously.In our studies,we used low-toxic compounds with the same alkyl length but different structures: stearic acid,oleic acid (OlA) and octadecylphosphonic acid [32].
The present work is a continuation of our previuos research.The aim of this study is to investigate and compare the stability of the hydrophobic and protective properties of the coatings of the above–mentioned compounds on commercially pure magnesium (Mg90) with different types of morphology.In addition,in order to improve the protection of Mg90 by organic acids,we have also studied the possibilities of their layer-by-layer adsorption with vinyltrimethoxysilane.
2.Experimental section
2.1. Materials
The studies were carried out on samples of commercially pure magnesium grade Mg90 containing Mg—99.9%,Fe—up to 0.04%,Mn—up to 0.03%,Al—up to 0.02%,Ni—up to 0.001 %,Cu — up to 0.004 %,Si — up to 0.009%,Сl— up to 0.005 %.Protective coatings were formed in ethanolic solutions of stearic acid (SA,Sigma Aldrich,98.5 %),oleic acid (OlA),octadecylphosphonic acid (ODPA,Sigma Aldrich,97 %) and/or vinyltrimethoxysilane H2C=CH–Si(–OCH3)3(VTMS).
2.2. Formation of anticorrosion hydrophobic and superhydrophobic coatings
The protective and hydrophobic properties of coatings formed on five different types of magnesium surface were studied:
– "smooth" (type 1);
– textured by chemical etching (type 2);
– laser textured (type 3–5).
In the first case,rectangular coupons with dimensions of 20×35×5 mm3were polished with silicon carbide (SiC) papers (KLINGSPOR,Poland) from 240 to 1000 grit successively and then degreased with acetone.Before the formation of surface roughness accordingto type 2–5,Mg90 plates were subjected to alkaline phosphate degreasing.The samples were treated for 10 min in a solution containing 45 g/L NaOH and 10 g/L Na3PO4at a temperature of 60 °C [33],followed by rinsing with distilled water.The degreased and rinsed Mg90 plates were dried in a drying oven at 65 °C.
Atype 2surface was obtained by etching Mg90 plates in a 2% aqueous solution of nitric acid (HNO3) for 10 min.
Laser texturing of Mg90 was carried out using an ytterbium short-pulse fiber laser XM-30 (Kazan,Russia).Laser scanning was performed on a grid in two passes.The parameters of treatment for each surface type (type 3–5) are shown in Table 1.After laser treatment,the samples were cleaned by ultrasonication in isopropanol for 5 min to remove particles formed on the surface and weakly bound to the metal surface.The plates were then exposed on air for 30 min.As a result of this preparation,the Mg90 surface acquired SHP properties.

Table 1Laser texturing parameters.
WhereVis the scanning speed;Pis the laser power;λis the wavelength;υis the radiation frequency;dis the diameter of the laser beam;lis the distance between two adjacent linear trajectories.
Samples of Mg90 prepared by any of the above-methods described were treated in an ethanolic HPA solution.In the case of SA,OlA,and VTMS,the concentration was 0.01 M and in the case of ODPA,it was 0.001 M.The treatment time in CI solutions was 60 min.The coupons were then dried in an oven at 65 °C for 30 min.
2.3. Sample surface characterization
The surface roughness of the Mg90 samples was measured by the stylus method using a Model-130 profilometer (Proton,Russia).As a result,the values of the roughness parameters were obtained:Rzis the height of the profile irregularities at ten points (the sum of the average absolute height values of the five largest profile projections and the depths of the five deepest depressions of the profile within the base sampling length),μm;Rais the mean arithmetic deviation of the profile,μm;Sis the average step of the local projections of the profile within the base length,μm.Based on the obtained parameters,the roughness class was determined according to GOST 2789 [34]and ISO 1302–2002 [35].
2.4. Study of protective and hydrophobic properties of coatings
2.4.1.Voltammetric measurements
The protective after–effect of hydrophobic and SHP coatings on Mg90 were investigated by the polarization potentiodynamic method using an IPC–Pro-MF potentiostat/galvanostat(Russia)at a potential scan rate of 0.0002 V/s.Polarization curves were recorded using a three-electrode clamp cell.Measurements were carried out in a borate solution with pH 9.2 (0.05 M Na2B2O7× 10 H2O) containing 0.001 and 0.01 M NaCl.The reference electrode was a two–key silver–chloride electrode,and the auxiliary electrode was a pyrographite plate.The electrode potentials,E,measured versus a saturated silver-chloride electrode were converted to the normal hydrogen scale.The working area of the electrodes was 1.02 cm2.All electrochemical tests were carried out under static conditions at room temperature of the solution (23±2 °C) and natural aeration.The effectiveness of the Mg90 protection was judged on the basis of a number of parameters that were determined from the polarization curves:free corrosion potential difference,the values of local depassivation potential values and/or protective effect Eqs.(1) and(2).
whereEcor0,EcorCIare the free corrosion potential of the untreated sample and the preliminary passivated sample in CI solutions,respectively.
whereicor0,icorCIare the corrosion current density of the Mg90 samples without treatment and with organic acid films,respectively.
