Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
Jintao Dong ,Sainan Ji ,Yi Zhang ,Mengxia Ji ,Bin Wang ,Yingjie Li ,Zhigang Chen ,Jiexiang Xia ,*,Huaming Li
1 School of Chemistry and Chemical Engineering,Institute for Energy Research,Jiangsu University,Zhenjiang 212013,Jiangsu Province,China.
2 School of the Environment and Safety Engineering,Jiangsu University,Zhenjiang 212013,Jiangsu Province,China.
Abstract: Rapid increase in energy shortage and ecological environmental pollution has become a major issue that has been continuously drawing global attention,because it severely affects human health and limits sustainable social development.Various technologies have been developed and used to rationalize the utilization of new energy sources and pollution control.Among these technologies,photocatalysis has become a research priority in the field of environmental governance and energy development.Advantages such as low energy consumption,no secondary pollution,simple operation methods,and mild reaction conditions make photocatalysis an attractive choice.Notably,although photocatalysis is a promising approach for enhancing antibiotic removal and CO2 reduction efficiency,the industrialization and large-scale application of photocatalysts is limited because of issues such as low photo-absorption efficiency,redox capacity,and photogenerated electron separation or migration efficiency.The progress of current research on the regulation of composition/structure and performance of photocatalysts has promoted the exploration of efficient and practical modification strategies to construct photocatalyst composites with improved performance by facilitating light absorption/utilization and enhancing photocatalytic surface/interface reaction performance.Among the many common modification strategies,the construction of a Z-scheme heterojunction can enhance the light absorption ability and significantly reduce the recombination rate of photogenerated electron-hole pairs.Additionally,this strategy maintains the strong reduction/oxidation ability of photogenerated electrons/holes to facilitate the oxidation of environmental pollutants and conversion to clean energy.In this study,Z-scheme MnO2/BiOBr (MO/BiOBr) composites were effectively constructed using a mechanically assisted ball-milling process.In situ X-ray photoelectron spectroscopy under dark and light conditions confirmed that photoexcited electrons in MnO2 can migrate directionally to BiOBr through Mn3+/Mn4+ redox couple to create a Z-scheme transfer path.A similar conclusion can also be deduced from the results of electron spin-resonance spectroscopy and band structure analysis.The formation of a Z-scheme heterojunction between MnO2 and BiOBr,attributed to the Mn3+/Mn4+ redox couple from MnO2 and staggered energy band,enabled the space separation of oxidation and reduction centers.Furthermore,compared with BiOBr,MO/BiOBr composites exhibited enhanced light absorption and a markedly reduced photoinduced electron-hole pair recombination rate,as confirmed by ultraviolet-visible diffuse reflectance spectroscopy and photoluminescence spectroscopy.Thus,the MO/BiOBr composites exhibited exceptional photocatalytic performance toward ciprofloxacin (CIP) oxidation and CO evolution.The CIP removal efficiency of the MO/BiOBr composites reached 77.3% in just 60 min,which is 1.28 times higher than that of BiOBr (60.2%).Simultaneously,the photocatalytic CO2-to-CO performance of the MO/BiOBr composites (20.02 µmol·g-1·h-1) was found to be 2.20-fold higher than that of BiOBr (9.08 µmol·g-1·h-1).Photocurrent measurement and electrochemical impedance spectroscopy indicated that the MnO2/BiOBr Z-scheme heterojunction has higher interfacial electron transfer efficiency than pure MnO2 and BiOBr.Additionally,liquid chromatograph mass spectrometry and in situ Fourier transform infrared spectroscopy is conducted to study the generation of intermediates during the photocatalytic CIP removal and CO2 reduction process.The toxicity of CIP and corresponding intermediates after the photocatalytic degradation of the MO/BiOBr composites was evaluated using toxicity estimation software (T.E.S.T.) to analyze the actual physiological toxicity,based on indexes such as Daphnia Magna lethal concentration 50% (LC50,48 h),Fathead Minnow lethal dose 50% (LD50,96 h),mutagenicity,and bioaccumulation factor.Thus,this study proposed a novel and simplified approach for constructing a Z-scheme heterojunction to facilitate solar-derived antibiotic removal and fuel synthesis.
