Selective flotation of chalcopyrite from pyrite via seawater oxidation pretreatment
Wanqing Li,Yubiao Li,,Zhonghong Wang,Xu Yang,Wen Chen
a Hubei Key Laboratory of Mineral Resources Processing &Environment, Wuhan University of Technology, Wuhan 430070, China
b School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China
c Changsha Research Institute of Mining and Metallurgy Co.Ltd., Changsha 410012, China
Keywords:Seawater oxidation Chalcopyrite Pyrite Non-depressant flotation The coordination theory
ABSTRACT The flotation separation of chalcopyrite from pyrite has attracted increasing attention due to the consumption of vast water resources and depressants.This study proposed the seawater oxidation pretreatment for non-depressant flotation separation of chalcopyrite from pyrite,as an effective and environmentally friendly strategy.Without the addition of depressants,seawater oxidation for 3 d effectively depressed pyrite flotation,with the highest recovery difference greater than 70% and a selectivity index greater than 6 between chalcopyrite and pyrite.The surface investigation showed that pyrite surface was more readily oxidized to form hydrophilic Fe oxidants/oxyhydroxides,as compared to that of chalcopyrite.Further UV-visible spectrophotometer and Fourier transform infrared spectrum (FTIR)results indicated that xanthate was less adsorbed onto the treated pyrite surface,resulting in unfloatable particles.Chalcopyrite surface was changed slightly due to seawater oxidation,thereby insignificantly affecting its flotation.The coordination theory was further used to reveal the combination mechanisms between xanthate and pyrite or chalcopyrite.This study therefore provides a promising strategy to effectively separate chalcopyrite from pyrite,especially in the freshwater-deficient area.
1.Introduction
Copper(Cu),which plays an essential role in the economic construction process worldwide,is applied in semiconductors,machinery manufacturing,aerospace science,defense industry,etc.[1,2].The predominant Cu-bearing mineral of chalcopyrite(CuFeS2) accounts for approximately 70% of copper reserves on Earth[3].Due to good natural hydrophobicity,chalcopyrite is usually separated and concentrated via flotation [4].Xanthate is the most widely used collector for chalcopyrite flotation beneficiation but easily decomposed to generate toxic compounds such as carbon disulfide in acidic solution [5,6].Therefore,chalcopyrite flotation is commonly carried out under alkaline condition to reduce the decomposition of xanthate [7].However,the flotation process faces many challenges,especially when low-grade ores frequently associated with other sulfides such as pyrite (FeS2),deteriorating chalcopyrite flotation[8].Therefore,maintaining a high selectivity in chalcopyrite separation from pyrite is beneficial to achieve a good quality of Cu concentrate.
Inorganic or organic depressants are normally applied to selectively depress pyrite flotation,as shown in Table 1[8,9].However,the application of these reagents increases the potentials in environment pollution[9].In recent years,various oxidizing strategies have been carried out to achieve a selective flotation separation by changing the surface hydrophobicity of gangue minerals[9,10].For instance,the addition of oxidizing reagent (H2O2) oxidized chalcopyrite significantly and generated more hydrophilic products on chalcopyrite surface rather than molybdenite surface,thereby selectively separating molybdenite from chalcopyrite [10].Therefore,the application of oxidative methods was feasible to obtain selective flotation separation of chalcopyrite from pyrite.
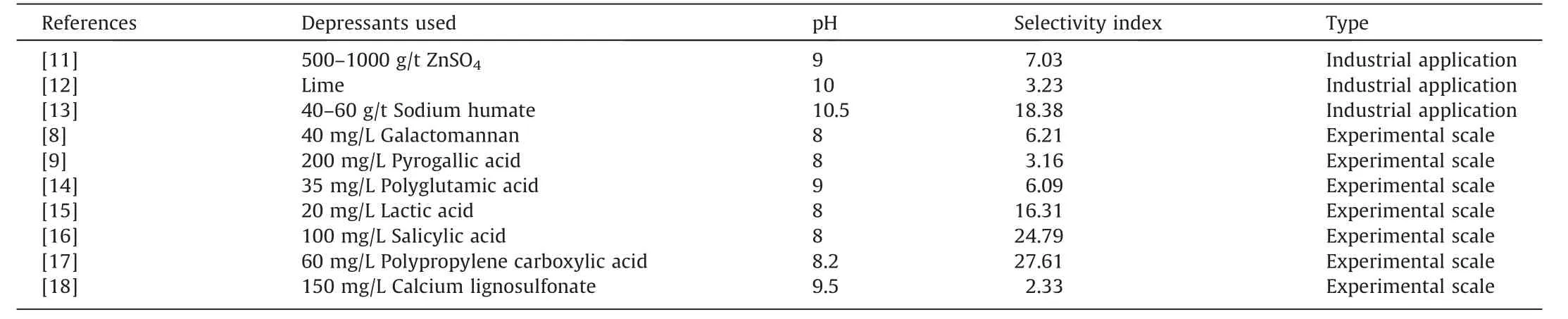
Table 1 The depressants used for chalcopyrite separation from pyrite.
Moreover,flotation is a highly water-intensive process,which obviously increases freshwater usage[19,20].Therefore,as the predominant water resource globally,seawater has attracted increasing attention.However,the separation flotation using seawater normally consumes more depressor,especially lime,to achieve a good selective depression,due to the pH buffer role of seawater[21].For instance,Castro [21] reported that the lime usage in seawater was ten times higher than that in freshwater when adjusting pulp pH from 7 to 11.In this case,it is very meaningful to reduce lime usage for chalcopyrite-pyrite separation flotation in seawater.
The objective of this work is therefore to achieve a selective separation of chalcopyrite from pyrite via seawater oxidation pretreatment without depressants.Different seawater oxidation durations were applied to single pyrite,chalcopyrite and a mixture of them,followed by non-depressant micro-flotation experiments.The underlying mechanisms were explored by surface hydrophobicity,Fourier transform infrared spectroscopy (FTIR),electrochemical and X-ray photoelectron spectroscopy (XPS)measurements.Furthermore,the interactions between collector and the surfaces of pyrite or chalcopyrite were calculated based on coordination theory.Therefore,this study not only sheds new lights on avoiding the use of depressors,but also provides a promising way for innovative utilization of seawater in effective separation of chalcopyrite from pyrite.
2.Experimental
2.1.Materials
Chalcopyrite sample was collected from Peru(Chile)while pyrite sample was obtained from Daye (China).After crushing,grinding and sieving,the high-purity samples were sized in the range of 75-150 μm with a D80of 131 μm,which were further cleaned in ethanol solution via sonication and then freeze drying.The prepared powders were then transferred into tubes and frozen in liquid nitrogen to minimize further surface oxidation.X-ray fluorescence spectrum (XRF) analyses showed a chalcopyrite purity of 90.5 wt%,with 25.1%Cu,31.8%Fe and 33.6%S while a pyrite purity of 98.6%,with 46.1% Fe and 52.5% S.All analytical grade reagents including NaCl,KCl,CaCl2,MgCl2,NaHCO3and MgSO4,were provided by Sinopharm Chemical Reagent Co.,Ltd.,Shanghai,China.Sodium butyl xanthate (SBX) was applied as the flotation collector.The seawater with a pH of 7.7-7.8 was prepared according to the reported seawater composition in literatures[7].Specifically,0.47 M NaCl,0.01 M KCl,0.01 M CaCl20.025 M MgCl2,0.0018 M NaHCO3,and 0.028 M MgSO4were added into ultrapure water for preparing seawater and the compositions of simulated seawater were showed in Table 2.

Table 2 Composition of simulated seawater used in this study.
2.2.Micro-flotation experiments
Each micro-flotation experiment (Fig.1) was carried out in an XFG II type of flotation machine (Wuhan Exploration Machinery Factory,Wuhan,China).1 g mineral sample (75-150 μm) was poured into 50 mL plastic tube with 25 mL seawater for oxidation pretreatment.Afterwards,the seawater-oxidized pulp was stirred at 1200 r/min for 1 min.100 ppm SBX was added in the subsequent 4 min.Thereafter,200 mL/min air was introduced and then the floated particles were collected as five concentrates at 1,3,5,8 and 10 min.Both concentrates and tailing were dried and weighed for flotation recovery calculation.After seawater oxidation pretreatment for different days,the pH of chalcopyrite pulp,pyrite pulp and that of 2 mixed minerals pulp remained in 7.4-7.8,indicating that seawater oxidation pretreatment affected the pulp pH slightly.Each experiment was carried out at least 3 individual times,and the average value was shown herein.In addition,a confidence interval of 95%was applied for the displayed error bars for flotation recovery.