2.4.2.Corrosion tests
The protective properties of films have been evaluated by the results of direct corrosion tests in a humid atmosphere with periodic moisture condensation and in a salt spray chamber (SSC) (Weiss SC/KWT 450,Germany).
In the first case,the prepared samples were exposed in glass cells filled with 40–50 mL of distilled water att=40–50 °C.The cells were placed in a drying oven,wheret=40±2 °C was maintained for 8 h.Then the heating was turned off,thus ensuring moisture condensation on the surface of the samples.The samples were examined hourly.During the tests,the time of the first corrosion damage appearance (τcor) was determined.
In the second case,a 5 % NaCl (pH 6.5–7.2) was used as an aggressive solution.The salt solution was sprayed inside the test sample chamber as a mist.The chamber has been operated continuously in a cyclic mode (each cycle consisted of 15 min of saline spray followed by a 45 min period in which the chamber was switched off).The tests were carried out att=35 °C and 95–100 % humidity.To determineτcor,the samples were examined hourly on the first day,and then 3 times a day.
2.4.3.Water contact angle measurements and its evolution over time
The presence of hydrophobic properties was assessed by measuring the static edge angle (θ) of a drop of distilled water.The stability of the hydrophobic properties was assessed on the basis of changes inθover time with constant contact of Mg90 samples with distilled water.The samples were kept in distilled water with stirring for 60 min and heating in a drying oven att=65 °C for 30 min.Theθvalues were calculated in a graphical editor from photographic images of a drop obtained using a laboratory setup with an integrated DCM300 camera (China).The drop volume for each measurement was 3–5 μL.
2.5. Scanning kelvin probe force microscopy (AFM/SKPFM)
The rectangular coupons with dimensions of 10×10×1 mm3were used for the AFM studies.The surface treatment of Mg90 consisted of several steps.First,the samples were preliminarily ground with silicon carbide(SiC) papers from 240 to 2000 grit.After grinding,the coupons were successively polished with diamond pastes with particle sizes ranging from 8 μm to 0.5 μm.The final polishing was carried out using a colloidal suspension of silicon oxide (O.P.S.,Germany) with a particle size of 0.05 μm.
The surface topography and surface potential (VCPD)were measured by two-pass power Kelvin probe microscopy(SKPM) in the amplitude modulation mode using an automated scanning atomic force microscope (SolverNexT II,NovaPhotonix LLC,Russian Federation) in an open atmosphere.We used a silicon probe with a conductive Pt coating(NS15/Pt) (resonant frequency 62.328 kHz,stiffness constant 4.5 N/m).The second pass height was 10 nm.The sample was grounded.Prior to the measurements,the probe was calibrated on a fresh surface of highly oriented pyrolytic graphite(HOPG).The work function of such a probe was 5.9 eV,which is in agreement with the literature data [36].
The obtained images were processed using the Gwyddion 2.61 software[37].The electron work function was calculated using the equation:
where:VCPDis the measured surface potential;WTIPis the electron work function of the probe material;WSAMPLEis the electron work function of the sample material;e is the elementary electric charge.
3.Results and discussion
3.1. Investigation of the protective properties of acids on a“smooth” surface
3.1.1.AFM/SKPFM studies
Maps of the surface topography and the surface potential distribution of Mg90 before and after treatment in acid solutions are shown in Fig.1.The initial surface of the sample is relatively homogeneous with some traces of polishing in the form of stripes and intermetallic inclusions,which do not significantly affect the surface potential distribution.

Fig.1.Topography and surface potential maps of Mg90 samples without (bare) and after treatment in ethanolic solutions of SA,OlA,and ODPA at various concentrations.
Treatment of Mg90 in 0.001 M ethanol solutions of the studied acids reduces its surface potential value.The ODPA adsorption did not affect the surface topography,indicating the formation of uniform thin acid films on the surface.A different picture was observed after exposure Mg90 in 0.001 M solutions of OlA and SA.Rounded particles of various sizes(up to 300 nm) were found on the surface,the value of the surface potential of which is slightly lower than for the rest of the surface.
Increasing the concentration of acid solutions to 0.01 M leads to an even greater decrease in the surface potential and an increase in the size of the above formations.The surface potential values of these particles are close to those of the rest of the surface.They are probably the corresponding acids.
The electron work function of characterizes the thermodynamic possibility of an electrochemical reaction [38–41].In a first approximation,its increase can be interpreted as an enhancement in corrosion resistance.The average value of the electron work function of the background sample,calculated by Eq.(3),is 3.50±0.01 eV.As can be seen from Table 2,the Mg90 samples with acid films have significantly higherWSAMPLEcompared to the background value.The maximum value of the electron work function corresponds to Mg90 treated in 0.01 M ODPA,which may indirectly indicate the high protective properties of such a coating.

Table 2The electron work function values and their standard deviations for the Mg90 surface with adsorbed organic acids from solutions of various concentrations.