Key Words: Z-scheme heterojunction; BiOBr; Ciprofloxacin removal; CO2 reduction; Photocatalysis
1 Introduction
The rapid development of technology and economy has brought great convenience to human life,but it concurrently causes astonishing fossil energy consumption,excessive pollutants emission and startling ecological environment destruction,which has led to serious energy shortages and environmental pollution problems1,2.So many scientists have made great efforts to handle environmental pollution and energy crisis.Recently,water pollution and ecological damage caused by antibiotics attracts urgent concerns in environmental pollution3.Antibiotics could function as significant role in clinical and animal husbandry ascribed to the high efficiency,broad-spectrum,convenient administration,and other advantages,but also antibiotics residual accumulated in water environment might produce substantial immunity and organicity damage for human body4.Photocatalytic antibiotic removal technology has attracted extensive attention of researchers due to its strong oxidation capacity,non-toxic,low energy consumption,and simple operation,and has become an effective method for wastewater treatment.Furthermore,excessive CO2emission is supposed to trigger environmental and climate problems,such as extreme weather,sea level rise,and global temperature rise,etc.5.The technology of converting carbon dioxide into fuel not only helps to reduce carbon emissions,but also can produce high calorific-value fuels and ease the energy crisis,which can kill two birds with one stone6,7.Various technologies have been developed to convert CO into fuels or high value-added chemicals,mainly including thermo-catalysis8,electrocatalysis9and photocatalysis10,11.Herein,photocatalytic CO2reduction has aroused extensive research interest ascribed to prominent characteristics,such as inexhaustible solar energy,no secondary pollution,and mild reaction conditions11.Herein,photocatalytic technology is low-carbon and sustainable route that can achieve environmental pollutant removal and energy conversion to fuels by the photocatalytic redox process.
While photocatalytic technology is promising and feasible for achieving efficient antibiotic removal and CO2reduction,the industrialization and large-scale application of photocatalyst is confined to three limiting factors,as (1) the light absorption range and utilization capacity,(2) the generation and migration performance of photogenerated carriers and (3) the oxidationreduction ability of photocatalyst12,13.Therefore,the enhancement of photogenerated electrons separation and light absorption efficiency is supposed to breaking the developmental “bottle-neck” of single photocatalyst.Nevertheless,the photocatalytic performance of single photocatalyst could hardly be enhanced by the simultaneous promotion of visible light absorption and oxidation-reduction ability.Although the reduction of the band gap is helpful to broaden the spectral response range of photocatalyst,and triggers a noteworthy problem that is detrimental to the photoexcited electron-hole separation process14.Heterojunction photocatalysts have attracted booming attention and extensive research applications attributed to the various outstanding characteristics such as amplified photo-absorption range and directional separation of photoinduced electrons and holes15.The universal and effective heterojunction construction method coupled by semiconductor photocatalyst mainly consists of Type-II and Z-scheme heterojunction16.Two (or more) semiconductors for constructing Z-scheme heterojunction also possess strong oxidation or reduction capacity,respectively17,18.Therefore,the Z-scheme heterojunction photocatalyst can not only efficiently accelerate photogenerated electrons transfer,but also can achieve space separation of oxidation or reduction active center for maintaining the strong redox capability of single photocatalyst.
As an environment-friendly and low-cost photocatalyst,Bismuth oxybromide (BiOBr) not only possesses suitable energy band position,but also maintains a layered structure composed of [Bi2O2]2+layers and double bromine atomic layers for accelerating photogenerated carrier separation19.However,the narrow light-absorption range and high photogenerated carriers’recombination rate of BiOBr remains tremendously restricted the carrier migration and photocatalytic conversion efficiency.Although BiOBr possesses appropriate conduction band (CB)position for CO2reduction,it cannot be satisfied with the oxidation potential for •OH generation attributed to valence band(VB) positions20.Hence,coupling with other materials for constructing Z-scheme heterojunction is of great scientific value and practical significance to optimize the oxidation ability and electron transfer ability of BiOBr21,22.As a transition metal oxide,MnO2has attracted attention ascribed to its stable structure,simple preparation method and narrow band gap,MnO2can demonstrate satisfactory photo-absorption capacity and staggered band positions with BiOBr.Furthermore,MnO2with exceptional photoelectrochemical performance also can provide efficient channels for electron transmission in the reaction process.Z-scheme heterojunction coupled with MnO2and Monolayer g-C3N4was constructed to achieve exceptional overall water splitting reaction by accelerating photogenerated carrier separation23.Furthermore,Zn0.5Cd0.5S/MnO2heterostructures prepared by Nagarajaetal.exhibited enhanced HMF oxidation and H2O reduction performance by the construction of Z-scheme heterojunction24.So,the introduction of MnO2is supposed to boost photoconversion,photoelectrons separation and redox characteristics of BiOBr by constructing Zscheme heterojunction.