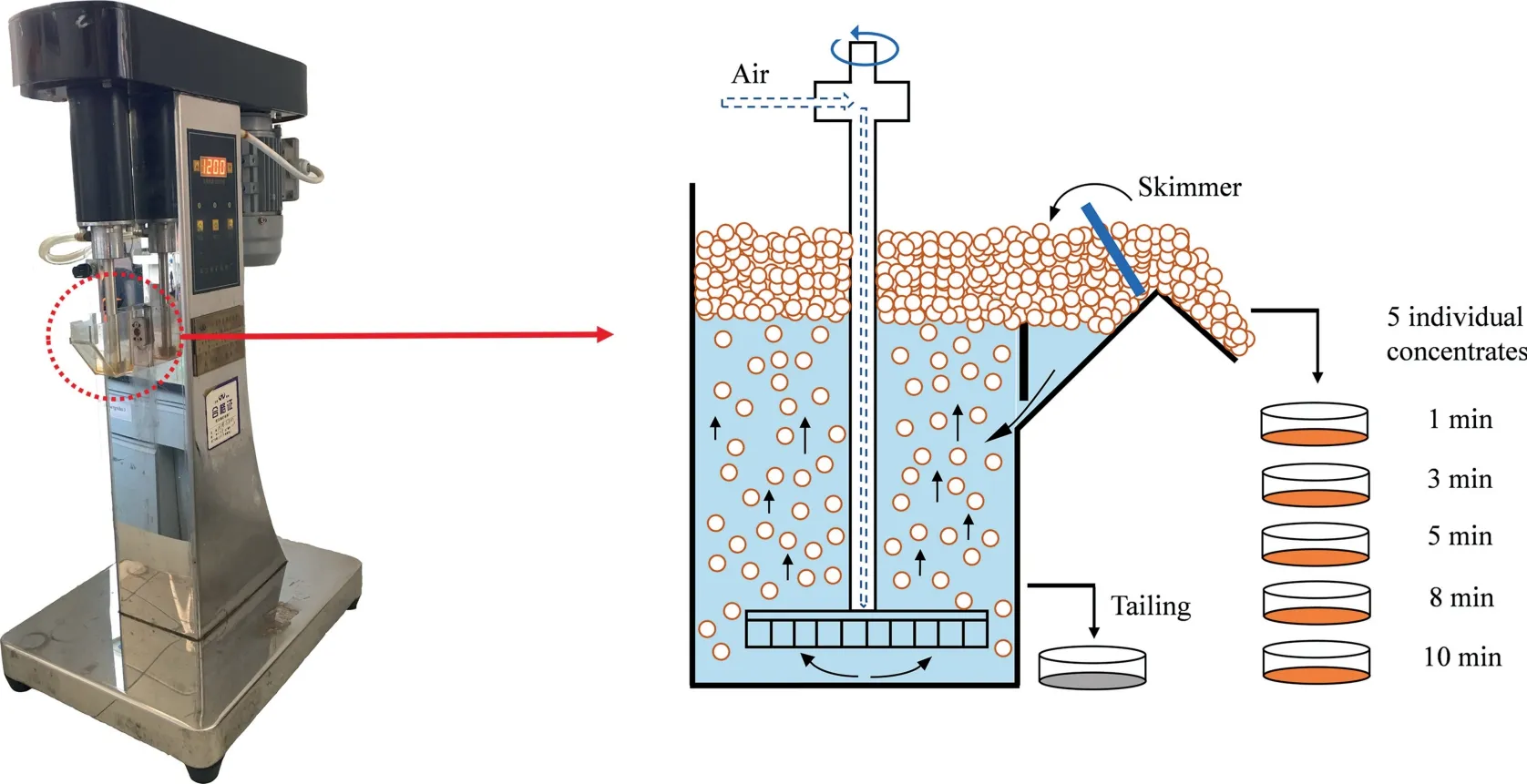
Fig.1.Diagram of micro-flotation experiment.
In addition,the separation performance of chalcopyrite from pyrite was further determined by selectivity index (SI),according to Eq.(1).Usually,the higher SI value indicates a better selection efficiency.
2.3.Contact angle measurements
The captive bubble method was employed to measure the contact angle,as shown in Fig.2.Chalcopyrite and pyrite slabs were polished consecutively using 600#,1000#,2000#and 5000#abrasive papers,ensuring a flat and smooth surface.The sample surface was then washed carefully by ultrapure water to remove any remaining impurities and then rapidly dried by a blower.After drying,the slab was immersed into seawater for different times.An air bubble (0.15 μL) was generated and attached on the mineral surface through a microliter syringe.Afterwards,the profile of contact angle was measured using the JC2000C1 version software (Shanghai Zhongchen Digital Technology Equipment Co.,Ltd.,Shanghai,China).This measurement was repeated at least 3 times under each condition,and the average values were reported in this study.After seawater oxidation pretreatment for different days,the seawater pH in the presence of chalcopyrite or pyrite remained in 7.4-7.8.In addition,the concentration of SBX used was 100 ppm,the same as that for the flotation.
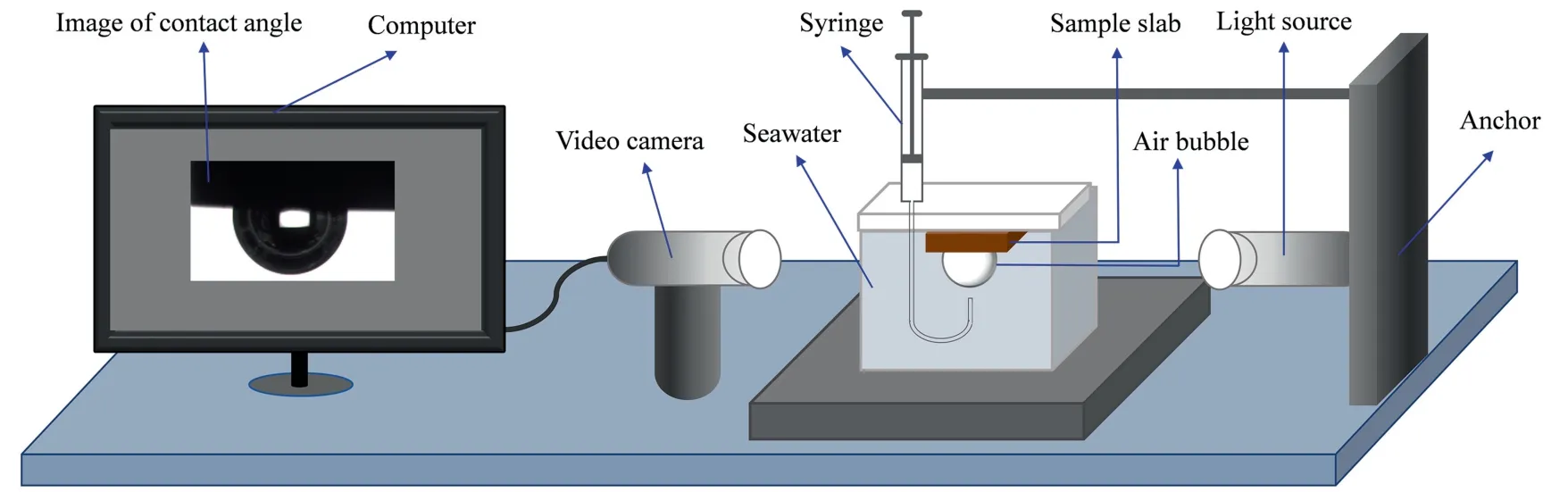
Fig.2.Schematic diagram of contact angle measurements.
2.4.Electrochemical measurements
Electrochemical measurements were performed using an electrochemical workstation (CHI660E,Shanghai Chenhua Instrument Co.,Ltd.,Shanghai,China).A typical three-electrode system was applied,which consisted of a reference electrode(Ag/AgCl),a counter electrode (platinum wire) and a work electrode (mineral electrode).The simulated seawater was used as the supporting electrolyte solution.The crystal chalcopyrite or pyrite cube was mounted into epoxy resin to prepare the working electrode,and only one flat surface was exposed.Before testing,the exposed surface of mineral electrode was polished following the same procedure as shown in Section 2.3,but prior to washing ultrapure water was used.After that,the dried slab surface was immediately transferred to the electrolyte solution.Specially,the sample was oxidized in electrolyte solution (seawater) for 3 d before test.The cyclic voltammetry (CV) measurement was conducted from -0.6 to 0.6 V (vs,open circuit potential,OCP) with a 0.02 V/s scan rate.After seawater oxidation pretreatment for different days,the seawater pH in the presence of chalcopyrite or pyrite remained in 7.4-7.8,close to that during flotation experiments.The diagram of electrochemical tests was showed in Fig.3.