3.1.2.Potentiodynamic measurements(Potentiodynamic polarization)
The anodic and cathodic polarization curves of commercially pure magnesium before and after treatment in ethanol solutions of the studied acids are shown in the Fig.2.It can be seen that the treatment of Mg90 with a “smooth” surface in 0.001 M ethanol solutions of CI leads to a decrease in theEcorvalue compared to the background.However,the corrosion current value decreases (Table 3),indicating the presence of some protective effect in the formed films.The corrosion potential of Mg90 treated in a solution of 0.001 M ODPA is less shifted to the cathodic side,suggesting that alkyl phosphonic acid is more effective as a CI compared to carboxylic acids.Fig.2 shows that increasing the concentration has a positive effect on theEcorvalue of Mg with SA and especially OlA films.At the same time,the opposite effect was observed for ODPA: the deterioration of the protective properties of the phosphonate is evidenced by both a decrease inEcorand an increase in corrosion current (Table 3).
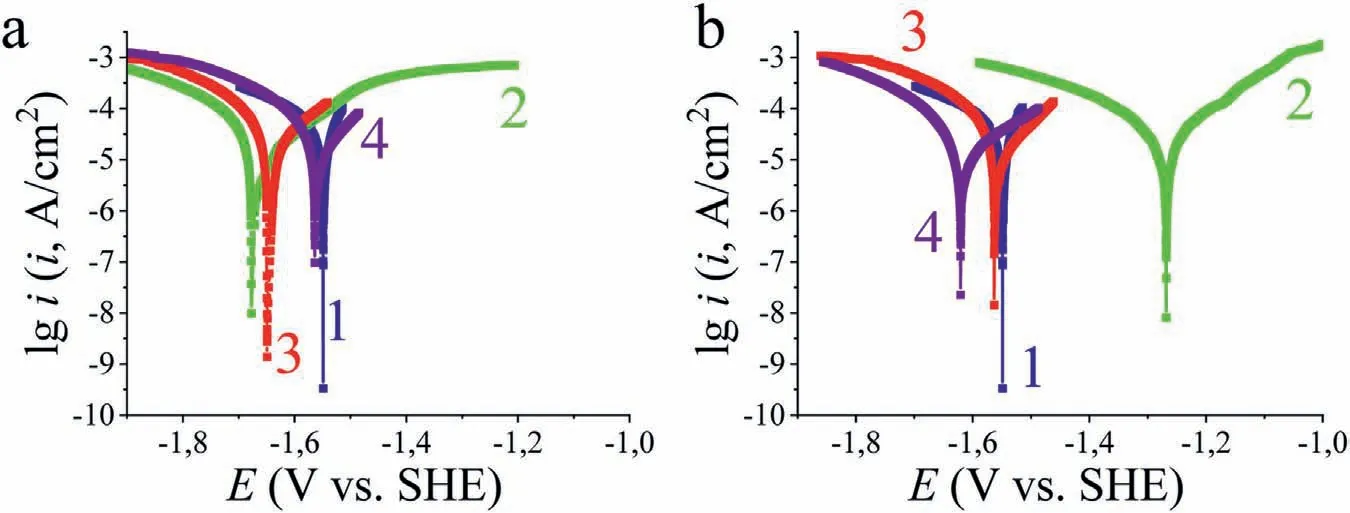
Fig.2.Potentiodynamic polarization curves of purified Mg90 in a borate solution at pH 9.2 containing 0.001 M NaCl,without (bare) and with preliminary modification in ethanol solutions of 0.001 M (a) or 0.01 M (b) acids.

Table 3Polarization parameters of “smooth” samples of Mg90 with adsorption layers of acids immersed in a borate solution containing 0.001 M NaCl.
Therefore,the using of ODPA solutions with a concentration of 0.001 to protect Mg90 is more advantageous not only from an economic point of view,but also from an experiment one.As for the carboxylates,the protective effectiveness of SA and OlA increased with increasing their concentration(C),as evidenced by the combination of Kelvin probe force microscopy and polarization measurements.Subsequently,to modify the textured surface of Mg90 and achieve the SHP state,we used solutions of modifiers with the following concentrations 0.01 M for SA and OlA and 0.001 M for ODPA.
3.2. Study of the water-repellent and protective properties of acids on a textured Mg90 surface
3.2.1.Effect of Mg90 surface pretreatment on water-repellency
3.2.1.1.Sample surface characteristics.As we have previously shown,without specific surface roughness-forming methods,the subsequent modification in solution of none of these acids enable to impart SHP properties to the surface[32].In order to obtain SHP coatings,we have selected the optimal modes for surface preparation of Mg90 and measured the roughness parameters.In the previous work,the profilograms of 5 surface types and their corresponding values ofRzand S were presented.In the present paper,we have supplemented these data by providing theRavalues and roughness class (Table 4).

Table 4Roughness parameters of Mg90 specimens after different methods of surface preparation.
The Mg90 surface after grinding and etching corresponds to one roughness class according to ISO [35],but theRzand S values of the etched Mg90 surface are 1.7 and 1.8 times higher.
TheRzvalue of various magnesium surfaces increases in the ordertype 1<type 2<type 3<type 4<type 5and reaches the highest value during laser processing with low scanning speed combined with high power.According to the data of profilometric measurements,the average roughness value of samples with the surface roughnesstypes 4and5are 19 and 25 μm,respectively.The cross-sections confirm the order of magnitude of these values (17 and 30 μm,respectively).
