CO2 gas stripped off membranous residual oil from pore surfaces: Effects of temperature,pressure and wettability
Tao Yu,Haixiang Hu,*,Qi Li,Yongsheng Tan,Liang Xu,Xiaomin Cao
a State Key Laboratory of Geomechanics and Geotechnical Engineering,Institute of Rock and Soil Mechanics,Chinese Academy of Sciences,Wuhan,430071,China
b University of Chinese Academy of Sciences,Beijing,100049,China
Keywords:Molecular dynamics simulation CO2 flooding Wettability Interaction energy Residual oil CO2 utilization CO2 capture utilization and storage (CCUS)
ABSTRACT The threshold values of CO2 gas stripped off membranous residual oil from the pore walls are not clear under different temperatures,pressures and wettability conditions.The extent to which temperature,pressure and wettability influence CO2 flooding for enhancing the recovery of residual oil in membranous formations also remains uncertain.Therefore,further quantitative characterization is entailed.In this study,the molecular dynamics method was employed to explore CO2 flooding under different temperatures,pressures and wettability conditions,aiming to enhance the production of membranous residual oil.The results reveal that the interaction energy between CO2,decane molecules and pore walls exhibits a decrease with increasing temperature and an increase with increasing pressure,respectively,in distinct wettability scenarios.When the temperature was at or below 363 K and the pressure was not lower than 40 MPa,CO2 gas could detach the membranous residual oil from the pore walls in the waterwet systems.When the temperature was equal to 363 K and the pressure remained under 40 MPa,or the temperature surpassed 363 K,CO2 gas failed to detach the membranous residual oil from the pore walls in the water-wet systems.For the mixed-wet and oil-wet systems,CO2 molecules could not detach the membranous residual oil from the pore walls.The hierarchy of influence regarding temperature,pressure and wettability on the competitive adsorption capacity of CO2 and decane molecules on the pore walls emerged as follows: wettability >temperature >pressure.The findings of this study offer valuable insights into the application of CO2 gas flooding for the exploitation of membranous residual oil on pore walls.
1.Introduction
CO2capture utilization and storage (CCUS) is widely studied to reduce CO2emissions in the atmosphere around the world(Li et al.,2014;Hepburn et al.,2019;Richard et al.,2021;Turan et al.,2021).After long-term exploration and development of oil and gas reservoirs,the structure and reservoir attributes are well understood,and the reservoir space is large.Meanwhile,the upper part of the oil and gas reservoirs has stable cap development.In addition,CO2injection into oil and gas reservoirs for CO2geological storage can fully use of early development knowledge of geologic bodies and existing production wells and other equipment without a large amount of secondary investment.The combination of CCUS technology and enhanced oil recovery (EOR) technology can further reduce the economic cost of CO2geological storage.Therefore,CO2-EOR technology has become a current research hotspot (Kuuskraa et al.,2017).With the exploration and development of oil and gas reservoirs shifting from shallow to deep layers,the development proportion of tight sandstone reservoirs is increasing.Micro-and nanopore throats are widely developed in tight sandstone reservoirs,and conventional water injection development faces many difficulties.However,CO2gas can be miscible with crude oil under reservoir conditions,increasing the flow capacity of crude oil,and the diffusion capacity of CO2is higher than that of the water phase(Zhou et al.,2020;Ge et al.,2022).Due to the apparent viscous fingering and crossflow during water flooding,the volume sweep efficiency of water flooding was low.However,after CO2was injected into the reservoir,not only could it diffuse and migrate in the regions not affected by water flooding,but it could also be miscible with crude oil,effectively suppressing the fingering phenomenon and resulting in a more uniform displacement front(Zhao et al.,2020).Therefore,CO2flooding development in tight sandstone reservoirs has more advantages than water flooding(Lin et al.,2016).
Due to the development of micro-and nanopore throats in tight reservoirs,the interface effect between fluids and pore walls is pronounced,causing lots of membranous residual oil retention on the pore walls during the development of tight sandstone reservoirs(Feali et al.,2012;Kuvshinov et al.,2018).Currently,chemical flooding is the main way to tap the potential of membranous residual oil.The recovery degree of membranous residual oil can be improved using surfactants to decrease the interfacial tension between fluids and reduce the oil contact area on the pore surfaces(Cao,2021;Li et al.,2021;Tan et al.,2022).At the same time,Fan et al.(2021) and Han et al.(2021) explored the flooding mechanism of the membranous residual oil through numerical simulation and believed that increasing the horizontal stress difference of the membranous residual oil can improve the flow ability of oil films on the pore walls.However,macromolecular polymers are generally added in the process of chemical flooding.Tight reservoirs with more micro-and nanopore throats can result in very high injection pressure and high flow resistance in pore throats.Therefore,chemical flooding suitable for conventional reservoirs is not suitable for tight reservoirs(Xiong et al.,2020).Because CO2gas can be miscible with crude oil,it has strong flow and diffusion capacity in the reservoir,improves the flow capacity of crude oil,and can effectively improve the displacement efficiency of small pores.Compared with chemical flooding,CO2flooding has more advantages in tapping the potential of residual oil in tight reservoirs(Makimura et al.,2013;Lashkarbolooki et al.,2019;Drexler et al.,2020).Currently,there are many studies on the mechanism of CO2-EOR.The research results of Cao et al.(2013) showed that the interfacial tension between CO2and oil decreased with the increase in pressure.With the increase of temperature,the interfacial tension of CO2oil increases,and the minimum miscibility pressure of CO2oil increases.Li et al.(2022) studied the CO2flooding of cores with different apertures under different pressures through NMR technology.The obtained experimental results indicated that the crude oil in the large pores was easily displaced,and the residual oil mainly accumulated in the small pores.The displacement efficiency increased with increasing pressure.The final oil recovery was determined by the displacement efficiency of small pores.Bikkina et al.(2016) revealed that wettability had a significant impact on the CO2displacement effect through conventional core displacement experiments.Zeng et al.(2018) studied the influence of wettability on the oil recovery of CO2flooding using NMR technology,and the obtained results found that the oil recovery of the water-wet cores was the highest,followed by mixed-wet cores,and oil-wet cores had the lowest oil recovery.Hemmati-Sarapardeh et al.(2013) explored the effect of different crude oil components on the minimum miscible pressure of CO2and crude oil and found that the minimum miscible pressure of CO2and crude oil increased with the proportion of heavy components increasing in crude oil.In summary,the main factors affecting the CO2displacement effect were temperature (Cao et al.,2013),pressure (Cao et al.,2013;Li et al.,2022),wettability (Bikkina et al.,2016;Zeng et al.,2018),pore diameter(Li et al.,2022)and crude oil composition(Hemmati-Sarapardeh et al.,2013).However,there are relatively few research results on the impact of these factors on CO2gas stripping off oil films from pore walls.Fang et al.(2019a) used the molecular dynamics (MD) method to probe the mechanism of CO2flooding at 323 K and 20 MPa,and found that the displacement effect of CO2gas was perfect,and the membranous residual oil on the pore surfaces could be completely peeled off.In another study,Fang et al.(2017) used the MD method to reveal the mechanism of CO2gas stripping off the membranous residual oil from pore surfaces at different temperatures under 20 MPa.The obtained results showed that when the temperatures were 303 K,323 K and 343 K,CO2molecules could better strip off the membranous residual oil from the pore walls;while when the temperatures were 363 K and 383 K,the oil films could not be completely stripped,and more oil molecules were still adsorbed on the pore surfaces.Yu et al.(2022b)explored the influence of different wettability on CO2gas peeling off oil films from pore walls at 323 K and 20 MPa through the MD method.The obtained results showed that the membranous residual oil on the hydrophilic pore walls could be peeled off by CO2gas,but the membranous residual oil on the lipophilic pore walls could not be peeled off by CO2gas.According to the research results of CO2flooding for the membranous residual oil on pore walls,the potential of membranous residual oil under different temperature,pressure and wettability conditions needs to be further understood,and the threshold value of temperature,pressure and wettability affecting the CO2gas stripping off the oil films from the pore walls is not clear.Meanwhile,the influence degree of temperature,pressure and wettability on CO2flooding to enhance the recovery of membranous residual oil is also unclear,and needs further quantitative characterization.