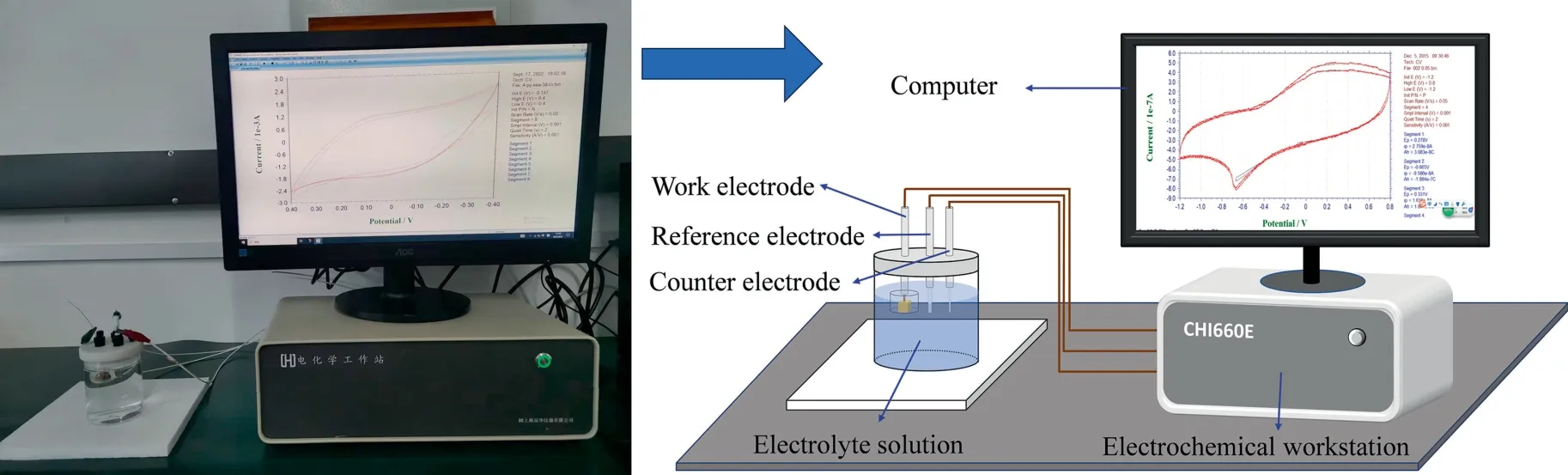
Fig.3.Schematic diagram of electrochemical measurements.
2.5.Uv-visible spectrophotometer measurements
An UV-visible spectrophotometer from AOE Instruments(Shanghai) Co.,Ltd.,Shanghai,China was applied to measure the residual SBX concentration in the solution.Once 1 g of 75-150 μm mineral powder was poured into 25 mL seawater,the suspension was oxidized for different times.Prior to the adsorption test,the initial 100 ppm of SBX was added into the suspension and treated for 14 min,the same as the flotation time.Subsequently,the suspension was filtered and the obtained solution with remaining SBX was diluted for five times for the UV-visible spectrophotometer measurements.After seawater oxidation pretreatment for different days,the pH of chalcopyrite pulp and pyrite pulp remained within 7.4-7.8,the same as that during the flotation experiments.
2.6.FTIR measurements
The FTIR spectra of SBX on sample surface was assessed from 4000 to 400 cm-lat a resolution of 4 cm-1using a Nicolet iS-10 FTIR spectrometer (Thermo Fisher Scientific Inc.,Waltham,MA,USA).50 mg fine mineral particles with a particle size smaller than 5 μm was poured into 50 mL seawater for oxidation,followed by stirring and reacting with 100 ppm SBX for 30 min,where the sample treating method was similar to the reported studies[22].After filtering,the sample was washed softly for 3 times using ultrapure water,followed by freeze drying.Finally,2 mg dried mixture and 200 mg spectral grade KBr was pressed into a flake for FTIR measurements.After seawater oxidation pretreatment for different days,the pH of chalcopyrite pulp and pyrite pulp remained at 7.4-7.8,close to that during the flotation experiments.
2.7.XPS measurements
After oxidation in seawater for different times,the treated minerals pulps were filtered,washed softly with ultrapure water to remove the surface substances adsorbed and finally freeze-dried for XPS tests.The ESCALAB 250Xi type of spectrometer (Thermo Fisher Scientific Inc.,Waltham,MA,USA) with a monochromatic Al X-ray source was used to determine the XPS spectra.All XPS survey spectra scans were run from 1350 to 0 eV with a pass energy of 100 eV and a step size of 1.0 eV.In addition,each XPS spectra scan was run with a pass energy of 30 eV and a step size of 0.1 eV.
3.Results and discussion
3.1.Flotation recovery
Fig.4 showed the influence of oxidation time on the flotation recovery of single chalcopyrite and pyrite.A high flotation recovery was presented when chalcopyrite was not pretreated by seawater(Fig.4a),in agreement with reported studies[4,7].The chalcopyrite was floated significantly within the initial 3 min,achieving a cumulative recovery greater than 85.0%.Afterwards,chalcopyrite recovery was raised slowly and eventually remained at 91.5% at 10 min,suggesting a fast flotation kinetics of chalcopyrite.After seawater oxidation for 3 d,the flotation kinetics of chalcopyrite were reduced,with the cumulative recovery decreased to 87.0%.Further increase in oxidation time (4 d) reduced flotation kinetics and recovery (82.0%).These suggested that the oxidation time shorter than 3 d had a slightly negative impact on chalcopyrite recovery in seawater.
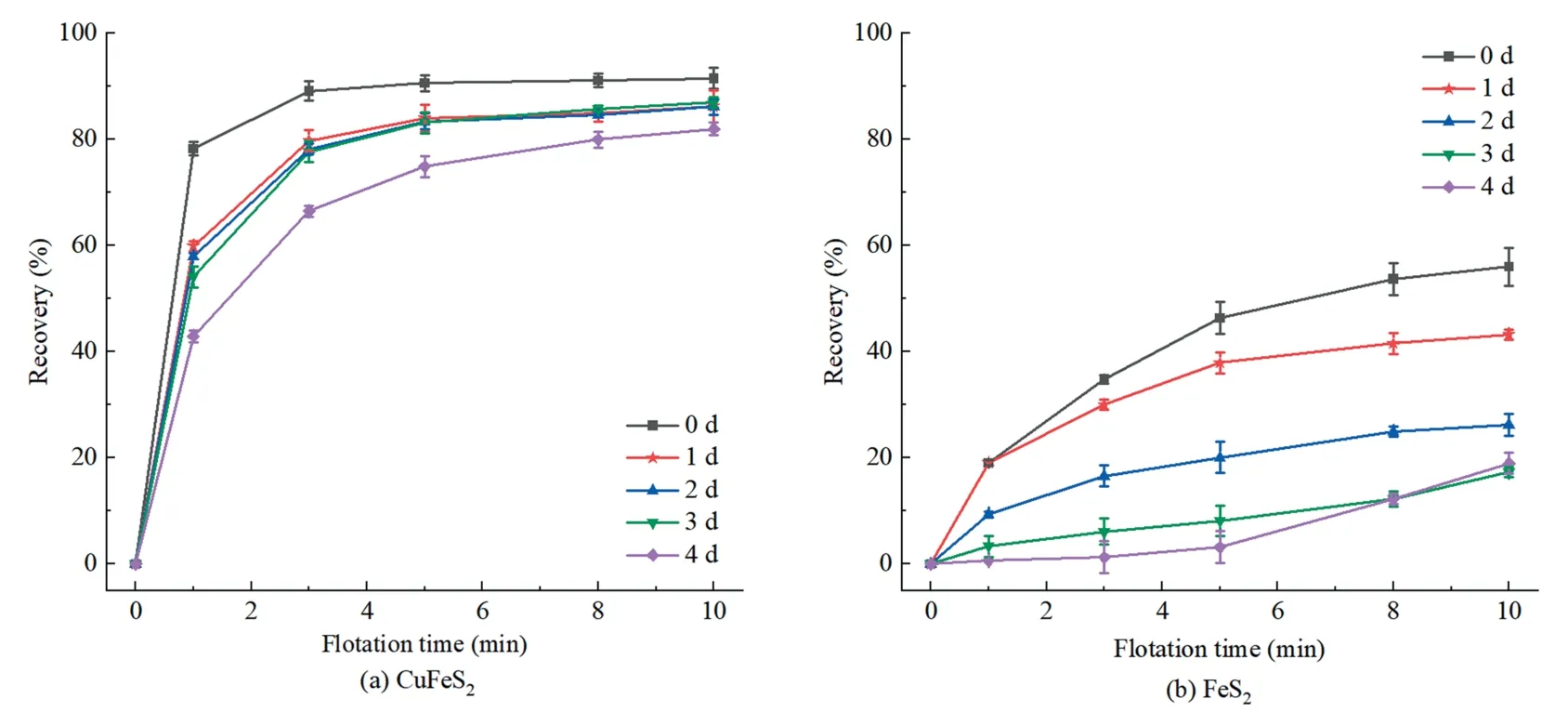
Fig.4.Effects of oxidation time on flotation recovery of chalcopyrite and pyrite.
Fig.4b showed a flotation recovery of 56.0% for pyrite within 10 min when seawater treatment was not applied,apparently lower than that of chalcopyrite.In addition,pyrite recovery decreased to 43.2%,26.2% and 17.3% when oxidizing for 1,2 and 3 d,respectively.The reduced pyrite recovery after seawater pretreatment was probably ascribed to the formation of hydrophilic products (i.e.,FeOOH and Fe2(SO4)3) on pyrite surface when exposed to weak acidic/alkaline or neutral solution[23].However,pyrite flotation recovery was changed slightly when oxidation time longer than 3 d.
Fig.4 also showed an increased recovery difference from 35.5%to 69.7%between chalcopyrite and pyrite flotation as a function of time (≤3 d).These differences in flotation recovery were probably due to various hydrophilic species formed during oxidation treatments,further verified by XPS.These therefore indicated the potential applicability of seawater oxidation pretreatment as a selective depressing method to separate chalcopyrite from pyrite.