The interaction of a laser beam with the surface of a Mg90 sample is accompanied by local melting,oxidation,explosive ablation,intense evaporation of magnesium,followed by deposition and accumulation of surface stresses.In addition,particles formed in the plasma as a result of laser ablation of the surface layers are partially deposited on the treated surface.Probably,at high laser scanning speed (Type 3,Fig.3)smaller surface structures are formed,therefore,they are not visible in the image at this magnification,as well as surface roughness.A decrease inVfrom 400 to 100 mm/s makes it possible to maintain the molten state of the surface layers much longer in the presence of oxygen (Type 4,Fig.3).This leads to the formation of a thicker oxide layer on the Mg surface,which becomes visible in the cross-section image.When the laser beam passes again during melting,the protective oxide film is destroyed and moved by convection to the inner layers,while the oxidation of the freshly exposed top layer begins again.The metal goes through several stages of melting and solidification,which leads to excessive oxidation and the formation of a thick surface oxide layer covered with a layer of nanoparticles ejected from ablation craters.It can be seen in Fig.3 that the combination of high power and low speed intensifies the above-mentioned processes and contributes to the formation of such layer with a thickness of 80–130 μm.We hypothesize that the dark spots in the image are voids that have been formed as a result of a large number of passes.It should be noted that the Mg90 surface had a dark gray color after such preparation.
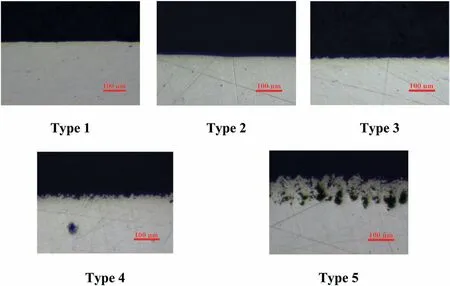
Fig.3.The cross–section image of Mg90 with different types of roughness.
3.2.1.2.Water contact angle measurements.Fig.4 shows images of water droplets obtained on the surface of Mg90 after various surface preparation.The stability of the waterrepellent properties of the coatings was assessed by immersing the samples in distilled water and measuringθ,according to the method described in Section 2.4.3 (Fig.5).
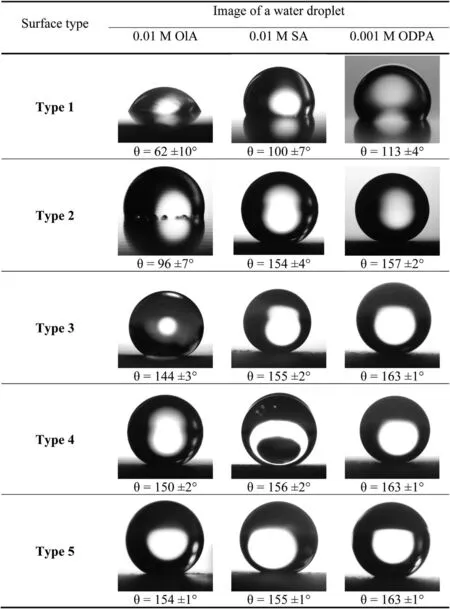
Fig.4.Images of water droplets on the surface of Mg90 with various surface treatment options.

Fig.5.Change in θ during exposure time in distilled water on Mg90 samples pre-modified in ethanol acid solutions after different surface preparation methods:a — type 2;b — type 3;c — type 4;d — type 5.
The SA film formed on the surface of etched Mg90 exhibited low stability of the SHP properties.This is confirmed by a sharp decrease inθ(Fig.5a).Phosphonic acid was found to be the best modifier for Mg90 with this surface type: its coating retains water-repellent properties for a longer time when in contact with water,and the measurement error ofθon the sample before exposure to water was lower.
The pre–laser texturing of the samples has a favourable effect on the initial value ofθ,and the long–term persistence of the SHP properties of surfaces modified with both SA and ODPA (Fig.5b).As shown earlier,increasing the roughness by reducing the scanning speed of the marker from 400 to 100 mm/s allows the SHP condition of the Mg90 surface to be achieved even when OlA is used as a modifier.Such a coating retains its properties for 4 h of exposure in distilled water (Fig.5c).
Changes in laser power have different effects on the SHP properties of the studied acids films.An increase in the surface roughness promotes the formation of slightly more resistant in water OlA coatings,while the stability of SA coatings has significantly decreased.The influence of this parameter on SHP properties of ODPA films is ambiguous.On the one hand,the immersion time in water until the complete loss of SHP state of Mg90 surfaces after treatment in phosphonic acid is the same for bothtype 4and5surface roughness of samples.On the other hand,in the second case (as shown in Fig.5d),there is a noticeable area ofθdecrease followed by stabilization,while in Fig.5c,θdecreased uniformly.The statistical error on textured samples slightly increased over time compared to etched coupons.This is likely because of the more uniform surface roughness and distribution of the modifier.
3.2.2.Influence of Mg90 surface pretreatment on the protective properties of acid films
3.2.2.1.Voltammetric measurements.Fig.6 shows the anodic polarization curves of Mg90 with different roughness types(surface morphology) before and after modification in ethanolic solutions of acids.These curves were obtained in the same borate solution with 0.001 M NaCl,according to the procedure described above (Section 2.4.1).