Herein,a novel Z-scheme system by the coupling of MnO2and BiOBr was established with mechanically assisted ball milling method.Ascribed to photo-absorption enhancement and the construction of Z-scheme heterojunction,MnO2/BiOBr(MO/BiOBr) composites exhibited significantly enhanced photocatalytic antibiotics removal and CO2reduction performance.Photoelectrochemical measurement and photoluminescence (PL) spectroscopy demonstrates that MO/BiOBr Z-scheme heterojunction possesses rapid electrons separation and migration performance.The enhancement mechanism and degradation products analysis for ciprofloxacin(CIP) was revealed by ESR and LC-MS measurement.Additionally,insituFTIR spectra was measured for investigating by analyzing the intermediates generation in photocatalytic CO2reduction process.Consequently,this study inaugurates a novel method for Z-scheme heterojunction construction for boosting antibiotics removal and fuel synthesis derived by solar energy.
2 Experimental
2.1 Chemicals
All chemicals were identified as analytical grade and were used directly without separation and purification.Ethanol (95%),Na2SO4(99%),KMnO4(99%),Bi(NO3)3·5H2O (99%),KCl(99.5%),K3Fe(CN)6(99.0%),K4Fe(CN)6·3H2O (98.5%) and 1-hexadecyl-3-methylimidazolium bromide ([C16mim]Br,99%)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Ciprofloxacin (98%) was obtained from Shanghai Macklin Biochemical Technology Co.,Ltd.
2.2 Preparation of MnO2
According to previously reported methods23,firstly,40 mg Na2SO4was injected into 35 mL deionized water,2 mL of KMnO4solution (30 mg·L-1) was injected into above solution during the stirring process and then kept stirring for 1 h.Then the mixed solution was poured into 50 mL autoclave and heat it for 12 h at 120 °C.Finally,MnO2was collected by high-speed centrifugation after vacuum dry.
2.3 Preparation of BiOBr and MO/BiOBr composites
The synthesis process of MO/BiOBr composites is shown in Fig.1a.485.1 mg Bi(NO3)3·5H2O and 387.4 mg [C16mim]Br was dispersed in 20 mL ethanol,then 43.63 mg MnO2was injected into the dispersion and stirred for 0.5 h.Then the mixture after stirring was transferred in a 50 mL into agate tube and was treated by ball milling process at 1200 r·min-1for 1 h.The product obtained by ball milling was dried by repeated washing with ethanol and deionized water,marked as MO/BiOBr composites.And pure BiOBr was prepared without MnO2by similar method.
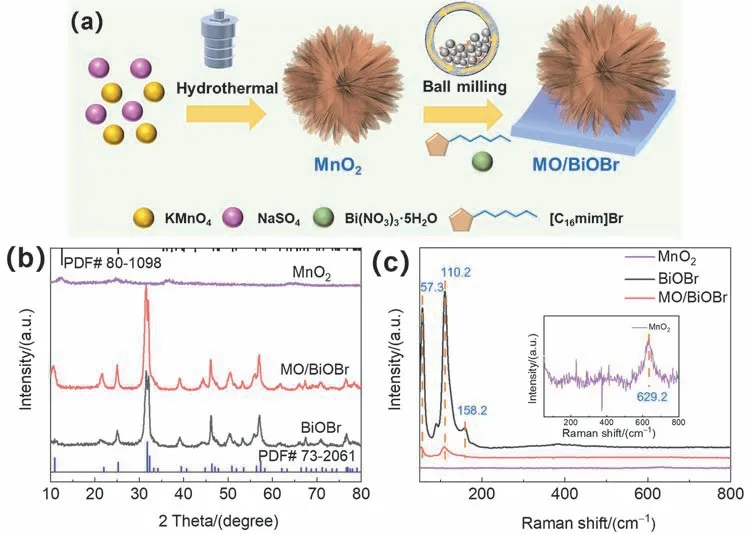
Fig.1 (a) Synthesis of MO/BiOBr composites via ball milling method; (b) XRD patterns and(c) Raman spectra of MnO2,BiOBr and MO/BiOBr composites.