Fig.5 showed the recovery difference obtained from the mixed chalcopyrite and pyrite flotation after seawater treatment.It can be seen that both chalcopyrite and pyrite showed a high flotation recovery at 0 d,i.e.,95.8%and 63.2%,respectively,where the selectivity index(SI)was lower than 4,indicating a poor separation efficiency of chalcopyrite from pyrite.In contrast,a very good separation was achieved at 3 d,with a difference of 70.6%,where a higher SI value of 6.53 was observed.In contrast,the poorest separation of chalcopyrite from pyrite was observed when treating both minerals in ultrapure water for 3 d,where the lowest SI value of 1.88 was present.These findings indicated that seawater pretreatment can separate chalcopyrite from pyrite effectively,which has great significance for efficient separation of copper sulfide from gangue minerals and the comprehensive utilization of seawater.
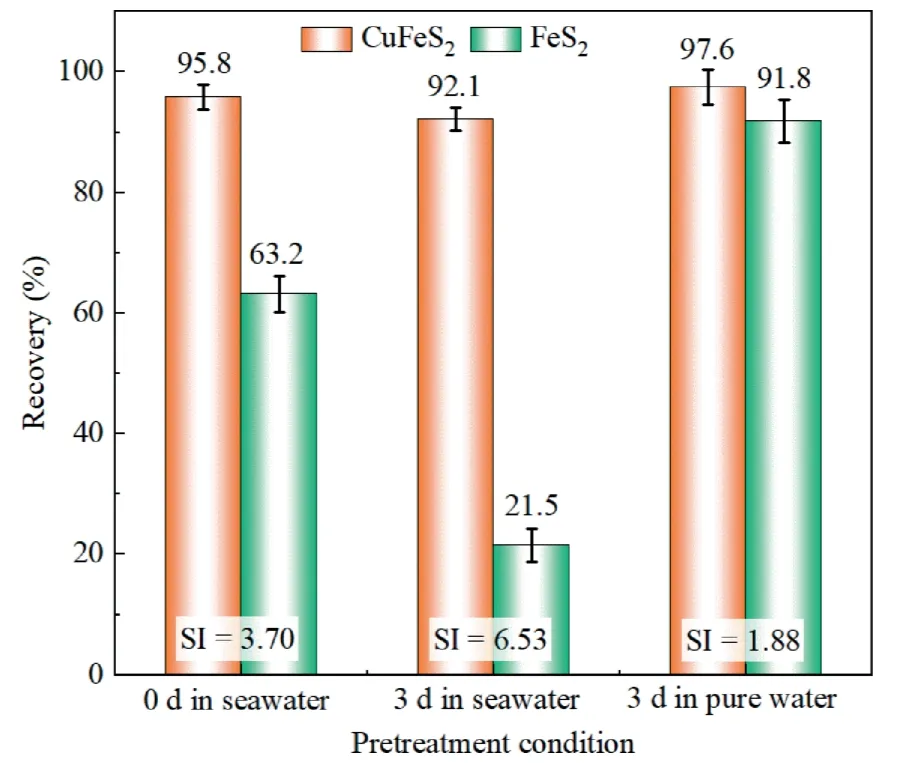
Fig.5.Effects of pretreatment on flotation recovery of mixed chalcopyrite and pyrite.
3.2.Surface hydrophobicity
Usually,a high hydrophobicity corresponds to a good floatability [7].As shown in Fig.6,the chalcopyrite surface showed the highest contact angle,indicating a good hydrophobicity,consistent with the highest flotation recovery(Fig.4).When chalcopyrite was treated in seawater for 1 d,the contact angle decreased from 91°to 83°.With increasing time from 1 to 4 d,the contact angle remained within a very narrow range from 80° to 84°.These indicated a relatively weak influence of seawater oxidation on surface hydrophobicity of chalcopyrite.
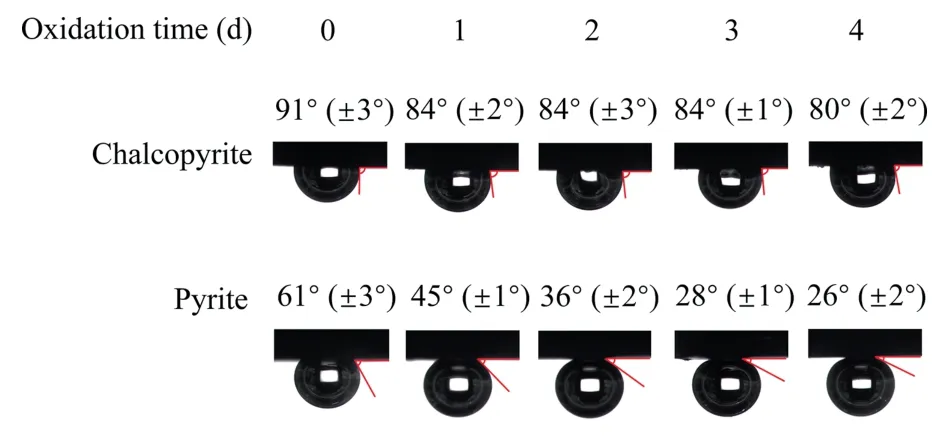
Fig.6.Contact angles of chalcopyrite and pyrite surfaces.
But a gradually decreased contact angle was found on pyrite surface when time increased from 0 d to 4 d.For instance,the natural contact angle of pyrite was 61° without seawater treatment.With the extension of time to 3 d,the contact angle of pyrite dropped to 28°,whereas further increased time influenced the contact angle of pyrite inapparently(26°).In addition,the highest difference (56°) on both minerals was found at 3 d,consistent with the single pyrite flotation.These results therefore suggested that seawater oxidation selectively reduced the hydrophobicity of pyrite surface,thereby depressing its flotation recovery.As compared to chalcopyrite,a more significant decrease in hydrophobicity was found on pyrite surface in seawater,more likely due to different oxidation rates between chalcopyrite and pyrite when exposed to aqueous solution.This will be further investigated in the following electrochemical and XPS analyses.
3.3.Electrochemical analyses
Fig.7 showed the electrochemical characteristics of chalcopyrite and pyrite during different treating times.During positivegoing potential scanning,the voltammogram of chalcopyrite showed a weak anodic peakA1between 0.2 and 0.4 V,probably attributing to the slight oxidation of chalcopyrite according to Eq.(2),as reported in previous study [24].The cathodic peakC1in the negative-going direction represented the reverse of Eq.(2).After treating for 3 d,although no new peaks were observed on chalcopyrite curve,the existing peaks increased slightly,suggesting that seawater treatment increased the oxidation degree of chalcopyrite.
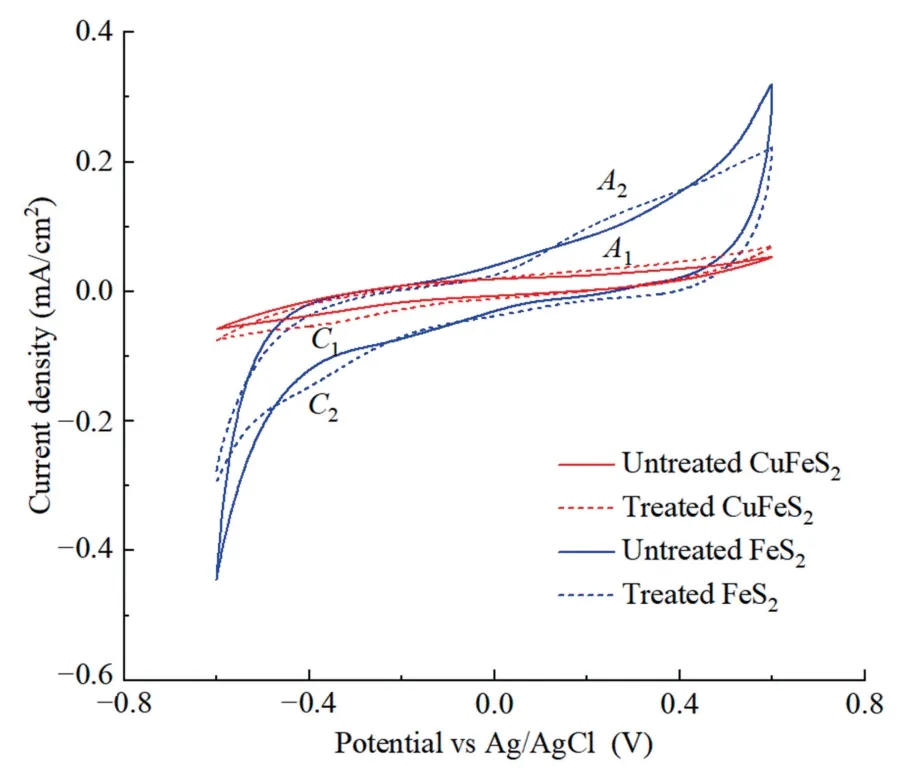
Fig.7.Effects of seawater pretreatment on CV curves of chalcopyrite and pyrite.
A wider current density was found on the CV curve of pyrite,indicating that pyrite was more readily oxidized than chalcopyrite when exposing to seawater.Anodic peakA2at around 0.4 V represented the oxidation of pyrite based on Eq.(3) [25].According to Eq.(4),partial Fe(OH)3can be divided into Fe2O3and H2O.However,the current density of seawater-treated pyrite narrowed as compared to that of untreated pyrite,indicating a reduced oxidation capacity,due to the coverage of oxidation species formed on pyrite surface.