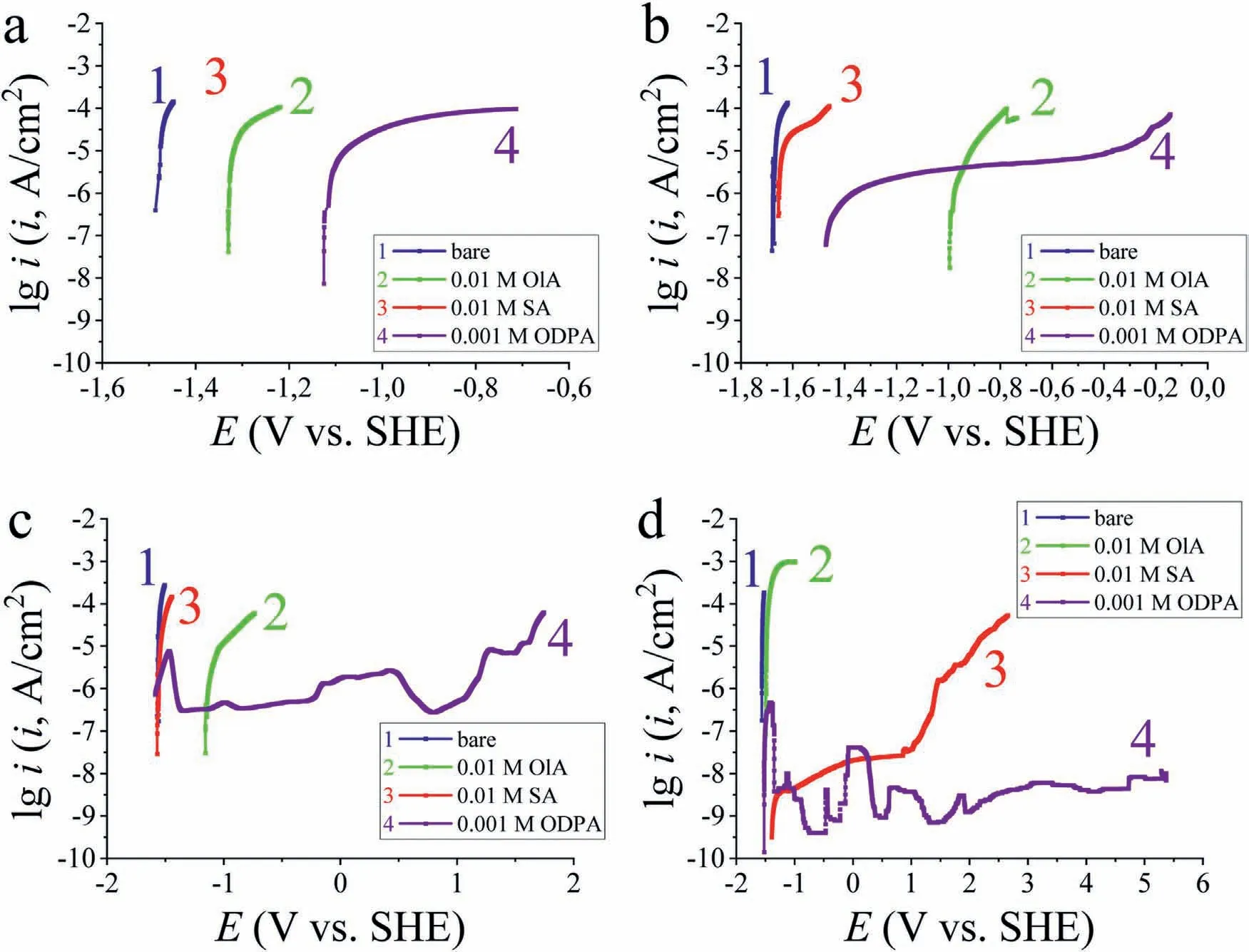
Fig.6.Anodic polarization curves of Mg90 in borate solution at pH 9.2 conteining 0.001 M NaCl,after different surface preparation methods: a — Type 2;b — Type 3;c — Type 4;d — Type 5,without (bare) and with subsequent modification in ethanolic acid solutions.
On the polarization curve (Fig.6a),there is a rapid increase in current density for both the ground and etched Mg90,which is attributed to active metal dissolution.This surface treatment increases theEсorby 0.063 V,compared to“smooth” Mg90,indicating an inhibition of the anodic process.This can be caused by the removal of impurities during the etching process,as well as the formation of a thicker and more uniform oxide-hydroxide layer during washing and subsequent drying.
It is known that increase in the surface roughness of a metal leads to an enhanced tendency for local damage.This is due to the increased wettability of hydrophilic surfaces[42,43],which in turn facilitates the access of aggressive ions to the surface.The more negative Mg90 potentials observed after laser texturing atV=400 mm/s andP=9 W are in agreement with this thesis (Fig.6b,Table 5).

Table 5Polarization parameters of textured Mg90 samples without (bare) and with acid adsorption layers immersed in borate solution containing 0.001 M NaCl.