The process of CO2flooding to enhance the production capacity of membranous residual oil is mainly the interface behavior between CO2molecules and oil films on pore walls in tight sandstone reservoirs,while traditional experimental methods and numerical simulation methods cannot directly quantitatively evaluate the interface behavior between fluids and pore walls.Therefore,this study explored CO2flooding to improve the production capacity of membranous residual oil on pore walls under different temperatures,pressures and wettability through the MD method.Because the membranous residual oil in the pore channels is mainly adsorbed on the pore walls,the influence of aperture was not considered in this study.At the same time,considering the complexity of crude oil components and the computing power,this study simplified the crude oil components.First,the changes in the relationship between the strength of the interface effect and temperature,pressure and wettability were characterized by the interaction energy between fluids and pore surfaces(Einteraction(CO2-SiO2)andEinteraction(decane -SiO2)).Second,based on the density distribution(DD)of CO2and decane molecules on the pore walls,the changes in the strength of CO2and decane molecules interaction with the pore walls under different temperatures,pressures and wettability were revealed.Finally,the competitive adsorption capacity of CO2and decane molecules on the pore walls was quantitatively evaluated by the difference in theEinteraction(CO2-SiO2)andEinteraction(decane -SiO2),and the temperature,pressure and wettability threshold values of the membranous residual oil stripped by CO2gas from the pore walls were determined.Meanwhile,the influence degree of temperature,pressure and wettability on the membranous residual oil stripped from pore walls by CO2gas was quantitatively evaluated.Based on the simulation results,the threshold values of CO2gas stripping off the membranous residual oil from the pore walls under different temperatures,pressures and wettability were clarified,which could provide a reference basis for CO2gas flooding to improve the production capacity of membranous residual oil on pore walls.
2.Models and methods
2.1.Model construction
To illustrate the productive potential of membranous residual oil during CO2flooding in the tight sandstone reservoirs,and clarify the effect of temperature,pore pressure and wettability on the competitive adsorption of CO2and oil molecules on the pore walls,CO2/SiO2and decane/SiO2systems with different temperatures,pressures and wettability were established.First,the CO2and decane systems with different temperatures,pressures and wettability were established by the Amorphous cell module of Materials Studio software;Then,the quartz surface models were constructed in the Build module by using the quartz crystal model that came with the software;Finally,the CO2and decane components were assembled with the quartz surface models in the Build module to construct the CO2/SiO2and decane/SiO2systems,and all the systems were periodic inX,YandZdirections.Many research results showed that reservoir wettability included water-wet,mixed-wet and oil-wet.Therefore,this study considered the three wettability as influencing factors(Robin et al.,1995;Grattoni et al.,2003;Yadali Jamaloei et al.,2011).In this study,the ranges of temperatures and pressures were selected to cover tight sandstone reservoirs as far as possible.Therefore,the temperature systems include 303,323,343,363 and 383 K,and the pore pressure systems include 20,30,40,50 and 60 MPa.Fig.1 shows the initial conformation diagram of the interaction between the fluids and pore walls.The number of CO2(Table A.1 in Appendix A) and decane (Table A.2 in Appendix A) molecules was converted from the temperature-pressure-density curve according to the NIST database.The size of the CO2gas and oil films was approximately 85 Å × 24.5 Å × 30 Å (Fig.A1 in Appendix A) (Fang et al.,2019b).Decane (Liu et al.,2016;Fang et al.,2019c;Moh et al.,2022) represents crude oil and quartz crystal (Chiquet et al.,2007;Iglauer et al.,2015;Ahmadi et al.,2022) represents sandstone in each system.The quartz surfaces were hydroxylated and methylated,respectively,to represent the water-wet and oil-wet pore walls,while the mix-wet surfaces were represented by quartz surfaces with uniform distribution of hydroxyl and methyl groups(Hautman et al.,1991;Yu et al.,2022c).The size of the pore walls was approximately 85 Å × 24.5 Å × 17.5 Å.An invisible rigid graphene barrier was placed over the CO2and decane components to prevent CO2and decane molecules from escaping into the vacuum layer and maintain the volume of CO2and decane components.Meanwhile,a 25 Å vacuum layer was constructed above the graphene barrier.
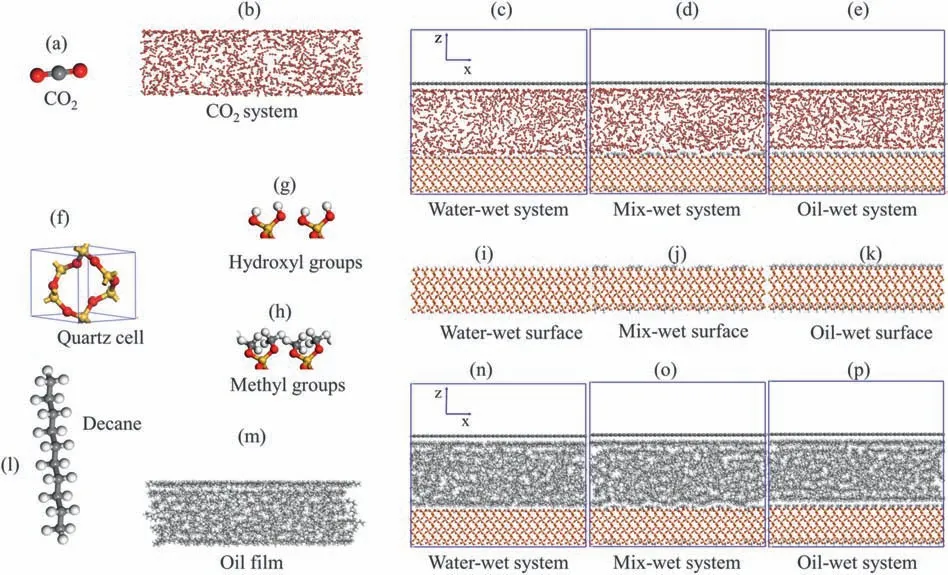
Fig.1.The initial conformation diagram of the interaction between the CO2 and decane molecules and pore walls:(a)CO2 molecule,(b)CO2 system,(c)CO2/SiO2 water-wet system,(d) CO2/SiO2 mixed-wet system,(e) CO2/SiO2 oil-wet system,(f) Quartz cell,(g) Hydroxyl groups,(h) Carboxyl groups,(i) Water-wet surface,(j) Mixed-wet surface,(k) Oil-wet surface,(l) Decane molecule,(m) Oil films,(n) Decane/SiO2 water-wet system,(o) Decane/SiO2 mixed-wet system,and (p) Decane/SiO2 oil-wet system.The colors of each atom in the figure are as follows: gray indicates C,red indicates O,white indicates H,and yellow indicates Si.
2.2.Simulation process
First,2 ns equilibrium molecular dynamics simulations were performed at different temperatures and pressures for the CO2and decane systems under the NVT ensemble to obtain reasonable equilibrium conformations through the Forcite module,respectively.Taking CO2and decane systems at 303 K as an example(Fig.A2 in Appendix A),the systems with different pressures reached equilibrium after 1 ns,and the pressures of all the systems fluctuated above and below the specified pressures with small fluctuations.Therefore,in this study,the conformation of CO2and decane systems under the specified pressure was selected from the equilibrium conformation of the latter 1 ns to study the adsorption energy.Then,the selected conformation was placed on the quartz surfaces through the Build module,and built an invisible rigid graphene baffle above the CO2and decane systems (Fig.A3 in Appendix A).Finally,the CO2/SiO2and decane/SiO2systems under the NVT ensemble were performed 2 ns equilibrium molecular dynamics simulations to calculate the adsorption energy.Statistical analysis was conducted through the last 1 ns of molecular motion trajectory data in each system(Chang et al.,2018;Yu et al.,2022c).All systems were calculated under the COMPASS force field (Sun,1998;Liu et al.,2015;Wu et al.,2016).The temperature of all systems was constrained via the Nose-Hoover function (Evans et al.,1985).The Coulomb and Lennard-Jones 9-6 potential functions were used for electrostatic interaction and van der Waals force (vdWF) calculations,respectively.The time step of the MD calculation was 1 fs,and the truncation radius of all systems was 12.5 Å during the simulation.In the whole simulation process,the atoms on the quartz walls were fixed except for hydroxyl and methyl groups.