2.4 Photocatalytic CIP removal
The photocatalytic performance of as-prepared MnO2,BiOBr and MO/BiOBr composites was evaluated by CIP under visible light illumination.The photocatalytic degradation experiments were carried out in a Pyrex photocatalytic reactor equipped with a 300 W Xe lamp (λ> 400 nm) at 30 °C for preventing CIP thermal decomposition.In typical photodegradation experiments,30 mg photocatalyst was applied to evaluate its performance by CIP (20 mg·L-1) dispersions,respectively.The CIP concentration was measured by TU-1810 UV-Vis spectrophotometer (Beijing Purkinje GENERAL Instrument Co.,Ltd.) at the corresponding maximum absorption wavelength(276 nm),respectively.
2.5 Photocatalytic CO2 reduction measurement
The photocatalytic CO2reduction performance of MnO2,BiOBr and MO/BiOBr composites was carried out in a 500 mL all glass automatic on-line trace gas analysis system (Labsolar-6A,Beijing Perfectlight).Totally,20 mg sample was added into 50 mL deionized water and ultrasonically dispersed.The system was vacuum-treated and then pumped into high-purity CO2up to 80 kPa.All experiments were carried out in constant temperature (5 °C) with a circulating cooling water system to prevent thermo-catalysis effects.The PLS-SXE300D Xenon lamp source (Beijing Perfectlight) was applied as light source.
2.6 Apparatus
BiOBr and MO/BiOBr composites were prepared by MSKSFM-3 ball milling equipment (Hefei Kejing).The crystallographic structure of MnO2,BiOBr and MO/BiOBr composites was measured by Shimadzu XRD6100 X-ray powder diffraction (Japan) operated at 30 kV and 30 mA.Transmission electron microscope (TEM) images of MnO2,BiOBr and MO/BiOBr composites were performed using JEOL JEM-2100F (Japan) worked at 200 kV.The composition and valence of the samples were analyzed by X-ray photoelectron spectroscopy (XPS) measurements (ESCALAB250Xi,Thermo Fisher DXR,America).UV-Vis DRS spectra was measured on Shimadzu UV-3600 spectrophotometer (Japan).Raman Spectrometer (Thermo Fisher DXR,America) was employed to analyze the molecular structure.The PL spectra was measured by QuantaMaster™ 40 QuantaMaster & TimeMaster Spectrofluorometer (Photon Technology International,Inc.,America).InsituFTIR spectra were recorded on a Nicolet iS50 FT-IR spectrometer (America) equipped with a liquid nitrogen cooled HgCdTe (MCT) detector.InsituFTIR spectra were performed in the praying mantis DRIFTS accessory and reaction chamber.ESR spectra was measured on Bruker model EPR JESFA200 spectrometer (America).The various intermediates appeared in photocatalytic CIP removal process was analyzed by Thermo LXQ LC/MS measurements (America).
2.7 Photoelectrochemical measurements
All the photoelectrochemical (PEC) measurements were carried out in the universal three-electrode measurement system:the prepared samples modified tin oxide conductive (ITO) glass employed as the working electrode,a Ag/AgCl electrode functioned as the reference electrode and a Pt-wire auxiliary electrode worked as the counter electrode.The photocurrent response was measured in a PEC system constructed by the CHI760E electrochemical workstation and a 300 W xenon PLSSXE300D high brightness source (Beijing Perfectlight) as the excitation light source.The electrochemical impedance spectroscopy (EIS) analysis was analyzed at 0.24 V in the configured electrolyte consisted of 5 mmoL·L-1[Fe(CN)6]3-/[Fe(CN)6]4-equimolar and 0.1 mol·L-1KCl with the 0.1 Hz to 100 kHz frequency range.The Mott-Schottky plots of all samples were tested in 0.2 mol·L-1Na2SO4solution.