The protective after-effect of the adsorption acid layers also varied depending on the pretreatment of the Mg surface.As described in Section 3.1.2 (Fig.2),in the absence of surface pretreatment,the adsorption of OlA from its ethanol solution(C=0.01 M),contributed to the greatest shift of the potential to the anodic side.The results of the direct corrosion tests (Fig.7) also support the higher protective effectiveness of hydrophilic OlA film on the “smooth” Mg90 surface compared to the other investigated acids.If on the “smooth” surface of Mg90,the OlA coating formed from itsC=0.01 M solution provided more effective protection than ODPA,the situation has slightly changed asRzincreased (Fig.6).As can be seen in Fig.6a and Table 5,the most positive value ofEcorfor etched Mg90 (type 2) was observed when it was further modified in 0.001 M ODPA solution.In fact,this treatment outperformed even the use of carboxylic acid.The high effectiveness of ODPA coating on this type of surface is also confirmed by corrosion tests in a humid atmosphere (Fig.7,[32]).Among the investigated carboxylic acids,the SHP coating of SA increases theEcorto a lesser extent than OlA (by 0.072 and 0.156 V,respectively,as shown in Fig.6a).This could be attributed to the lower stability of the SHP properties of the former and the higher inhibitory efficiency of the latter acid in the borate solution.Probably for the same reason,the free corrosion potential of Mg90 with hydrophobic OlA coating formed ontype 3surface is also more positive than in the case of SA (Fig.6b).It is possible that the more negative values ofEcorobserved for Mg90 with ODPA films (Table 5)compared to OlA could be attributed to a smaller phosphonate layer thickness due to a difference in concentration of the passivation solutions used.Since no similar phenomenon was observed on etched Mg90,another possible reason could be that the oxide-hydroxide layers formed during etching and laser treatment differ not only in thickness but also in composition,which in turn affects the acid adsorption.However,despite theEcor,there is an extended plateau on the anodic polarization curve of Mg90 with ODPA.This is attributed to the presence of a gas interlayer at the solid/liquid interface which is a consequence of the SHP surface state.Further,the graph shows the currents increase at potentials more positive than-0.6 V (Fig.6b),which,as on zinc coating[44],can be caused by breakdown of the most vulnerable areas of this layer.The absence of a similar plateau in the anodic polarization curve of Mg90 with SA film may be due to the low stability of the SHP properties of this acid film.
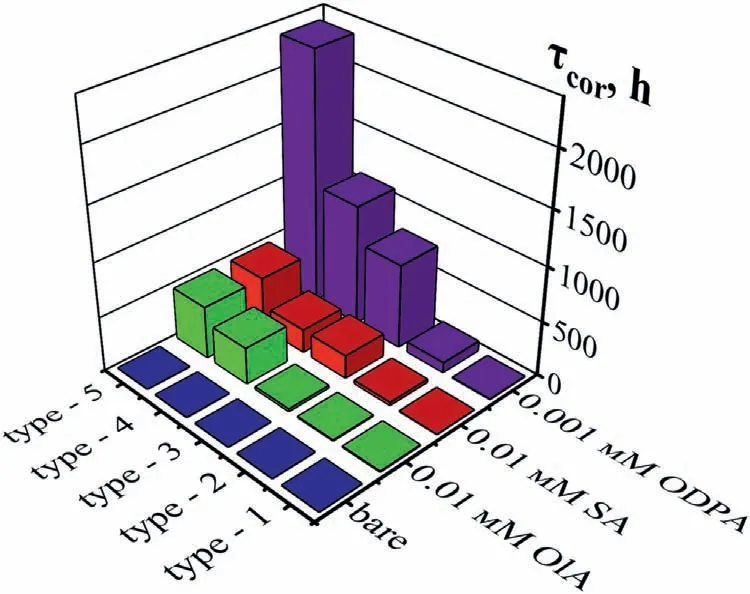
Fig.7.Corrosion test results under conditions of H=100 % and daily moisture condensation of Mg90 specimens without (bare),with hydrophobic and SHP coatings.
On the anodic polarization curve of Mg90 with ODPA films in Fig.6d,oscillations of the current density can be observed,with theivalues in these areas not exceeding 5 × 10-7A/cm2and in most cases being less than 5 × 10-8A/cm2.There was no significant increase in current density until the limit of measurement instrument range was reached.Nevertheless,it cannot be called passive because the abovedescribed fluctuations can be caused by both surface oxidation processes and the formation and repassivation of fine pitting.It would be more correct to call this region a quasipassive region.This is mainly due to the superhydrophobic state,which is described by the Cassie-Baxter model [43].According to this model,the actual contact area between the surface and the electrolyte is extremely small.Local destruction of the protective film can occur in the most vulnerable areas,such as the ridges of a highly textured metal surface or at the electrode edges.It should be noted that in this case the surface of the electrode still remains an SHP.This is evidenced by the presence of a mirror–like film on its surface,which is caused by presence of air.When the polarization was switched off,the sample potential was-1.429 V (during 14 h).When the cell was left disconnected for 14 h and then another curve was recorded on the same sample,tno similar peaks were observed.However,breakdown was observed at potentials more positive than 0.5 V.A similar behavior was noted for Mg90 coated ODPA with a smaller surface roughness (Fig.6c).Although,the current density in this curve is higher and the local depassivation potential can be seen in the graph.