3.Results and discussion
3.1.CO2 molecules interaction with pore walls
To evaluate the strength of the interaction between CO2and pore surfaces,the interaction energies of CO2-SiO2in different systems were calculated as follows(Rai et al.,2011;Xu et al.,2013;Yang et al.,2021):
whereEinteraction(M-N)is the interaction energy between components M and N;E(M-N)is the gross energy of the components M and N;E(M)andE(N)are the gross energy of the component M and N,respectively.
Fig.2 exhibits the influence of temperatures and pressures on theEinteraction(CO2-SiO2)under different wettability systems.Fig.2a,b and c exhibits the variations inEinteraction(CO2-SiO2)with different temperatures and pressures in the water-wet,mixed-wet and oil-wet systems,respectively.Regardless of the changes in wettability and pore pressures (Fig.2a-c),Einteraction(CO2-SiO2)decreased with increasing temperature.This occurred because as the temperature increased,which intensified the Brownian motion among CO2molecules and increased the ability to detach from the adsorption layer,decreasing the number of CO2 molecules near the quartz surfaces,and thus reducing theEinteraction(CO2-SiO2).To further reveal this phenomenon,the DD of CO2molecules along the vertical direction of pore walls under different temperatures and pressures in different wettability systems was calculated.Due to the similar distribution characteristics of CO2on the pore walls in different wettability systems,this study focused on the influence of temperatures at 20 MPa and 60 MPa in the water-wet systems on the DD characteristics of CO2molecules on the pore walls as an example for analysis (Fig.3),with the DD characteristics of CO2molecules at other pressures in Fig.A4 in Appendix A.In both low pressure(Fig.3a)or high pressure(Fig.3b)systems,the DD curve of CO2exhibited a clear peak,with the peak value decreasing as temperature increased,indicating that CO2molecules formed an adsorption layer on the pore walls at different temperatures,and the DD of CO2molecules in the adsorption layer decreased with increasing temperature.Therefore,in different wettability and pressure systems,regardless of temperature change,CO2molecules could form an adsorption layer near the quartz surfaces,signifying a strong interaction.As the temperature increased,the number of CO2molecules in the adsorption layer decreased,resulting in a reduction inEinteraction(CO2-SiO2)decreased.Meanwhile,Einteraction(CO2-SiO2)increased with increasing pressure across all wettability of the pore surfaces and temperature conditions(Fig.2a-c),because the collision opportunities between CO2molecules and pore surfaces increased with increasing pressure.Taking the influence of pressures at 303 K and 383 K in the water-wet systems on the DD characteristics of CO2molecules on the pore walls as an example to further reveal the cause of this phenomenon(Fig.4),and the DD characteristics of CO2molecules at other temperatures in the systems were shown in Fig.A5 in Appendix A.Similar to Fig.3,regardless of low or high temperature,the DD curves of CO2molecules also had a clear peak,with the peak value increasing with increasing pressure,indicating that CO2molecules formed an adsorption layer on the pore walls under different pressures,and the DD of CO2molecules in the adsorption layer increased with increasing pressure.Therefore,regardless of pressure changes,CO2molecules could form an adsorption layer near the quartz surfaces in different wettability and temperature systems.As the pressure increased,the number of CO2molecules in the adsorption layer decreased,resulting in an increase inEinteraction(CO2-SiO2).
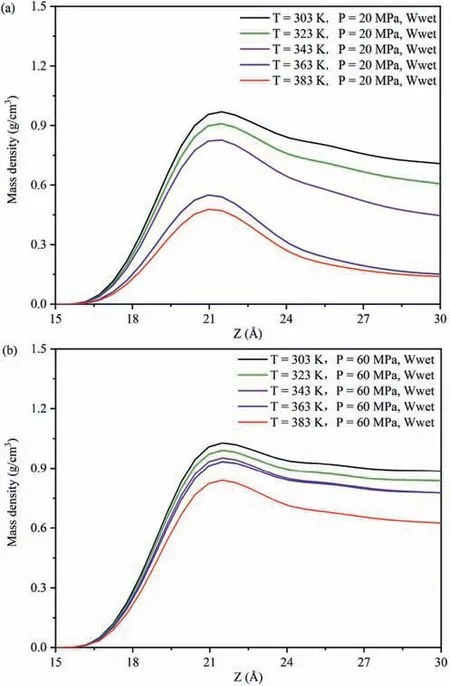
Fig.2.Effect of temperature and pressures on Einteraction(CO2 -SiO2): (a) Water-wet systems,(b) Mixed-wet systems,and (c) Oil-wet systems.
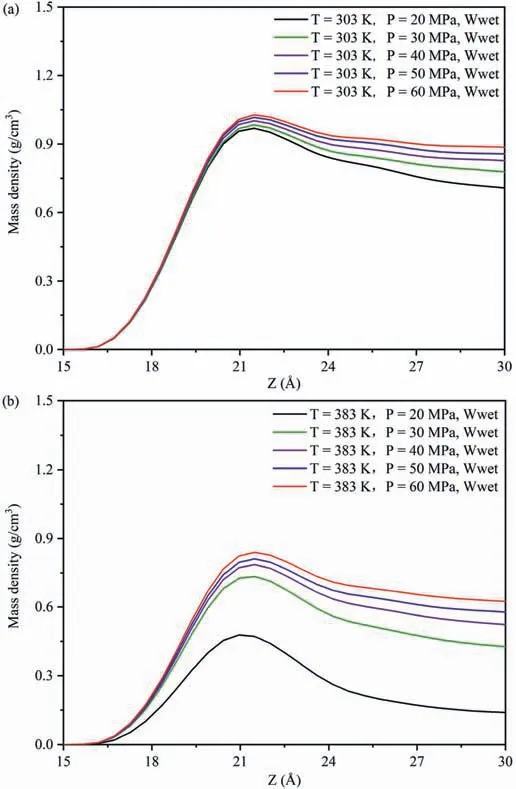
Fig.3.Effect of temperature on the distribution characteristics of CO2 molecules: (a)20 MPa,and (b) 60 MPa.
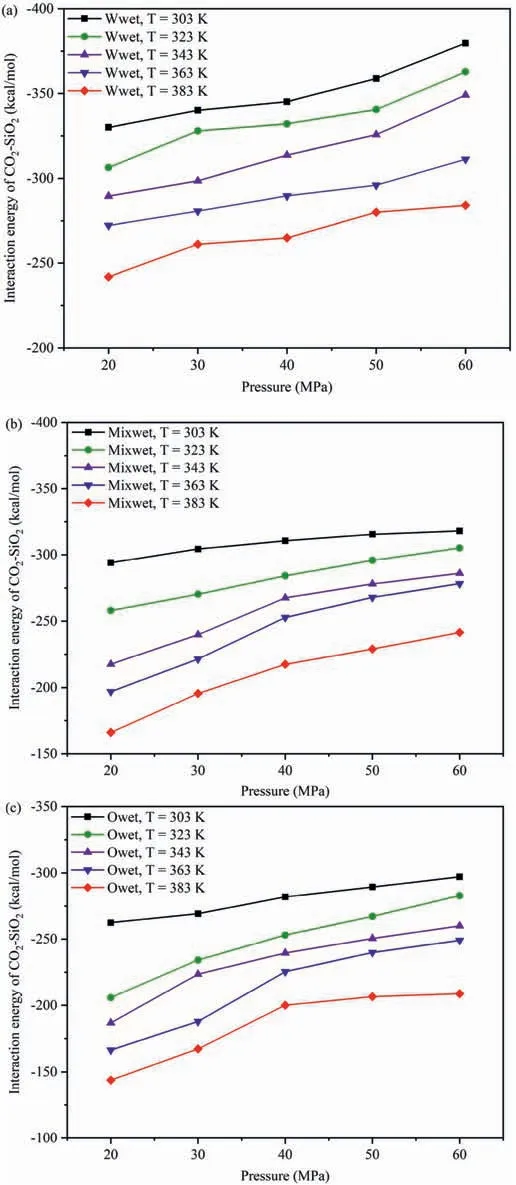
Fig.4.Effect of pressure on the distribution characteristics of CO2 molecules:(a)303 K,and (b) 383 K.