3 Results and discussion
X-ray diffraction (XRD) patterns were carried out for investigating the crystallographic structure of as-prepared MnO2,BiOBr and MO/BiOBr composites (Fig.1b).The obvious characteristic peaks located at 12.5°,25.2° and 36.2° are observed in MnO2,which can be assigned to (001),(002) and(110) lattice plane of the δ-MnO2standard card (JCPDS No.80-1098)25.Both the main diffraction peaks of BiOBr and MO/BiOBr composites can match with the BiOBr standard card(JCPDS No.73-2061),which demonstrates that BiOBr and MO/BiOBr composites have been successfully obtained and the introduction of MnO2has no destructive effect on crystal structure26.The molecular structure of MnO2,BiOBr and MO/BiOBr composites was further investigated by Raman spectra with a 532 nm laser excitation.As exhibited in Fig.1c,the three phonon modes of pure BiOBr can be observed.The obvious scattering peaks at 57.3,110.2,158.2 cm-1can be assigned toA1gvibration mode of Bi―Br bond,internalA1gandE1gstretching mode27.It is worth noting that the peaks for all phonon modes of MO/BiOBr composites are decreased remarkably,two weaker peaks seated at 57.3 and 110.2 cm-1can be discovered in MO/BiOBr composites.This result implies that strong interaction between MnO2and BiOBr emergesviaball milling process.
The microstructure of MnO2,BiOBr and MO/BiOBr composites was analyzed by TEM measurement.As shown in Fig.2a,b,as-prepared BiOBr has nanosheets microstructure and MnO2exhibits a nanoflower-like microstructure with a diameter of about 100 nm.Fig.2c exhibits that MO/BiOBr composites consists of BiOBr nanosheets and MnO2nanoflowers,and a distinct interface was formed between BiOBr nanosheets and MnO2nanoflowers under the impingement,extrusion,and friction force in ball milling process (Fig.2d).Consequently,MO/BiOBr composites were successfully fabricated by ball milling method.

Fig.2 TEM images of BiOBr (a),MnO2 (b) and MO/BiOBr (c,d) composites.
The surface chemical compositions and valence states of BiOBr and MO/BiOBr composites was measured by XPS spectra.The high-resolution XPS spectra of MnO2/BiOBr in the dark or under 300 W Xe lamp irradiation with a 400 nm filter were carried out.The distinct characteristic peaks located at 159.3 and 164.6 eV can be assigned to Bi 4f7/2and Bi 4f5/2orbits(Fig.3a),which is corresponding to Bi3+in MO/BiOBr composites28.And two broad peaks at 157.3 and 162.5 eV can match with Bi(3-x)+originated from the formation of oxygen vacancies29.After illumination,obvious shift is not found in the peaks corresponding to Bi 4f7/2and Bi 4f5/2orbits,but the peak intensity of Bi(3-x)+significantly enhances,illustrating that the more Bi(3-x)+forms after the injection of photogenerated electrons.The Br 3dspectrum (Fig.3b) of MO/BiOBr composites is deconvoluted to two peaks,which is ordinarily related to the Br 3d3/2(69.5 eV) and Br 3d5/2(68.4 eV)30.The high-resolution O 1sspectrum (Fig.3c) is imitated to two peaks located at 529.9 and 531.2 eV corresponding to lattice oxygen(Olat),and the surface adsorbed oxygen around oxygen vacancy(Oads)29,31.An obvious phenomenon is observed that Xe lamp irradiation introduces an obvious negative shift of binding energy for both Br 3dand O 1s,indicating the electron density around Br and O is enhanced by photoinduced electrons accumulating.The above result indicates that photogenerated electrons may transfer to BiOBr in MO/BiOBr composites32,33.The high-resolution Mn 2pspectrum of MO/BiOBr composites(Fig.3d) is split to four peaks.Wherein,two sharp peaks seated at 643.4 and 654.9 eV are assigned to Mn4+(2p3/2and 2p1/2,respectively),and the two broad peaks located at 641.6 and 652.6 eV can match with Mn3+(2p3/2and 2p1/2,respectively),which confirms the coexistence of Mn3+and Mn4+in MO/BiOBr composites34.After irradiation,the rate of peak area(AMn4+/(AMn4+ +AMn3+)) has fallen from 0.455 to 0.366,suggesting that Mn3+/Mn4+redox couple might form after illumination.Consequently,Mn3+/Mn4+electron pairs can serve as electron transport chains between the MnO2and BiOBr attributed to constructing Z-scheme heterojunction.The XPS analysis further verified that the MO/BiOBr composites were successfully fabricated by mechanical ball milling method.TheinsituXPS measurement confirms that photoinduced electrons in MnO2may transfer to BiOBr through Mn3+/Mn4+redox couple for forming Z-scheme transfer mechanism.