After analyzing the results of the electrochemical tests,it can be inferred that the protective properties of the SHP coatings enhance with increasing of surface roughness parameters.The hydrophobic properties of the compounds themselves played minor role in this case.Thus,ODPA provides the most effective protection,even though its molecules are less hydrophobic than those of SA and OlA,as evidenced by the partition and distribution coefficients (see Table 6).This is because phosphonic acids can firmly bind to the oxide–hydroxide layer of magnesium in both mono– and bidentate ways.
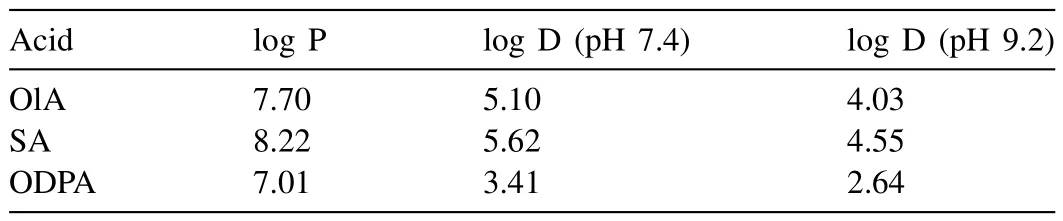
Table 6The partition (log P) and distribution (log D) coefficients.1
3.2.2.2.Corrosion tests.In the previous study,corrosion test results were presented for Mg90 samples with surfacetypes1–4,on which acid films had formed,under conditions of 100 % humidity and periodic moisture condensation [32].By comparing them with the data obtained on the5thsurfacetype(Fig.7),we can conclude that the efficiency of all corrosion inhibitors increases with increasing surface roughness.
It is known that the Wenzel model describes the surface wettability in the absence of treatment with low surface energy substances [43].Under these conditions,an increase in roughness enhances the hydrophilicity of the samples,leading to a reduction in the metal’s corrosion resistance.The surfaces of the laser–treated Mg90 plates without adsorption of HPA were superhydrophilic.This would also explain the shorter first corrosion time and the more negativeEcorvalues on the textured samples compared to the“smooth”and etched ones.
whereθwis the contact angle on a rough surface,i.e.,the apparent contact angle;r is the roughness coefficient,that denotes the ratio of the actual contact area to the geometric projected area.Theθis the liquid drop contact angle on a perfectly flat solid surface,which is also known the intrinsic contact angle.
The adsorption of acids on a surface with a developed morphology leads to a transition from a hydrophilic state to a superhydrophobic state.As a result,the Wenzel equation is no longer acceptable and the wettability of such these samples obeys the Cassie–Baxter equation [43,46]:
whereθcis the apparent boundary angle in the Cassie–Baxter model,andfrepresents the solid-liquid contact fraction.
As expected,hydrophobicity was improved with increase in surface roughness increasing (Section 3.2.1.2).In the case of the5thsurfacetype,the developed morphology combined with the HPA allowed a large amount of air to be trapped,which supported the liquid droplet.As a result,the access of aggressive ions to the metal was hindered.According to the Direct Corrosion Test results,the treatment of textured Mg90 (type 5) provided the longest lasting protection under both wet atmospheric conditions (Fig.7) and SSC conditions(Fig.8).Simultaneously,asθdecreases,a gradual transition to the Wenzel model was observed during the testing period for all SHP coatings.

Fig.8.Corrosion test results in SSC of Mg90 specimens,with roughness type 5,without (bare) and with superhydrophobic coatings.
Octadecylphosphonic acid chemically adsorbs strongly on the surface of textured magnesium,thereby providing a stable superhydrophobic state of the Mg90 surface.This state has a high resistance that it cannot be broken by anodic polarization (up to 5.0 V).As a result,a plateau with very low anodic current densities can be seen on the polarization curves.However,this can be significantly reduced by increasing the NaCl concentration in the borate solution from 0.001 to 0.010 M(Fig.9).The appearance of the potentiodynamic polarization curve of Mg90,with a film formed in a 0.001 M ODPA solution,changes with increase in NaCl concentration in borate solution.Specifically,oscillations on its anodic branch disappear and pitting becomes noticeable atEbd=-0.715 V.This fact determines the necessity of improving the protective properties provided by the acid layer.As previously established,one of the ways to enhance the protective properties of CIs can be their co–application with trialkoxysilanes(TAS),either in the form a composition or by layer-by-layer deposition [33,47].
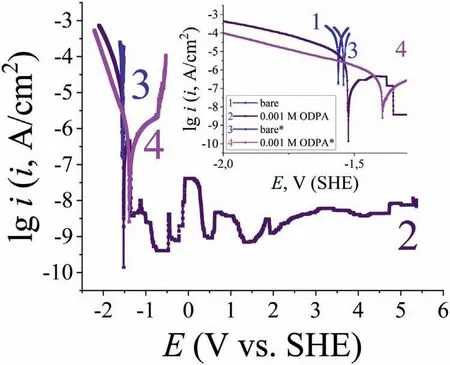
Fig.9.Potentiodynamic polarization curves of textured Mg90 (type 5) in a borate solution at pH 9.2 containing 0.001 M (no index),0.01 M (denoted by *) NaCl,without (bare) and after pre-modification in ethanol ODPA solution.