The effect of wettability onEinteraction(CO2-SiO2)under different temperatures and pressures was compared,and the influence of different pressures and wettability onEinteraction(CO2-SiO2)at 303 K and 383 K was analyzed as an example (Fig.5).In different wettability systems,the strength ofEinteraction(CO2-SiO2)followed the order of water-wet system >mixed-wet system >oil-wet system under the same temperature and pressure conditions,aligning with the DD characteristics of CO2molecules in the adsorption layer depicted in Fig.6.Fig.6 presents different wettability effecting on the distribution characteristics of CO2molecules in the adsorption layer on the pore walls.Fig.6a presents the DD curves of CO2on the pore walls of different wettability systems at 20 MPa,303 and 383 K,while Fig.6b presents the DD curves of CO2on the pore walls of different wettability systems at 303 K,20 and 60 MPa.At 20 MPa(Fig.6a),regardless of whether the temperature is high or low,the DD of CO2molecules in the adsorption layer on the pore surfaces followed the order: water-wet system >mixed-wet system >oilwet system.In water-wet systems,as CO2molecules interact with the pore surfaces though hydrogen bonds in addition to vdWF(Yu et al.,2022a,2022c),whereas in oil-wet systems,CO2molecules could not form hydrogen bonds with pore surfaces,and the interaction force of the adsorption layer was only vdWF (Yu et al.,2022b).At 303 K (Fig.6b),regardless of whether the pressure is high or low,the DD of CO2molecules in the adsorption layer on the pore surfaces maintained the same order,consistent with the results in Fig.5.
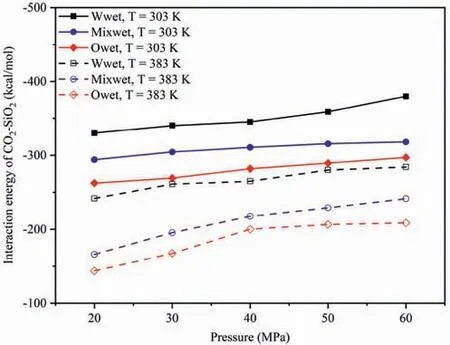
Fig.5.Effect of wettability on the Einteraction(CO2-SiO2)under different temperatures and pressures.
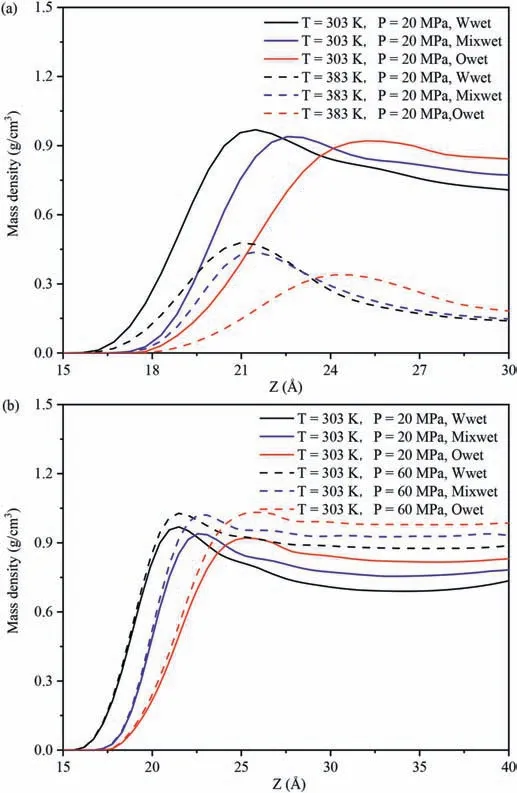
Fig.6.Effect of wettability on the distribution characteristics of CO2 molecules:(a)The wettability effect on the distribution characteristics of CO2 molecules under different temperatures,and (b) The wettability effect on the distribution characteristics of CO2 molecules under different pressures.
The change degree δ of interaction energy was defined to quantitatively evaluate the influence degree of temperature,pressure and wettability onEinteraction(CO2-SiO2).The change degreeδofEinteraction(CO2-SiO2)under various conditions was calculated based on the interaction energy of -330.04 kcal/mol under 303 K and 20 MPa in the water-wet system,as follows:
Tables 1-3 show the change degree δ ofEinteraction(CO2-SiO2)under different temperatures and pressures in the water-wet,mixed-wet and oil-wet systems,respectively.In Table 1,at 303 K,δchanged slowly with increasing pressure.At 20 MPa,δchanged relatively quickly with increasing temperature.Other temperature and pressure conditions in Table 1 show that theδof Einteraction(CO2-SiO2)was also more sensitive to temperature than pressure in the water-wet systems.Tables 2 and 3 also show that theδofEinteraction(CO2-SiO2)was more sensitive to temperature than pressure in the mixed-wet and oil-wet systems.According to the change in δ with pressure under 303 K and different wettability in Tables 1-3,it can be determined that theδofEinteraction(CO2-SiO2)was more sensitive to wettability than pressure.According to the change inδwith temperature under 20 MPa and different wettability in Tables 1-3,it can be determined that theδofEinteraction(CO2-SiO2)was more sensitive to wettability than temperature.In summary,the factors influencing theδofEinteraction(CO2-SiO2)follow the order of wettability >temperature >pressure.
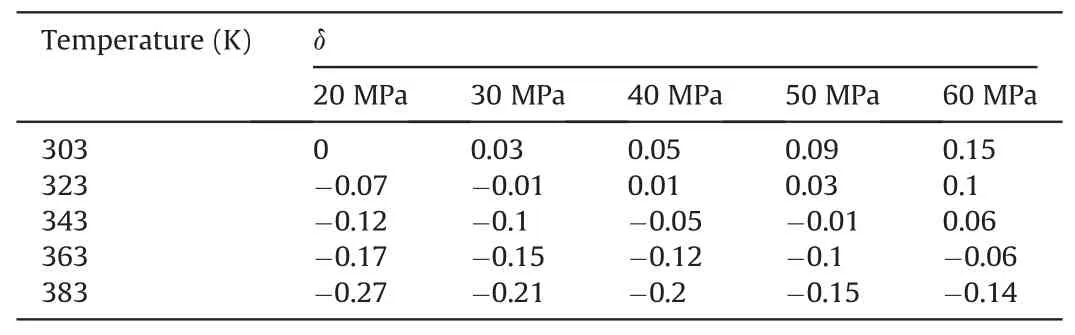
Table 1The change degree δ of Einteraction(CO2-SiO2)under different temperatures and pressures in the water-wet systems.
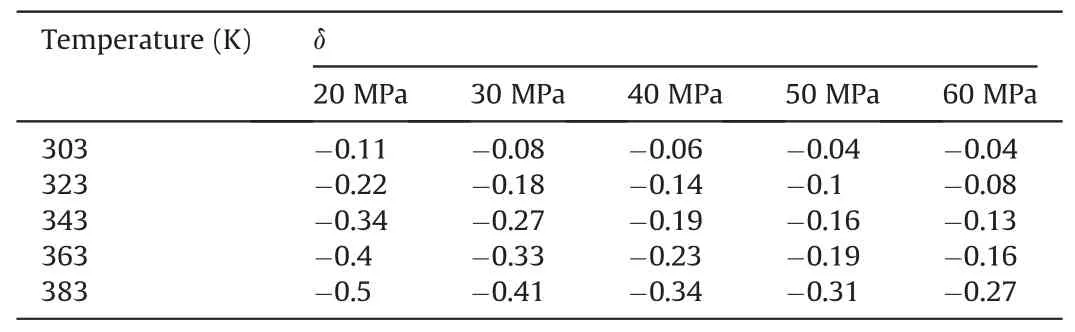
Table 2The change degree δ of Einteraction(CO2-SiO2)under different temperatures and pressures in the mixed-wet systems.