3.4.SBX adsorption
Fig.8 showed the UV-visible spectra of the remaining SBX in solution when 100 ppm SBX was applied.Once SBX was added into seawater and reacted for 14 min(same to the flotation procedure),the UV-visable absorption spectrum changed insignificantly compared to the initial value,indicating no interaction between xanthate and seawater.When 100 ppm SBX was added into seawater in the presence of mineral particles,only 28.0% of SBX was remained in the pulp for un-oxidized chalcopyrite,indicating that most of SBX was adsorbed onto chalcopyrite surface.The residual SBX slightly increased to 36.5% when SBX reacting with the oxidized chalcopyrite,indicating insignificant influence of seawater oxidation for the adsorption between SBX and chalcopyrite particles.In contrast,the SBX concentration in pyrite pulp increased from approximately 30.0%to 87.5%after seawater treatment,significantly depressing the adsorption of SBX on pyrite surface.This also explains the decreased recovery in pyrite flotation(Fig.4).Therefore,seawater oxidation for 3 d slightly influenced the interaction between SBX and chalcopyrite,but obviously preventing the adsorption of SBX on pyrite surface.
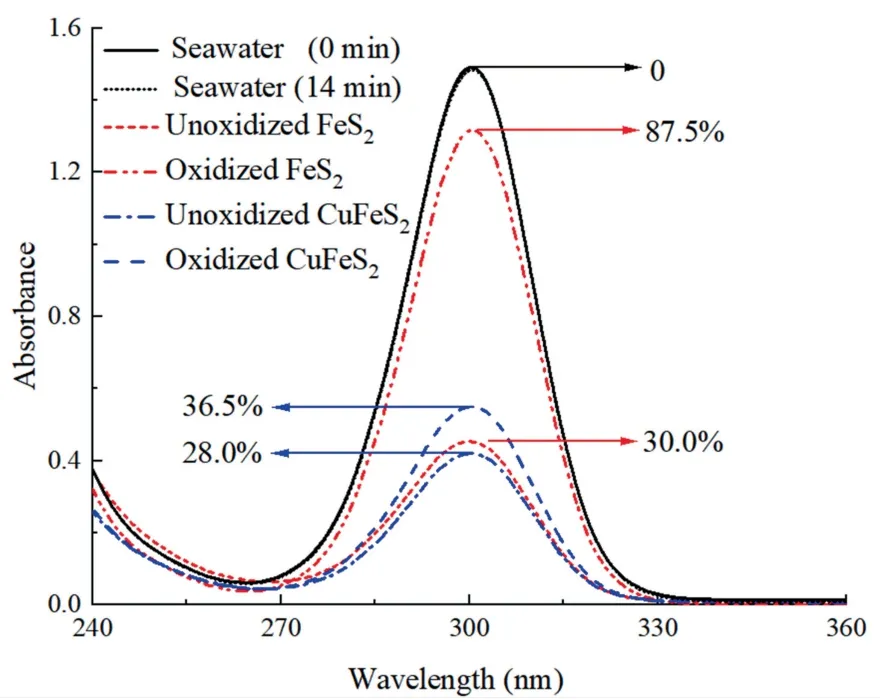
Fig.8.UV-visible absorption spectra of remaining SBX in solution when 100 ppm SBX was added.
3.5.FTIR analyses
Fig.9 showed the FTIR spectra of SBX and untreated/treated chalcopyrite and pyrite surfaces reacted with SBX,with the main characteristic peaks shown in Table 3.When the un-oxidized chalcopyrite treated with SBX,the band located at 1038.4 cm-1(C=S stretching vibration) while the bands at 1084.4 cm-1(C—O—C symmetric stretching vibrations of SBX) appeared [26].The C—O—C asymmetric stretching vibration peaks were observed at 1170.2 and 1270.3 cm-1.In addition,the band at about 1386.6 cm-1corresponded to —CH deformation vibration from SBX.These indicated that SBX was adsorbed on chalcopyrite surface.During chalcopyrite flotation,SBX reacted with Cu sites on chalcopyrite surface to form Cu xanthate [1,27].After oxidation for 3 d,these peaks of SBX shifted slightly on chalcopyrite surface,indicating that the seawater treatment did not affect the adsorption between xanthate and chalcopyrite,consistent with the SBX adsorption results.
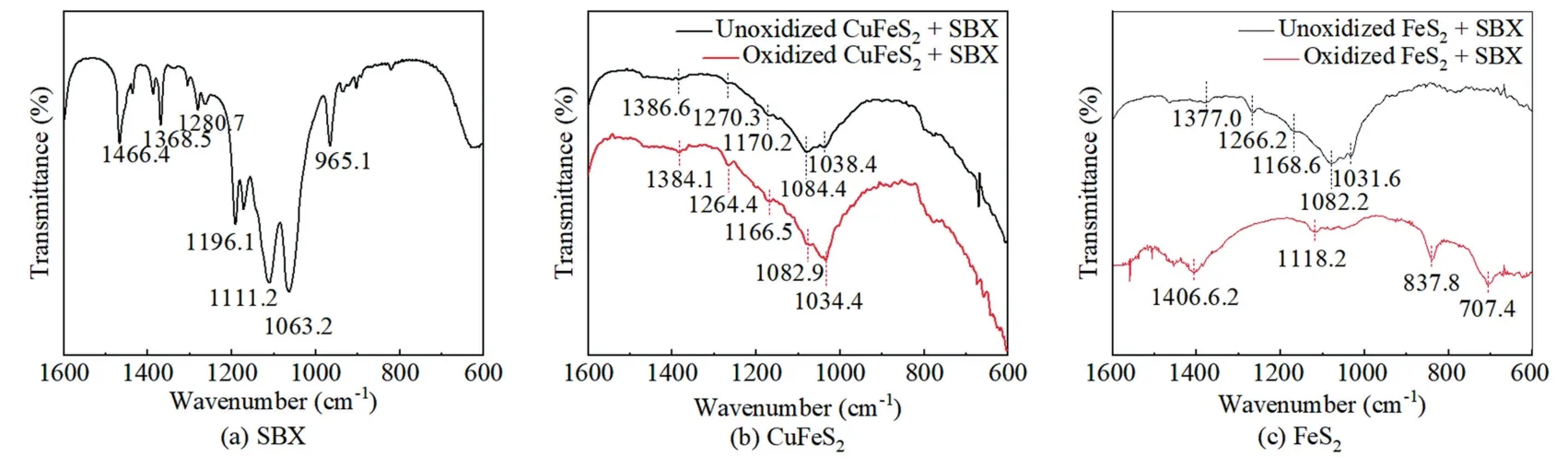
Fig.9.FTIR spectra of xanthate and minerals surfaces.
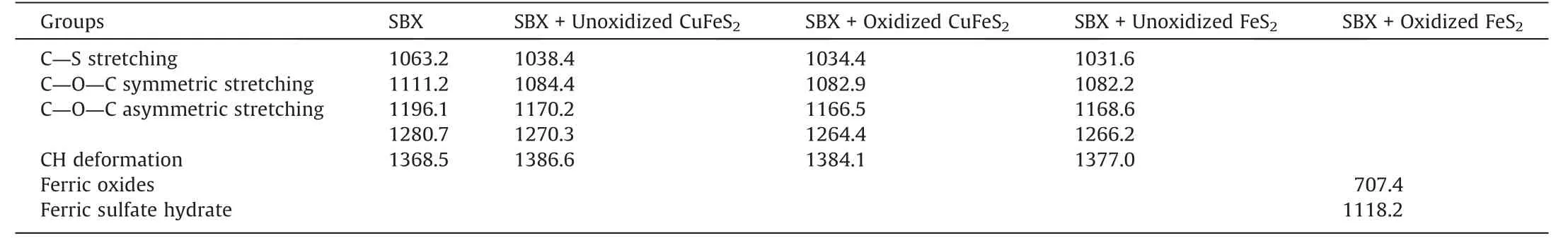
Table 3 FTIR spectral peak sites (cm-1) of SBX,chalcopyrite reacted with SBX and pyrite reacted with SBX.
Fig.9c showed that four bands of SBX at 1031.6,1082.2,1266.2 and 1377.0 cm-1appeared on pyrite surface,which were shifted to the left as compared to that of chalcopyrite.As reported by Zhang et al [26],the SBX was adsorbed on unoxidized pyrite surface via the reaction of BX-that derived from SBX at the Fe sites on pyrite.When the seawater oxidized pyrite was further treated by xanthate,all peaks of SBX disappeared while two new peaks attributing to ferric oxides and ferric sulfate hydrate appeared at about 707.4 and 1118.2 cm-1[8],respectively.This indicated that seawater oxidation played a strong role on pyrite surface,thereby preventing the attachment of xanthate on pyrite surface.Therefore,seawater oxidation is an efficient pretreatment for chalcopyrite separation from pyrite.
3.6.XPS analyses
3.6.1.Survey XPS
Fig.10 showed the XPS spectra of unoxidized and seawateroxidized chalcopyrite and pyrite.A relatively high O 1s peak was presented on both minerals,mainly due to the natural oxidation during grinding and flotation processes.Proper oxidation did not affect the surface hydrophobicity of chalcopyrite and pyrite,thereby showing good floatability.