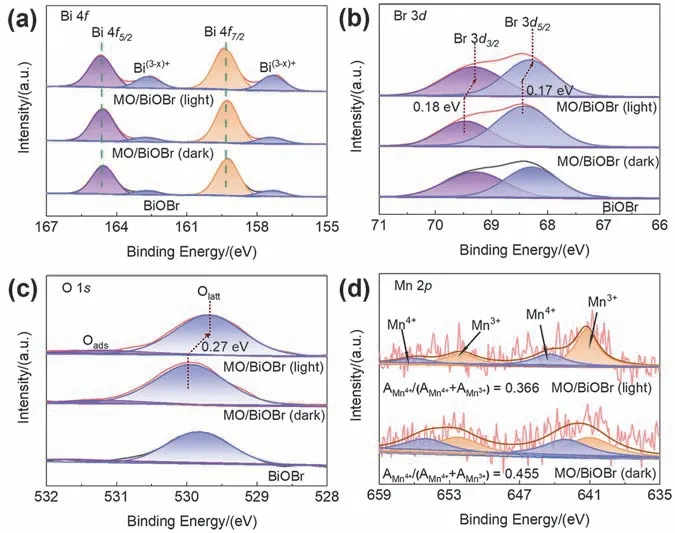
Fig.3 XPS spectra of BiOBr and MO/BiOBr composites: (a) Bi 4f,(b) Br 3d,(c) O 1s,(d) Mn 2p.
The optical absorption performance of BiOBr,MnO2and MO/BiOBr composites was determined by UV-Vis DRS spectroscopy.As exhibited in Fig.4a,the absorption edge of pure BiOBr is approximately about 430 nm.And the light absorption edge of MO/BiOBr composites red shifts to 500 nm with the introduction of MnO2and MO/BiOBr composites possess enhanced visible light absorption performance,indicating that the introduction of MnO2can broaden the visible light absorption.UV-Vis DRS spectra indicates that MO/BiOBr composites exhibit the enhanced light harvesting ability compared with pure BiOBr,which is beneficial for the generation of photogenerated carriers for boosting the photocatalytic activity of MO/BiOBr composites.PL spectra was applied for investigating recombination rate of photogenerated electron hole pairs35.The PL intensity of MO/BiOBr composites is significantly lower than that of BiOBr(Fig.4b),demonstrating that the recombination proportion of MO/BiOBr composites is significantly reduced ascribed to the addition of MnO2.
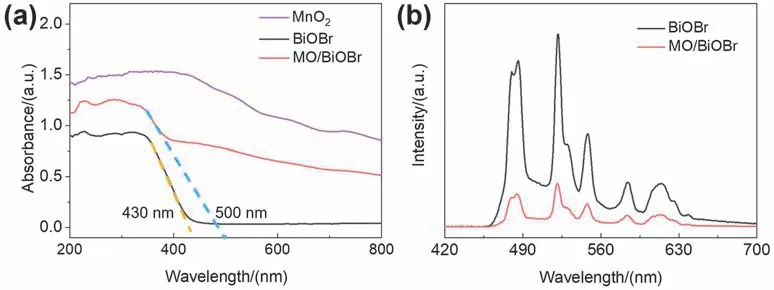
Fig.4 (a) UV-Vis DRS spectra of BiOBr,MnO2 and MO/BiOBr composites; (b) PL spectra of BiOBr and MO/BiOBr composites.
To investigate the promotion effect of MnO2introduction on the photocatalytic activity of BiOBr,CIP as a frequently-used fluoroquinolone antibiotic is chosen as the target pollutant to evaluate the photocatalytic degradation activity of BiOBr,MnO2and MO/BiOBr composites.CIP can hardly be directly oxidized without photocatalysts under illumination.The photocatalytic degradation removal efficiency of CIP by MnO2is merely 8.2% after 60 min,and only 60.2% CIP can be oxidized by BiOBr(Fig.5a).As the introduction of MnO2,MO/BiOBr composites exhibit the enhanced photocatalytic CIP degradation performance and achieves 77.3% CIP removal rate in 60 min under visible light irradiation,which almost reaches to 1.28-fold than that of BiOBr.Compared with previous reports (Table 1),MO/BiOBr composites possessed satisfactory CIP removal rate.As observed in Fig.5b,the reaction rate constants (k) of MnO2,BiOBr and MO/BiOBr composites was calculated as 0.00162,0.0167,0.0294 min-1,respectively.Cycling stability is generally regarded as essential factor for measuring application prospect of photocatalyst,and MO/BiOBr composites maintain comparatively stability after four cycles (Fig.5c).And MO/BiOBr composites preserve high crystallinity anddemonstrates that MO/BiOBr composites possess crystal structure stability (Fig.5d).