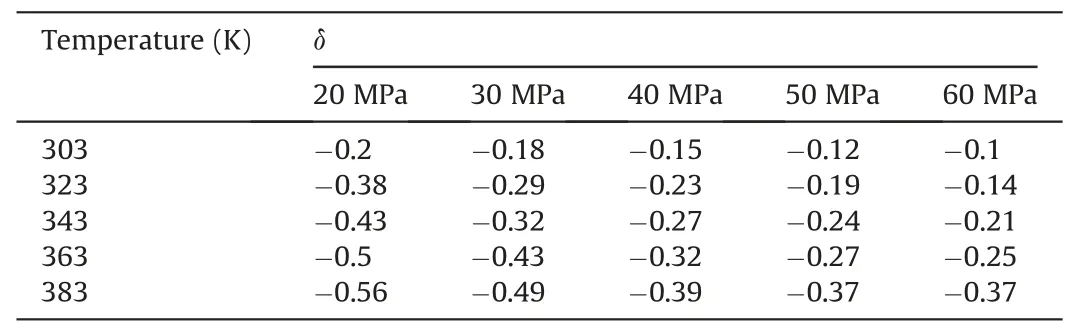
Table 3The change degree δ of Einteraction(CO2-SiO2)under different temperatures and pressures in the oil-wet systems.
3.2.Decane molecules interaction with pore walls
The interaction energies of decane-SiO2(Einteraction(decane -SiO2)) in different systems were used to evaluate the strength of the interaction between decane molecules and pore surfaces.Fig.7 shows the influence of temperatures and pressures on theEinteraction(decane-SiO2)under different wettability systems.Fig.7a,b and c show the changes in the relationship ofEinteraction(decane-SiO2)with varying temperatures and pressures in the water-wet,mixed-wet and oil-wet systems,respectively.Regardless of changes in the wettability of the pore surfaces and pore pressure(Fig.7a-c),Einteraction(decane-SiO2)decreased with increasing temperature,aligning with the variation characteristics of the density of decane molecules at different temperatures in the adsorption layer on the pore walls.Due to the similar distribution characteristics of decane on the pore walls across different wettability systems,this study analyzes the influence of temperatures at 20 MPa and 60 MPa in the water-wet systems on the DD characteristics of decane molecules on the pore walls as an example(Fig.8).The DD characteristics of decane molecules at other pressures in the systems are shown in Fig.A6 in Appendix A.Whether in low pressure(Fig.8a)or high pressure(Fig.8b)systems,the DD curves of decane exhibit a clear peak,and the peak value decreases with increasing temperature(see insert in Fig.8a and b),indicating that decane molecules form an adsorption layer on the pore walls at different temperatures,and the density of decane in the adsorption layer decreases with increasing temperature.The higher the density of the adsorption layer,the greater the number of decane molecules in the adsorption layer,signifying that the quartz surfaces possess a more robust adsorption capacity for decane molecules.Meanwhile,regardless of changes in the wettability of the pore surfaces and temperature changed (Fig.7),Einteraction(decane-SiO2)increased with increasing pressure,which was consistent with the variation characteristics of the density of decane molecules in the adsorption layer with pressures on the pore walls.The influence of pressures at 303 K and 383 K in the water-wet systems on the DD characteristics of decane molecules on the pore walls was taken as an example to further reveal this phenomenon (Fig.9),and the DD characteristics of decane molecules at other temperatures in the systems were shown in Fig.A7 in Appendix A.Similar to Fig.8,whether in low(Fig.9a)or high temperature (Fig.9b) systems,the DD curves of decane molecules also exhibited a clear peak,and the peak value increased with increasing pressure,indicating that decane molecules formed an adsorption layer on the pore walls under different temperatures,and the density of decane molecules in the adsorption layer increased with increasing pressure,resulting in an increase inEinteraction(decane -SiO2).
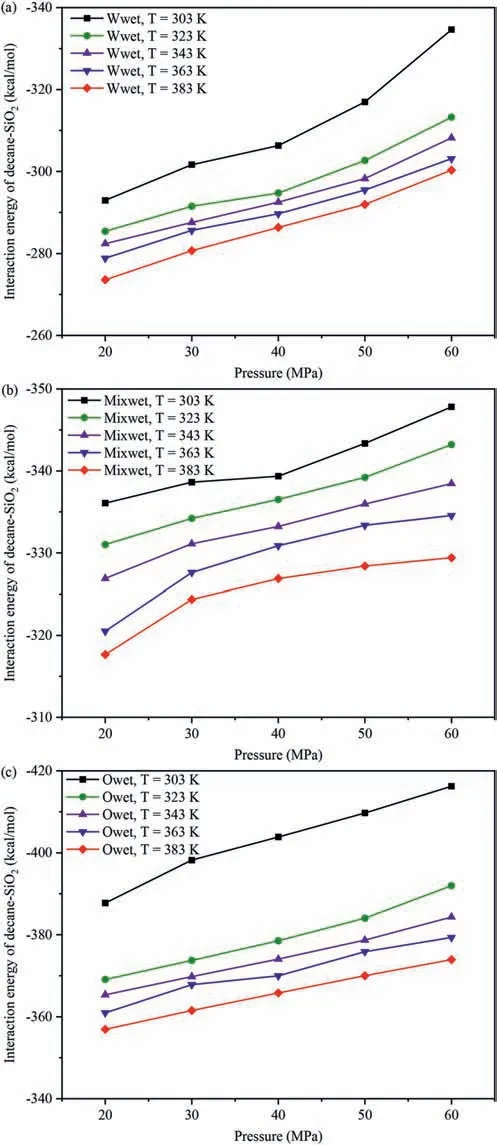
Fig.7.Effect of temperature and pressure on Einteraction(decane -SiO2):(a)Water-wet systems,(b) Mixed-wet systems,and (c) Oil-wet systems.
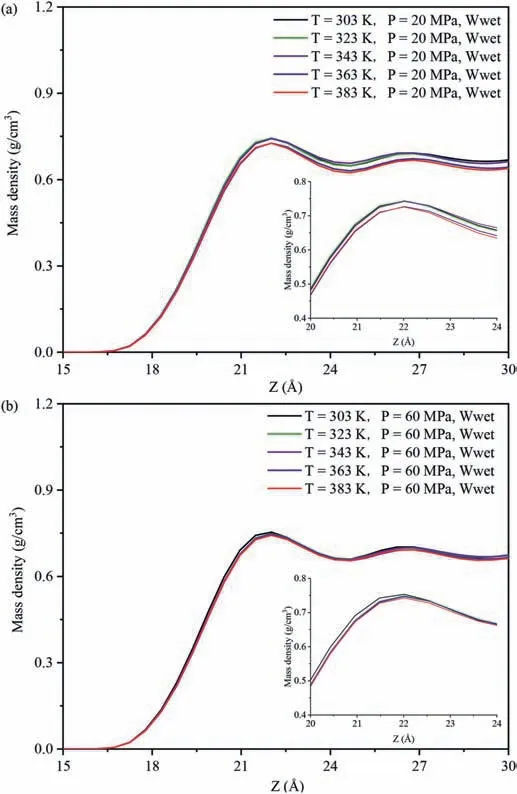
Fig.8.Effect of temperature on the distribution characteristics of decane molecules:(a) 20 MPa,and (b) 60 MPa.
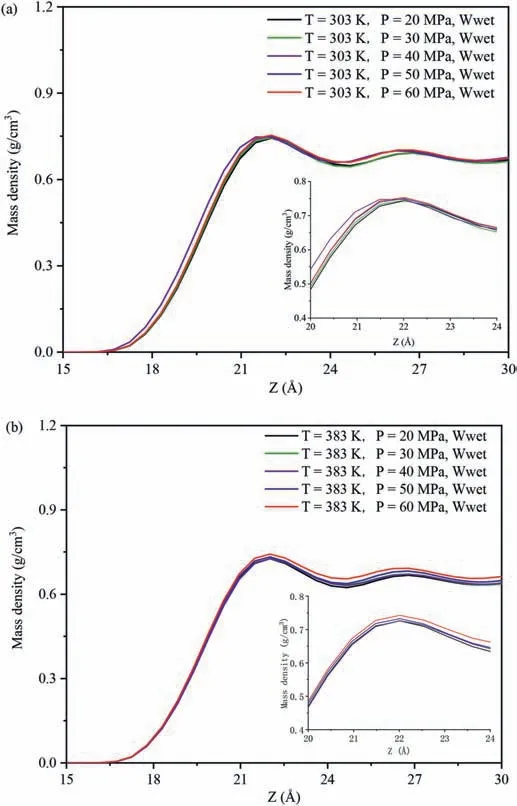
Fig.9.Effect of pressure on the distribution characteristics of decane molecules: (a)303 K,and (b) 383 K.