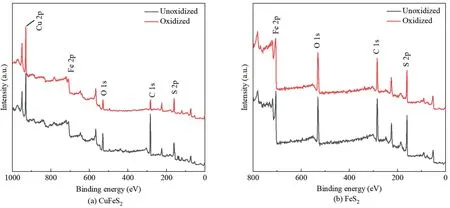
Fig.10.XPS spectra of minerals surfaces.
Table 4 showed the elemental concentration of both minerals under different conditions.After oxidizing for 3 d,the increased Cu,S concentrations were accompanied by reduced O and Fe concentrations,mainly attributing to the dissolution of oxidized ferric products on chalcopyrite surface [28].Specifically,the Cu/Fe ratio increased from 1.1 to 1.5 at 3 d,indicating that the Fe-deficiency layer was formed on chalcopyrite.As reported in Harmer et al[29],Fe was dissolved preferentially as compared to Cu,thereby forming the iron-deficiency layer.
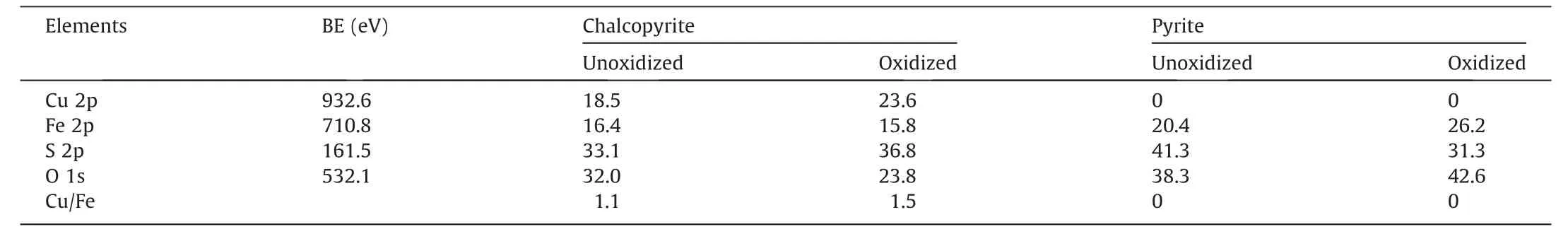
Table 4 Elemental concentration (%) on chalcopyrite and pyrite surfaces.
As noted,oxidizing for 3 d significantly increased pyrite surface O concentration to 42.6%,but decreased the S concentration from 41.3% to 31.3% remarkably (Table 4),confirming the increase in surface oxidation.Therefore,seawater oxidation pretreatment mainly facilitated the oxidation of pyrite surface.In addition,the reduced S concentration was probably due to the decrease of S species covered on pyrite surface.In summary,seawater pretreatment oxidized pyrite surface apparently,but not for chalcopyrite,beneficial to the flotation separation of these two minerals.The pH of seawater solution is normally 7.7-7.8,which has good buffer capability in neutralizing the H+produced by the oxidation of chalcopyrite and pyrite.In addition,the amount of the SO42-released due to chalcopyrite and pyrite oxidation is negligible as compared to the seawater possessing 2688 ppm SO42-(Table 2).Therefore,the dissolved SO42-during flotation process was not considered in this study.
3.6.2.High-resolution XPS spectra of chalcopyrite
The relative contents of Cu,Fe,S and O components were calculated according to the fitted peaks (Fig.11 and Table 5).Usually,the Cu2+structure presented a strong satellite at 7-10 eV above the main peak [30].Fig.11a showed only a wide but strong peak at 932-932 eV,corresponding to the structural Cu+in chalcopyrite.These indicated that the oxidation states of Cu and Fe in chalcopyrite were +1 and +3,respectively.
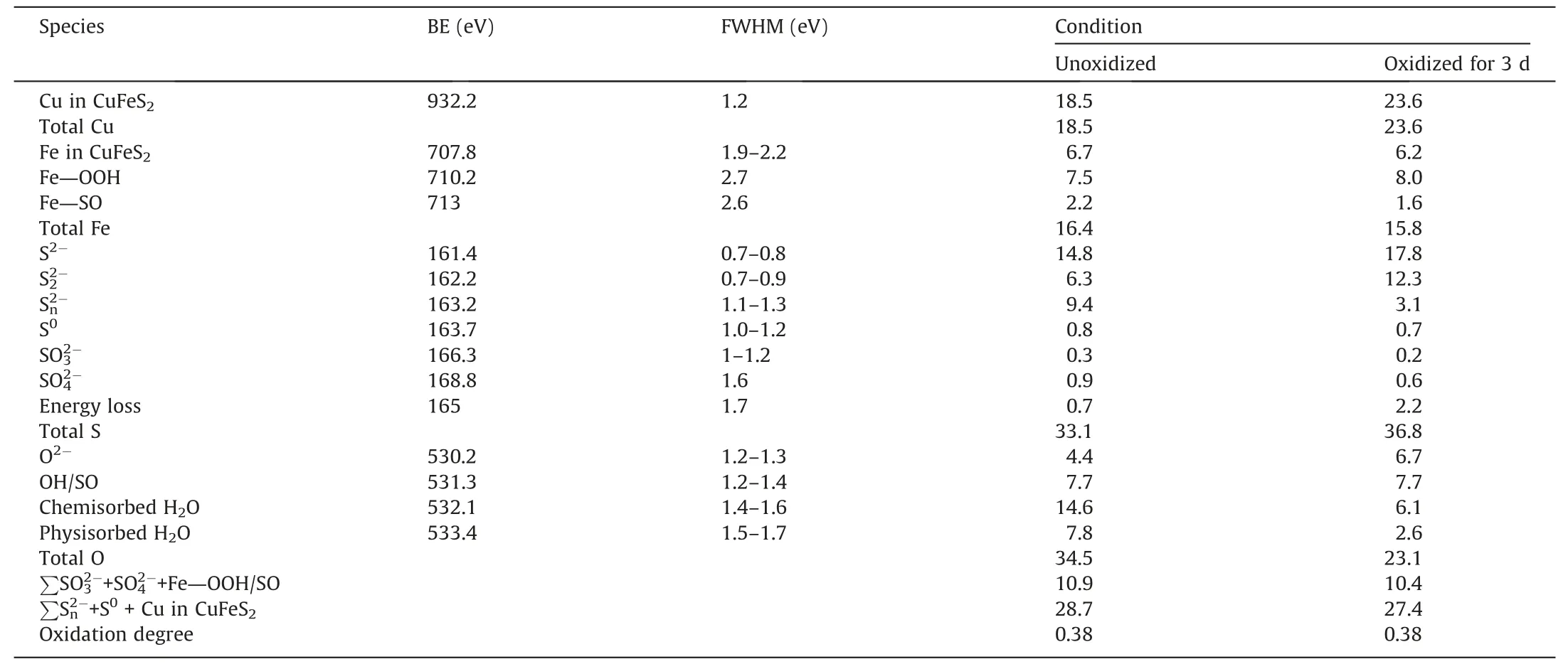
Table 5 Cu,Fe,S and O concentration of unoxidized and oxidized chalcopyrite surface.
Fig.11b showed that the peaks at about 707.8,710.2 and 713 eV corresponded to chalcopyrite (CuFeS2),ferric oxyhydroxides(Fe—OOH) and ferric oxysulfide (Fe—SO) species,respectively.Fig.11c showed seven S species on chalcopyrite surface,e.g.,monosulfide (S2-,161.4 eV),disulfide (S2-,162.2 eV),polysulfide(,163.2 eV),sulfur(S0,163.7 eV),sulfite(,166.3 eV),sulfate(,168.8 eV) and the S 3p →Fe 3d energy loss,165 eV) [28].Fig.11d showed four O peaks on chalcopyrite surface at around 530.2 (O2-),531.3 (OH-/),532.1 (chemisorbed H2O),and 533.4 eV(physiosorbed H2O),where hydroxide and sulfate species were assessed at (530.9±0.15) eV due to their overlap role [31].
Table 5 further showed that the Fe—OOH species on oxidized chalcopyrite surface slightly increased from 7.5% to 8.0%,mainly due to the slight increase of Fe oxidation,while the Fe—SO content decreased from 2.2% to 1.6%,mainly attributing to the dissolution of some Fe2(SO4)3and Fe2(SO3)3species from chalcopyrite surface during seawater pretreatment process[32].It is worth noting that the Fe oxidation species (Fe—OOH and Fe—SO) formed on unoxidized chalcopyrite surface,probably due to higher chemical reactivity of Fe as compared to Cu [33].These also indicated that chalcopyrite oxidation occurred even before seawater pretreatment,likely due to the slight oxidation during grinding process.
Table 5 showed that the concentrations of S0,andon chalcopyrite surface were 0.8%,0.3% and 0.9% for the unoxidized sample,respectively.The presence of these low contents of S oxidation species further confirmed the occurrence of surface oxidation [34].After chalcopyrite oxidation for 3 d,these three S species decreased from 2.0% to 1.5%,suggesting the dissolution of the oxidized S species due to seawater oxidation [7].In addition,the O2-increased from 4.4% to 6.7%,due likely to the increase of Fe oxides (e.g.,FeOOH),indicating more oxidation occurred after seawater pretreatment.