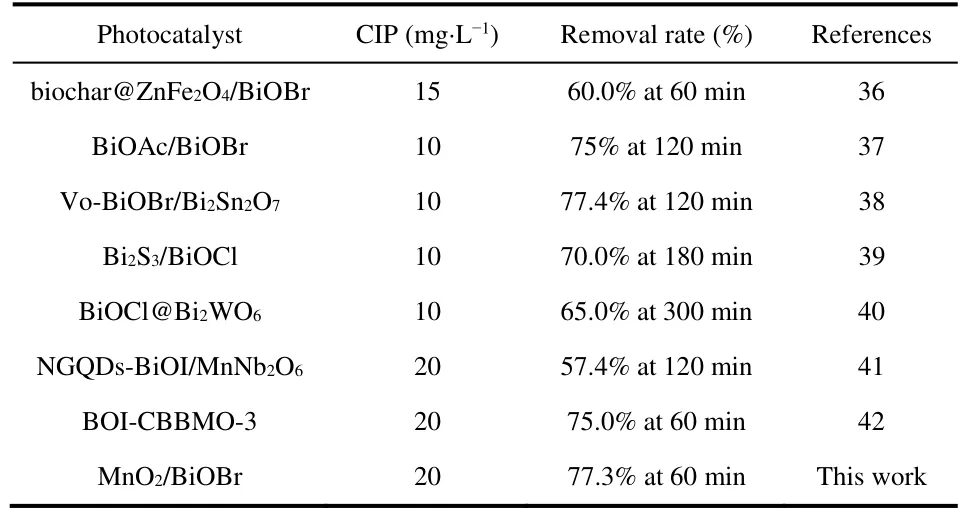
Table 1 Comparison of Bismuth-based materials for CIP degradation.
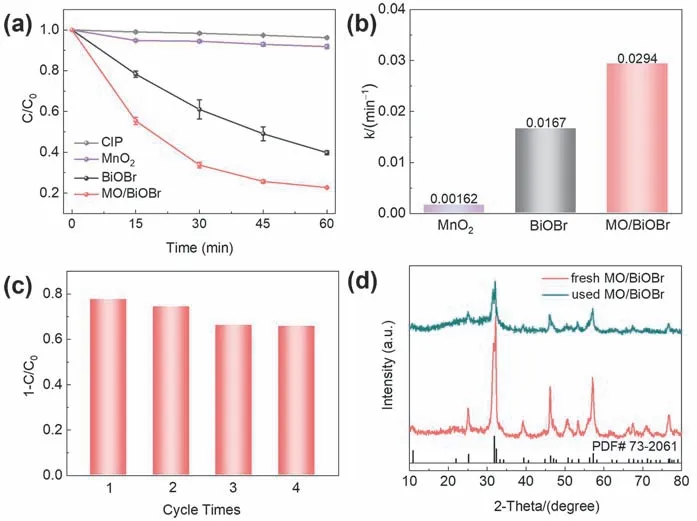
Fig.5 (a) Photocatalytic degradation curves and (b) the corresponding rate constant (k) of BiOBr,MnO2 and MO/BiOBr composites for CIP removal under visible light irradiation; (c) cycling experiments for the CIP photodegradation in the presence of MO/BiOBr composites under visible light irradiation; (d) XRD patterns of MO/BiOBr composites before and after the photocatalytic cycle experiments.