The effect of wettability onEinteraction(CO2-SiO2)under different temperatures and pressures was compared,and the influence of different pressures and wettability on theEinteraction(decane-SiO2)at 303 K and 383 K was analyzed as an example (Fig.10).In different wettability systems,the strength ofEinteraction(decane-SiO2)followed the order of water-wet system <mixed-wet system <oil-wet system at the same temperature and pressure,which was consistent with the DD characteristics of decane in the adsorption layer in Fig.11.Fig.11 presents the effects of wettability on the distribution characteristics of decane molecules in the adsorption layer on the pore walls.Fig.11a presents the DD curves of decane on the pore walls of different wettability systems at 20 MPa,303 and 383 K,and Fig.11b presents the DD curves of decane on the pore walls of different wettability systems at 303 K,20 and 60 MPa.At 20 MPa(Fig.11a),regardless of whether the temperature was low or high,the DD of decane molecules in the adsorption layer on the pore surfaces followed the order:water-wet system <mixed-wet system <oil-wet system.At 303 K(Fig.11b),regardless of whether the pressure was low or high,the DD of decane molecules in the adsorption layer on the pore surfaces followed the same order.Therefore,under the same pressure and temperature,the density of decane in the adsorption layer was the largest in the oil-wet systems,followed by the mixedwet systems,and was the smallest in the water-wet systems.This can be attributed to the interaction between decane and pore walls,where the vdWF of decane and pore walls was the largest in the oilwet systems,while the vdWF of decane and pore walls was the smallest in the water-wet system(Yu et al.,2022b).
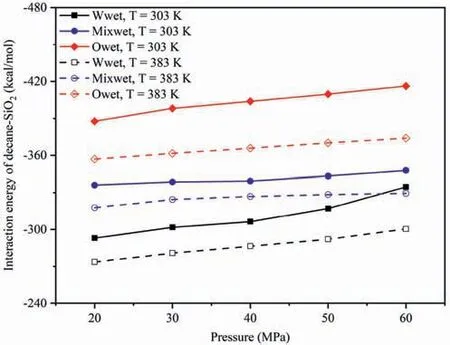
Fig.10.Effect of wettability on the Einteraction(decane-SiO2)under different temperatures and pressures.
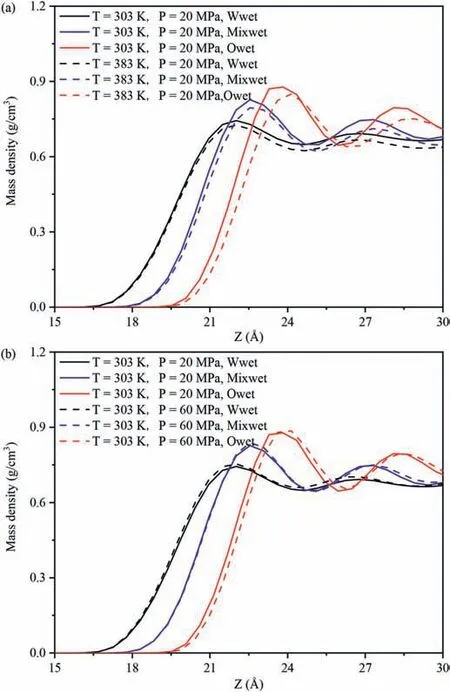
Fig.11.Effect of wettability on the distribution characteristics of decane molecules:(a)The effect of wettability on the distribution characteristics of decane molecules under different temperatures,and (b) The effect of wettability on the distribution characteristics of decane molecules under different pressures.
To quantitatively evaluate the influence degree of temperature,pressure and wettability onEinteraction(decane -SiO2),the change degreeδofEinteraction(decane-SiO2)under other different conditions was calculated based on the interaction energy of -292.95 kcal/mol under 303 K and 20 MPa in the water-wet system as follows:
The calculated results are shown in Tables 4-6.Tables 4-6 show the change degree δ ofEinteraction(decane-SiO2)under different temperatures and pressures in the water-wet,mixed-wet and oilwet systems.In Table 4,at 303 K,δchanged slowly with increasing pressure.At 20 MPa,δchanged more slowly with increasing temperature.Other temperature and pressure conditions in Table 4 show that the δ ofEinteraction(decane-SiO2)was more sensitive to pressure than temperature in the water-wet systems.Tables 5 and 6 also show that theδofEinteraction(decane-SiO2)was more sensitive to pressure than temperature in the mixed-wet and oil-wet systems.According to the change inδwith pressure under 303 K and different wettability conditions in Tables 4-6,it can be determined that theδofEinteraction(decane-SiO2)was more sensitive to wettability than pressure.According to the change ofδwith temperature under 20 MPa and different wettability conditions in Tables 4-6,it can be determined that theδofEinteraction(decane-SiO2)was more sensitive to wettability than temperature.In summary,the influence on theδofEinteraction(CO2-SiO2)was followed the order of wettability >pressure >temperature.
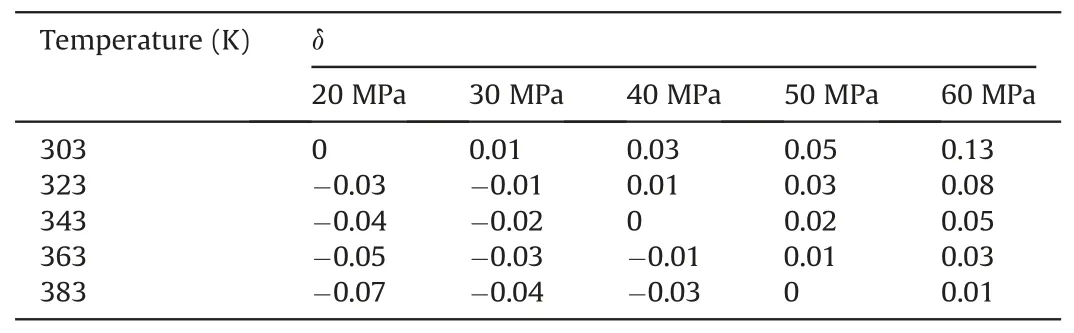
Table 4The change degree δ of Einteraction(decane-SiO2)under different temperatures and pressures in the water-wet systems.
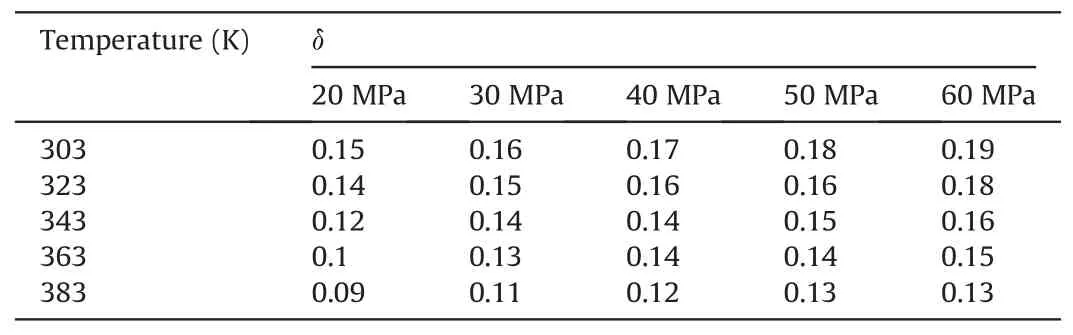
Table 5The change degree δ of Einteraction(decane-SiO2)under different temperatures and pressures in the mixed-wet systems.