The oxidation degree of mineral surface was further evaluated by calculating the proportion of formed hydrophilic species and hydrophobic species,as shown in Table 5.The higher ratio of hydrophilic substances and hydrophobic substances corresponded to a higher oxidation degree.The hydrophobic species included metal polysulfide,elemental sulfur and metal xanthate while the hydrophilic species included oxides and oxyhydroxides,sulfite and sulfate that generated on mineral surface.Prior to seawater pretreatment,the oxidation degree of chalcopyrite was 0.38,which did not change when chalcopyrite oxidized for 3 d,thereby slightly affecting chalcopyrite flotation.In other words,only slight oxidation occurred on chalcopyrite surface during seawater oxidation process,which influenced chalcopyrite recovery insignificantly(Fig.4).
3.6.3.High-resolution XPS spectra of pyrite
Fig.12 showed the high-resolution XPS spectra of Fe 2p,S 2p and O 1s on pyrite surface.Various peaks appeared on Fe 2p spectrum,including Fe(ΙΙ)—S,Fe(III)—S,Fe(III)—O,Fe(III)—OOH and Fe(III)—SO4peaks at 706.5-707.9,708.6-710.7,710.9,711.5 and 713.2 eV,respectively.Interestingly,the presence of Fe(III)—S located at 708.8 and 710.2 eV may result from the oxidation of Fe2+to Fe3+.The S and O species on pyrite surface were consistent to those on chalcopyrite surface.Although a high content of O content was present on the unoxidized pyrite surface,pyrite still showed a good floatability (Fig.4) as moderated surface oxidation enhanced the adsorption of collectors,resulting in a hydrophobic surface.
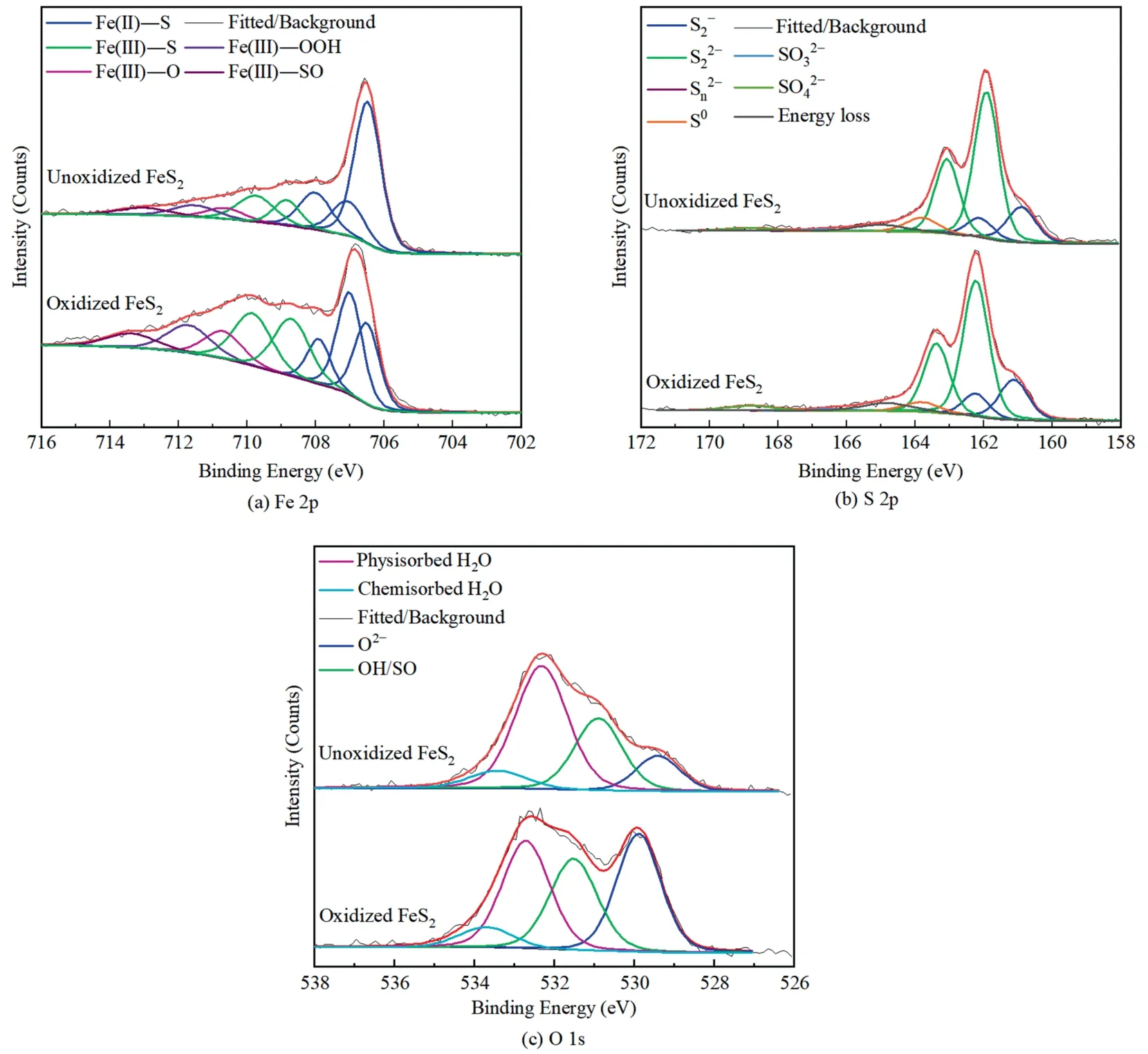
Fig.12.XPS spectra of unoxidized and oxidized pyrite.
The increase in Fe(III)—O and Fe(III)—OOH after oxidation indicated significant oxidation on pyrite surface,thereby decreasing its hydrophobicity and inhibiting the adsorption of xanthate [25].When pyrite was unoxidized,from the bulk pyrite dominated the S components,with the appearance of S0,,and.After oxidizing for 3 d,the concentration ofand S0decreased from 28.1%and 2.5%to 20.4%and 1.3%,respectively(Table 6).However,theincreased from 0.6%to 0.9%,indicating that the increased Fe(III)—SO species was Fe2(SO4)3.
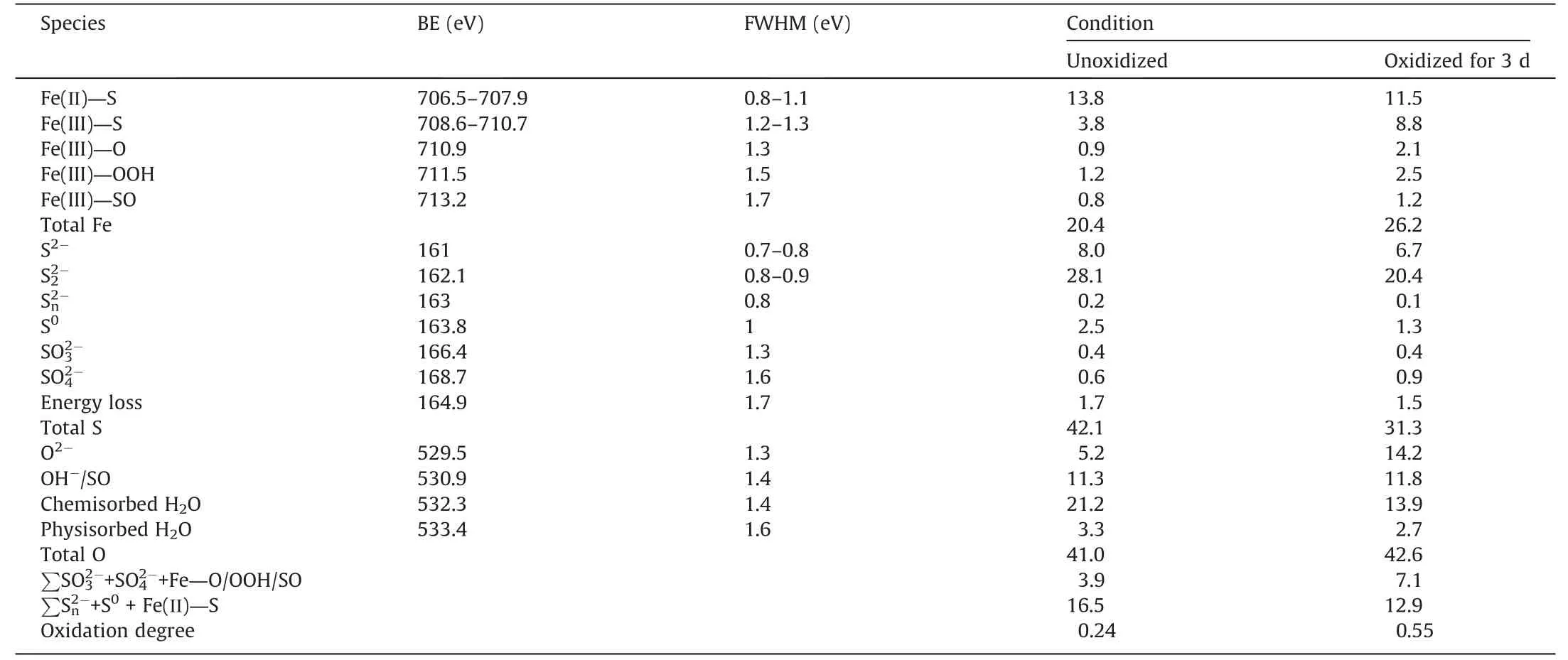
Table 6 Fe,S and O concentration (%) on pyrite surfaces.