3.3. Effect of VTMS on the protective and water–repellent properties of ODPA and SA films
The studies [47,48]demonstrate the possibility of enhancing the protective properties of superhydrophobic coatings of SA and ODPA by forming an additional TAS layer.In this work we used VTMS to improve the protection of SA and ODPA.All experiments were exclusively performed on textured Mg90 with surfacetype 5.The silane itself,when applied from ethanol solution (C=0.01 M),does not allow to achieve a SHP surface state,even on textured Mg90 (θ=143±4°).The modification of Mg90 in a mixture solution of VTMS both with SA and ODPA resulted in decrease to 151±1° and 162±1°,respectively,compared to individual acids.This phenomenon can be attributed to the competition in adsorption between the silane with lower hydrophobicity and the investigated acids from their mixtures.Likewise,layer-by-layer application of ODPA and TAS also failed toθ.However,the contact angle was increased by layer-by-layer application of VTMS and SA.The order of carboxylic acid and silane deposition had no significant effect on the hydrophobic properties of the surface.Theθvalues were 160±1° and 159±2°,respectively.
The stability of the SHP properties of the formed coatings was investigated by washing the samples in distilled water with agitation.Fig.10 shows the degradation kinetics of the water-repellent properties of the coatings over the exposure time.

Fig.10.Change of θ during exposure time in distilled water on Mg90 samples pre–modified in ethanolic solutions of 0.01 M SA,0.001 M ODPA and 0.01 M VTMS.2
A correlation has been found between the initialθvalue and the stability of the SHP properties of the coatings,whether SA and ODPA were used alone,in mixtures with VTMS,or by layer-by-layer application.In the case of phosphonate,silane did not allow for an increase in resistance in either mixed or layered formation of hydrophobic layers.The order of compounds application had an impact on the results.When VTMS was adsorbed first followed by ODPA,the resulting coating showed hydrophobic properties similar to those of ODPA itself.In contrast,the surface of Mg90 with an ODPA// VTMS film became hydrophobic after 13 h.For carboxylic acid,the combination of its layers with silane had a greater effect.The sequence of deposition had minimal impact on the resistance of the coating.In both scenarios,VTMS// SA and SA// VTMS,theθdecreased to a value below 150° within13 h and 12 h of exposure,respectively.
Fig.11 shows the results of direct corrosion tests performed on textured (type 5) Mg90 in SSC with poly–layer coatings.For clarity,we also duplicate the data obtained on Mg90 with the individual compound films (0.01 M SA and 0.001 M ODPA).
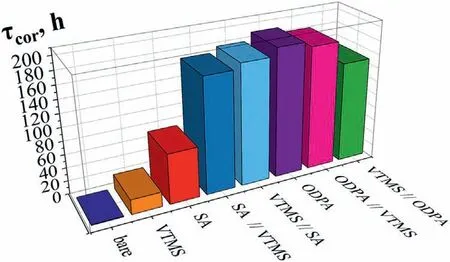
Fig.11.Corrosion test results in salt spray chamber of Mg90 specimens,with type 5 of surface roughness,without (bare) and with superhydrophobic coatings.3
According to the results of corrosion tests,SHP coatings prepared with the ODPA have better protective properties.Despite providing less prolonged protection in the salt spray chamber compared to acids,the modification of Mg90 with the5–thsurfacetypein 0.01 M VTMS solution,when applied by layer–by–layer deposition with SA,manages to improve Mg90 resistance by 2.3 and 2.4 times (SA// VTMS and VTMS// SA,respectively) compared to individually deposited SA,almost achieving ODPA results.There is no significant improvement in the corrosion resistance of Mg90 upon layer-by-layer application of VTMS with ODPA,as confirmed by the results ofθangle measurements.
4.Conclusions
1.Laser treatment of the surface,followed by modification in an ODPA solution,produces a stable superhydrophobic coating that provides long–lasting protection for technically pure magnesium,even under highly aggressive conditions of salt fog chamber.
2.The character of the anodic polarization curves of textured magnesium modified in 0.001 M ODPA solution is determined by the small contact area between the surface and electrolyte,as well as the low aggressiveness of the environment.When working with superhydrophobic coatings,it is important to exercise care when interpreting the results of potentiodynamic tests.The estimation of protective properties of such coatings should be carried out based on a complex set of studies,including direct corrosion tests.
3.The adsorption of VTMS on the laser–textured Mg90 surface from their ethanolic solutions (C=0.01 M)at room temperature followed by drying at 65 °C,in contrast to its use in mixtures or multilayer coatings,does not allow achieving the SHP surface state.
4.The water-repellent and protective properties of textured Mg90 are enhanced 1.5–fold and 2.3–2.4–fold,respectively,through its layer–by–layer modification in 0.01 M SA and 0.01 M VTMS solution compared to the individual treatment in SA solution.This practically achieves ODPA indicators.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Financing
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (122011300 078–1).
Acknowledgement
The authors would especially like to thanks PhD in Chemistry,Sergey Y.Luchkin for his help in interpretation of the scanning Kelvin Probe Force Microscopy data.
The authors also express they’re thanks to PhD in Chemistry,Alexander A.Chirkunov and Ilya A.Kuznetsov for their help in preparing the manuscript for publication.
 Journal of Magnesium and Alloys2023年9期
Journal of Magnesium and Alloys2023年9期
- Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