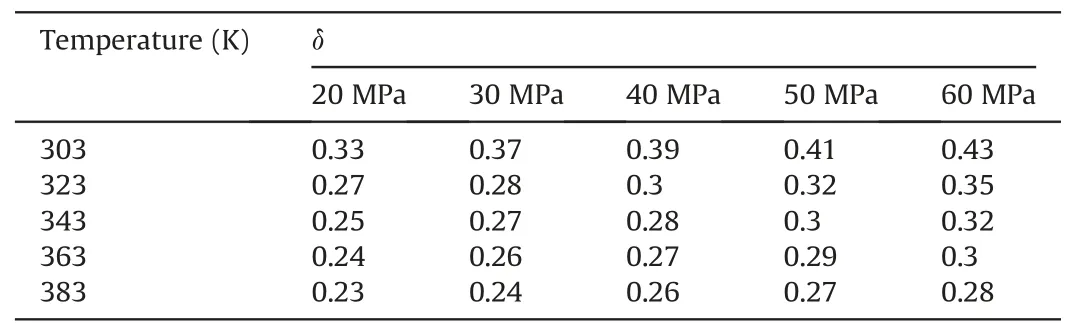
Table 6The change degree δ of Einteraction(decane-SiO2)under different temperatures and pressures in the oil-wet systems.
3.3.Competitive adsorption capacity of CO2 and decane molecules on pore walls
To evaluate the competitive adsorption capacity of CO2and decane molecules on the pore surfaces,the difference betweenEinteraction(CO2-SiO2)andEinteraction(decane-SiO2)in various systems was calculated.In addition,to quantitatively characterize the influence degree of temperature,pressure and wettability on ΔE(CO2/decane),the change degreeδof ΔE(CO2/decane)under different conditions was calculated.This calculation was based on the interaction energy of -37.09 kcal/mol at 303 K and 20 MPa in the water-wet system,as follows:
According to the aforementioned formula,when ΔE(CO2/decane)was negative,CO2molecules had a higher propensity for adsorption during the competitive adsorption process between CO2and oil molecules on the pore surfaces.Conversely,when ΔE(CO2/decane)was positive,decane molecules held the advantage in the competitive adsorption process between CO2and oil molecules on the pore surfaces.Tables 7-9 show the ΔE(CO2/decane)under different temperatures and pressures for water-wet,mixed-wet and oil-wet systems,respectively.For waterwet systems(Table 7),as temperature and pressure increased,the adsorption capacity of CO2molecules on the pore surfaces was enhanced compared to oil molecules.When the temperature was 303 K,323 K and 343 K,ΔE(CO2/decane)was negative regardless of how the pressure changes,indicating that under these conditions,CO2molecules could detach the membranous residual oil from the pore walls.When the temperature was equal to 363 K,and the pressure was lower than 40 MPa,CO2molecules could not detach the membranous residual oil from the pore walls.However,when the pressure exceeded 40 MPa,CO2molecules could detach the membranous residual oil from the pore walls.When the temperature was higher than 363 K,CO2molecules could not detach the membranous residual oil from the pore walls in the water-wet systems.For mixed-wet and oil-wet systems (Tables 8 and 9),the adsorption capacity of CO2molecules on the pore surfaces was enhanced compared to oil molecules with increasing temperature and pressure.However,regardless of the changes in temperature and pressure,ΔE(CO2/decane)remained positive,indicating that CO2molecules could not detach the membranous residual oil from the pore walls.
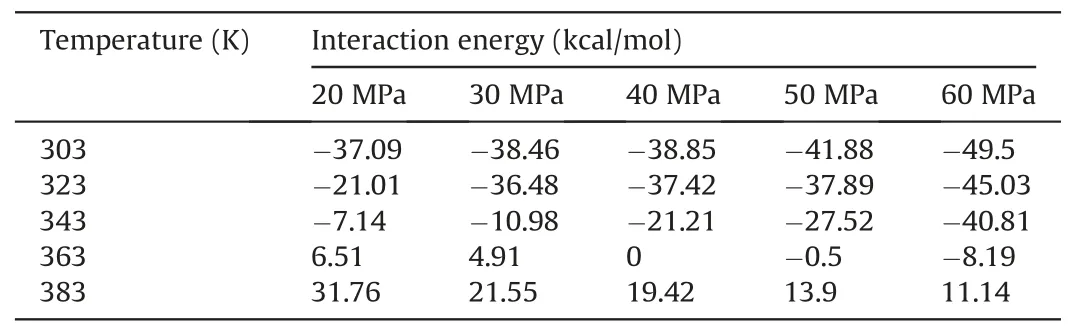
Table 7Comparison of the competitive adsorption capacity of CO2 and decane molecules on pore walls under different temperatures and pressures in water-wet systems.
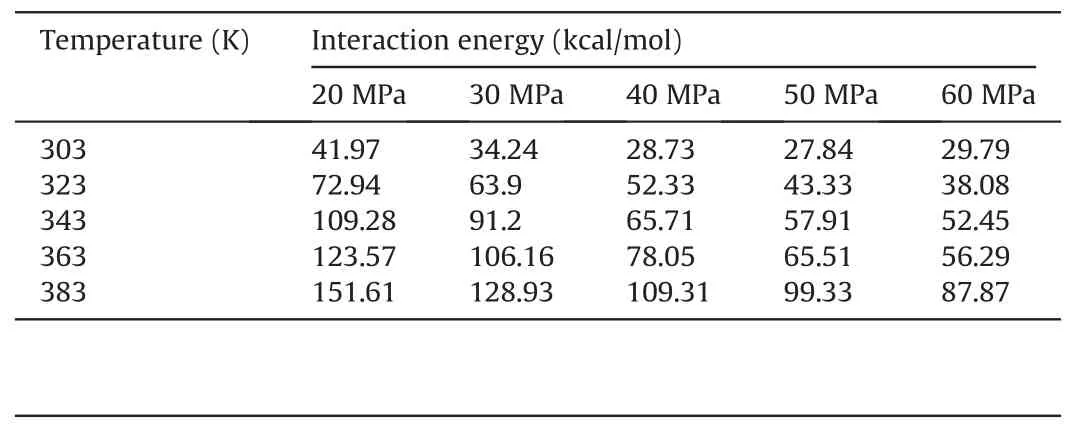
Table 8Comparison of the competitive adsorption capacity of CO2 and decane molecules on pore walls under different temperatures and pressures in mixed-wet systems.
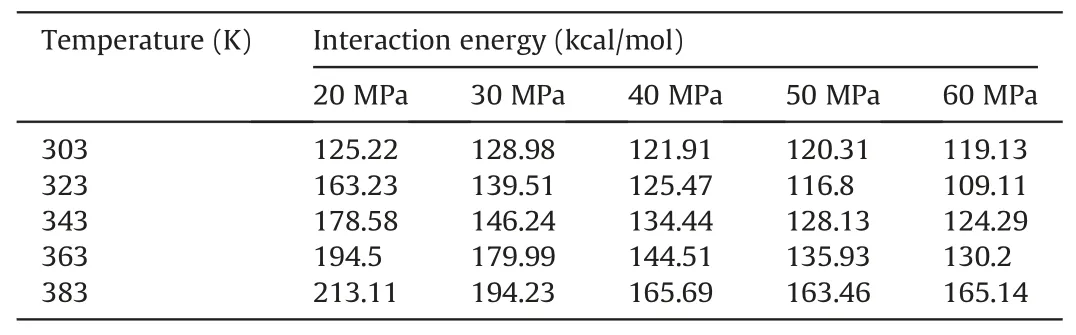
Table 9Comparison of the competitive adsorption capacity of CO2 and decane molecules on pore walls under different temperatures and pressures in oil-wet systems.
In Table 10,at 303 K,δchanged slowly with increasing pressure.At 20 MPa,δchanged more quickly with increasing temperature.Other temperature and pressure conditions in Table 10 show that theδof ΔE(CO2/decane)was more sensitive to temperature than pressure in the water-wet systems.Tables 11 and 12 also show that theδof ΔE(CO2/decane)was more sensitive to temperature than pressure in the mixed-wet and oil-wet systems.According to the change in δ with pressure under 303 K and different wettability in Tables 10-12,it can be determined that theδof ΔE(CO2/decane)was more sensitive to wettability than pressure.According to the change inδwith temperature under 20 MPa and different wettability in Tables 10-12,it can be determined that theδof ΔE(CO2/decane)was more sensitive to wettability than temperature.In summary,the influence on theδof ΔE(CO2/decane)followed the order of wettability >temperature >pressure.