In addition,the O2-and OH-/contents on pyrite surface increased significantly from 5.2% and 11.3% (unoxidized) to 12.2%and 11.8% (oxidized for 3 d),indicating more oxidation.As indicated in Section 3.5,the SBX was adsorbed at Fe sites on pyrite surface to improve the floatability of pyrite.However,the generation of abundant hydrophilic oxidation species on oxidized pyrite surface prevented the adsorption of SBX,further resulting in a low flotation recovery [34].Moreover,the oxidized pyrite showed a higher oxidation degree compared to the unoxidized particle(0.55 vs.0.24).In conclusion,seawater oxidation enhanced surface oxidation on pyrite than chalcopyrite,thereby selectively separating chalcopyrite from pyrite flotation.
3.7.Depressing mechanisms
Seawater contained various ions with relatively high concentration,including Ca2+,Mg2+and,which can act as activators or depressants for different minerals[7,35,36].In this study,the synergistic effect of seawater ions plays a dominant role to selectively depress pyrite from chalcopyrite flotation.The metallic ions (i.e.,Ca2+) presented in seawater have beneficial effects on flotation by depressing pyrite and improving the selectivity separation of Cu—S [35],where Ca2+and formed Ca complex have more affinity at the Fe site of pyrite than that of chalcopyrite [37],further confirming lower recovery of pyrite as compared to chalcopyrite.However,the difference between the recoveries of these two minerals was insufficient to separate them.Therefore,seawater oxidation pretreatment was applied to further increase the efficiency of selective separation.
When exposed to aqueous environment,the higher Fe(II)activity on pyrite surface was reported as compared to the Fe(III) activity on chalcopyrite surface due to different atomic structures [38],indicating more oxidation on pyrite surface than on chalcopyrite surface.These also indicated that the oxidized Fe species on the oxidized pyrite surface were mainly Fe2O3/FeOOH,which exhibited a greater affinity to H2O,compared with the fresh pyrite surface [25],thereby inhibiting the adsorption of xanthate on the oxidized pyrite.However,chalcopyrite surface only experienced slight oxidation during seawater oxidation,which influenced chalcopyrite recovery insignificantly,as per the schematic diagram in Fig.13.
Based on the coordination chemistry theory,the 5 d orbitals of coordinated metal ions on minerals surface played a vital role on the interaction of xanthate with these metal ions [25].As shown in Fig.14,the d orbitals of both Cu and Fe ions on chalcopyrite and pyrite surfaces are splitted into egorbitals (σ orbitals) and t2gorbitals (π orbitals) [39].Fig.14a shows that the Fe(II) in FeS2is a d6low spin ion due to three π electrons pairs in t2gorbitals and unoccupied orbitals in egorbitals.But the Fe(III) species(formed Fe2O3and FeOOH) in oxidized pyrite surface is a d5high spin ion due to unpaired electrons in each orbital[39].As for xanthate molecule,there are vacant orbitals in 3d orbitals and lone electrons pairs in S 3p orbitals,thereby coordinating with the vacant orbital from Fe 3d orbitals of pyrite [25].
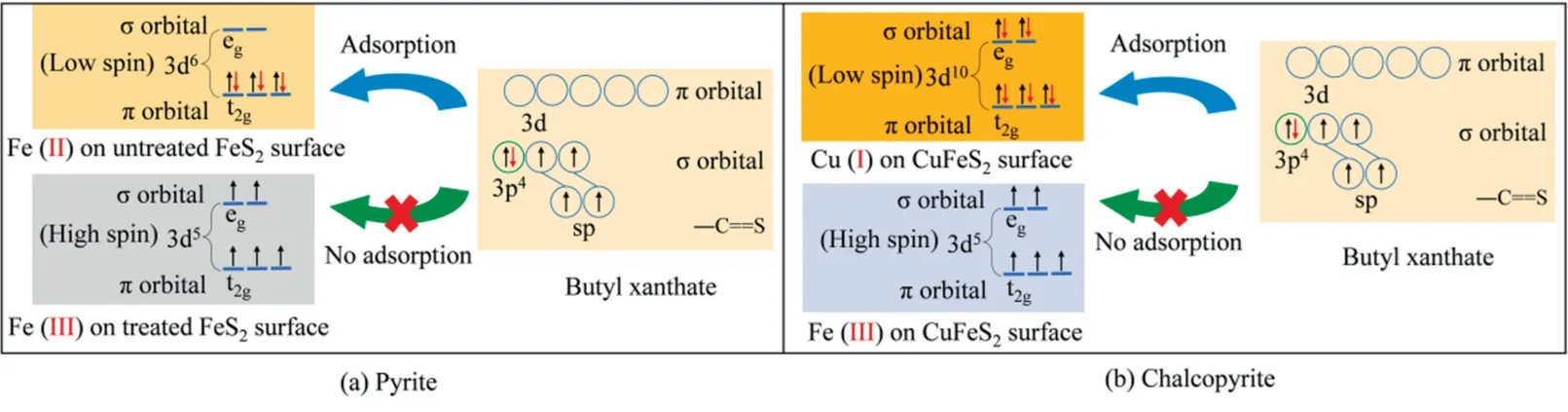
Fig.14.Coordination model of xanthate with metal ions on pyrite and chalcopyrite surfaces.
When the S atoms of xanthate coordinate with Fe(II) on pyrite,the σ bond and stronger back-bonding π bond are formed via the combination of 1 electron pair of xanthate with an egvacant orbital of Fe atom and 3 electron pairs in t2gorbitals of Fe atom with xanthate S 3d vacant orbital,respectively [25].Thus,Fe xanthate is successfully generated on the untreated pyrite surface.However,the formed FeOOH and Fe2O3cover on pyrite surface due to seawater oxidation.As neither electron pairs nor vacant orbitals are present in the d5orbital of high spin Fe(III),this type of electron configurations cannot form the σ bond or back-bonding π bond,thereby failing in forming Fe xanthate.Therefore,xanthate was not adsorbed on the oxidized pyrite surface.
Fig.14b shows the formation of Cu-xanthate on chalcopyrite surface.Cu(I) owing three electron pairs in t2gorbitals and 2 electron pairs in egorbitals presents a low spin of 3d10state.According to the discussion above,strong back-bonding π bond can be formed via coordinating the unoccupied π orbitals of xanthate and 5 π electron pairs of chalcopyrite,thereby improving chalcopyrite floatability.It should be noted that seawater oxidation treatment did not influence the Cu(I) state on chalcopyrite surface.Therefore,a good floatability remained even after oxidizing for 3 d.
4.Conclusions
(1) The micro-flotation results of single minerals indicated that seawater oxidation significantly improved the difference in flotation recovery between chalcopyrite and pyrite.87.0%recovery was obtained for chalcopyrite while 17.3%recovery was obtained for pyrite when seawater oxidation for 3 d.Furthermore,the micro-flotation results of the mixed minerals showed that 3 d oxidation pretreatment had excellent oxidation ability in selective separation of pyrite against chalcopyrite,with a recovery difference greater than 70%and a selectivity index greater than 6.
(2) Seawater oxidation seriously oxidized pyrite,generating abundant hydrophilic Fe(III) oxidation species (Fe2O3and FeOOH)on pyrite surface,reducing the contact angle of pyrite apparently,i.e.,from 61° to 28°.However,seawater pretreatment only slightly oxidized chalcopyrite,which did not affect the surface hydrophobicity and flotation recovery of chalcopyrite,with a high contact angle of 80°-91°.
(3) CV curve,UV-visible absorption spectra and FTIR crossconfirmed a difficult adsorption of xanthate on the oxidized pyrite,thereby lowing pyrite recovery.But xanthate was easily adsorbed on seawater-pretreated chalcopyrite surface.Therefore,chalcopyrite showed an excellent flotation recovery.
(4) The coordination chemistry theory further confirmed that the Fe(III) d5orbital (neither electron pairs nor vacant orbitals)exposed on oxidized pyrite surface can coordinate with the S atoms of xanthate.The Cu(I)3d10state with 5 electron pairs on oxidized chalcopyrite surface can coordinate with the S atoms of xanthate.Therefore,seawater pretreatment can be applied to depress pyrite flotation,thereby achieving a selective separation of chalcopyrite from pyrite.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.51974215).
- 矿业科学技术学报的其它文章
- Shear behaviours and roughness degeneration based on a quantified rock joint surface description
- Numerical modeling on strain energy evolution in rock system interaction with energy-absorbing prop and rock bolt
- Effect of particle gradation on pore structure and seepage law of solution in weathered crust elution-deposited rare earth ores
- Experimental study on instability mechanism and critical intensity of rainfall of high-steep rock slopes under unsaturated conditions
- Polysaccharides-based pyrite depressants for green flotation separation:An overview
- Mechanical properties and damage characteristics of solidified body-coal combination in continuous driving and gangue backfilling