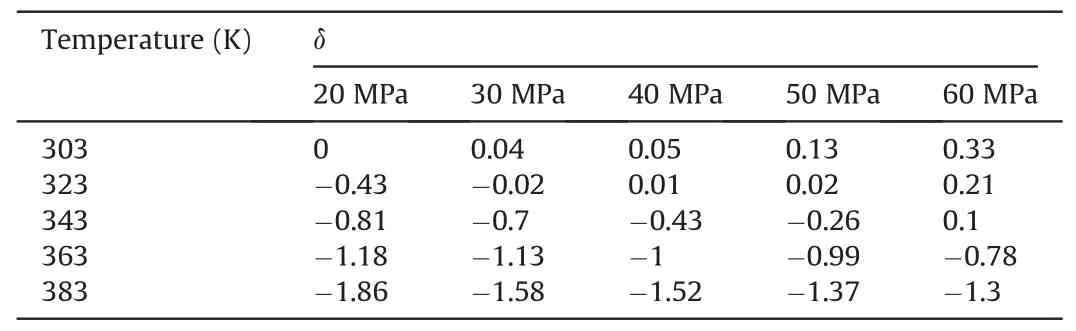
Table 10The change degree δ of ΔE(CO2/Decane)under different temperatures and pressures in the water-wet systems.
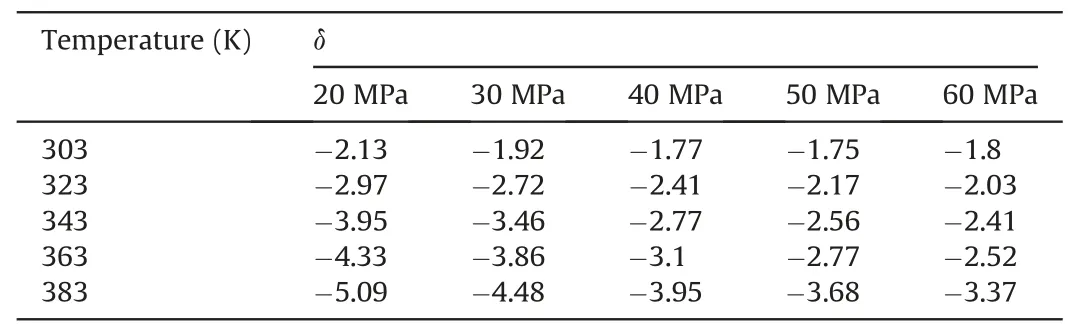
Table 11The change degree δ of ΔE(CO2/Decane)under different temperatures and pressures in the mixed-wet systems.
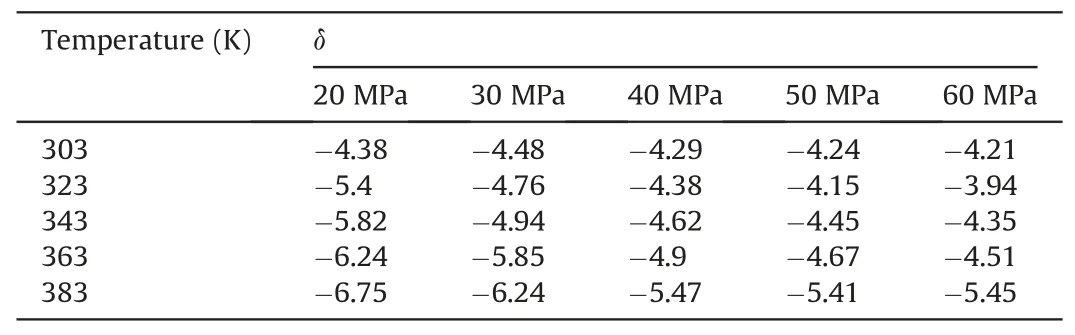
Table 12The change degree δ of ΔE(CO2/Decane)under different temperatures and pressures in the oil-wet systems.
4.Conclusions
In this study,the MD method was applied to explore the CO2flooding under different temperature,pressure and wettability conditions to improve the production capacity of membranous residual oil.First,the strength of the interaction between the fluids and pore walls was characterized by the interaction energy,and the microscopic distribution of CO2and decane molecules on the pore walls was analyzed to reveal the change in interaction energy;Then,the difference between the Einteraction(CO2-SiO2)and Einteraction(decane-SiO2)was applied to determine whether CO2gas could strip off the membranous residual oil from the pore walls.The following conclusions can be obtained in this paper:
(1) For the water-wet,mixed-wet and oil-wet systems,regardless of how the pressure and temperature changed,CO2and decane molecules could form a clear adsorption layer on the pore surfaces.Under the same wettability of pore surfaces,the densities of CO2and decane molecules in the adsorption layer decreased with increasing temperature and increased with increasing pressure.Under the same temperature and pressure conditions,the density of CO2molecules in the adsorption layer followed the order of water-wet system >mixed-wet system >oil-wet system,and the density of decane molecules in the adsorption layer followed the order of water-wet system <mixed-wet system <oilwet system.
(2) For the water-wet,mixed-wet and oil-wet systems,Einteraction(CO2-SiO2)andEinteraction(decane-SiO2)decreased with increasing temperature and increased with increasing pressure.At the same time,under the same temperature and pressure,the strength ofEinteraction(CO2-SiO2)followed the order of water-wet system >mixed-wet system >oil-wet system,and the strength ofEinteraction(decane-SiO2)followed the order of oil-wet system >mixed-wet system >water-wet system.
(3) For the water-wet systems,the capacity of CO2molecules adsorption on the pore surfaces was enhanced compared with oil molecules with increasing temperature and pressure.When the temperature was 303,323and 343 K,regardless of how the pressure changed,CO2molecules could detach the membranous residual oil from the pore walls.When the temperature was equal to 363 K and the pressure was below 40 MPa,CO2molecules could not detach the membranous residual oil from the pore walls,but when the pressure was beyond 40 MPa,CO2molecules could detach the membranous residual oil from the pore walls.When the temperature was above 363 K,CO2molecules could not detach the membranous residual oil from the pore walls in the water-wet systems.For mixed-wet and oil-wet systems,the capacity of CO2molecules adsorption on the pore surfaces was enhanced compared with that of oil molecules with increasing temperature and pressure.Regardless of the temperature and pressure changes,CO2molecules could not detach the membranous residual oil from the pore walls.
(4) The influence of temperature,pressure and wettability on the interaction energy was quantitatively evaluated via the change degreeδofEinteraction(CO2-SiO2)andEinteraction(decane -SiO2).For CO2/SiO2systems,the influence degree of temperature,pressure and wettability of pore surfaces onEinteraction(CO2-SiO2)is in the order of wettability >temperature >pressure.For the decane/SiO2system,the extent to which temperature,pressure and wettability of pore surfaces influenceEinteraction(decane-SiO2)is: wettability > pressure >temperature.
(5) The influence of temperature,pressure and wettability on the competitive adsorption capacity of CO2and decane molecules on the pore walls was quantitatively characterized via the change degreeδof ΔE(CO2/Decane),and the influence strength of temperature,pressure and wettability on ΔE(CO2/Decane)was obtained as follows:wettability >temperature >pressure.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Creative Groups of Natural Science Foundation of Hubei Province,China (Grant No.2021CFA030)and the National Natural Science Foundation of China(Grant Nos.41872210 and 41274111).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jrmge.2023.07.007.
 Journal of Rock Mechanics and Geotechnical Engineering2023年12期
Journal of Rock Mechanics and Geotechnical Engineering2023年12期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- The formation of orthogonal joint systems and cuboidal blocks: New insights gained from flat-lying limestone beds in the region of Havre-Saint-Pierre (Quebec,Canada)
- Numerical analysis of the effects of vesicle distribution characteristics on the engineering properties of volcanic rocks
- A hybrid attention deep learning network for refined segmentation of cracks from shield tunnel lining images
- 3D limit analysis of rock slopes based on equivalent linear failure criterion with tension cut-off
- Mutual impact of true triaxial stress,borehole orientation and bedding inclination on laboratory hydraulic fracturing of Lushan shale
- Unloading-induced permeability recovery in rock fractures