For further elaboration of performance enhancement by MnO2adjunction,the photocatalytic CO2reduction performance of BiOBr,MnO2and MO/BiOBr composites was carried out under 300 W xenon lamp irradiation.MO/BiOBr composites demonstrate satisfactory CO evolution performance (80.07 μmol·g-1) with 4 h measurement (Fig.6a,b),which is 2.2 and 19.8 times than that of BiOBr (36.31 μmol·g-1) and MnO2(4.04 μmol·g-1),respectively.Various control experiments were carried out for traceability of carbon source in CO evolution.The CO evolution performance was seriously constrained by setting condition (without light,catalyst,and CO2replaced by Ar),which demonstrates carbon source originates from inpouring CO2(Fig.6c).Furthermore,stability is an important evaluation factor for the catalytic activity of as-prepared photocatalyst.The photocatalytic CO evolution performance under continuous illumination was measured for assessing the stability of MO/BiOBr composites,and MO/BiOBr composites maintain continuation for CO2conversion in 24 h (Fig.6d),which demonstrates that MO/BiOBr composites would participate in long-term application and possess anticipated industrialization prospects.Compared to previous reports (Fig.6e),the MO/BiOBr composites exhibit satisfactory photocatalytic CO generation performance attributed to the MnO2involvement employing full-spectrum simulate illuminant with the comparison of various bismuth oxyhalide-based materials(named as R43 to R53)43–53.Consequently,MO/BiOBr composites demonstrate simultaneous improvement of photocatalytic CIP oxidation and CO2reduction performance,which may ascribes to heightened photoelectrochemical performance by introducing MnO2.
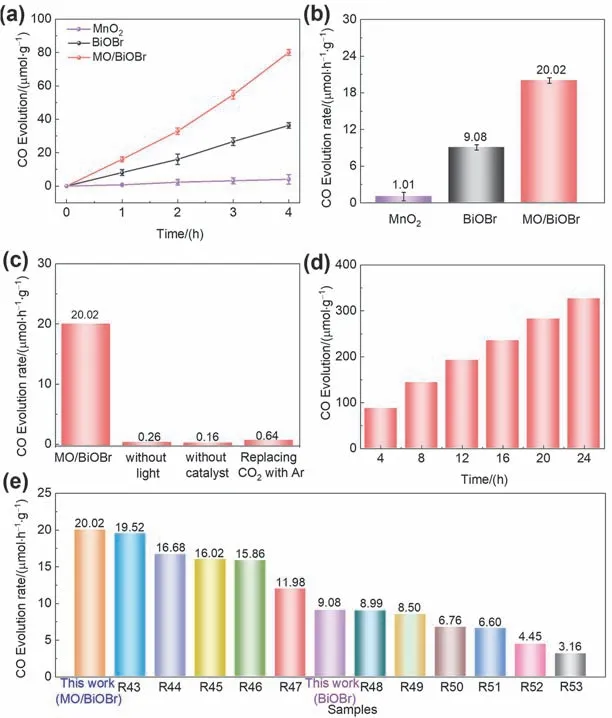
Fig.6 (a) The time courses of photocatalytic CO evolution and (b) the CO2 photoreduction rate in 4 h using MnO2,BiOBr and MO/BiOBr composites; (c) the CO2 photoreduction experiments of the MO/BiOBr composites,MO/BiOBr composites without light;without catalyst,MO/BiOBr composites in Ar for replacing CO2; (d) the stability of MO/BiOBr composites; (e) comparison of photocatalytic CO2 performance of BiOBr and MO/BiOBr composites with various bismuth oxyhalide-based materials.
The main active species involved in the photocatalytic CIP degradation of MO/BiOBr composites were investigated by DMPO spin-trapping ESR spectra54.As shown in Fig.7a,DMPO-•O2-signal cannot be observed in BiOBr and MO/BiOBr composites without illumination.Under the illumination conditions,DMPO-•O2-signals generated during the reaction in the MO/BiOBr composites are much stronger compared with the weak signal in BiOBr,which can prove that the introduction of MnO2is significant in boosting light absorption and photogenerated electron separation capability to generate •O2-for oxidizing CIP.Whether in dark or light,DMPO-•OH signals has not significantly observed in BiOBr.It is worth noticing that MO/BiOBr composites possess the demonstrable DMPO-•OH quadruple peaks with equal distances for charactering •OH,which can demonstrate that the introduction of MnO2could be conducive to •OH generation in MO/BiOBr composites (Fig.7b).Consequently,the above results can determine that •OH and•O2-active species synergistically contribute to the enhanced photocatalytic activity in MO/BiOBr composites.
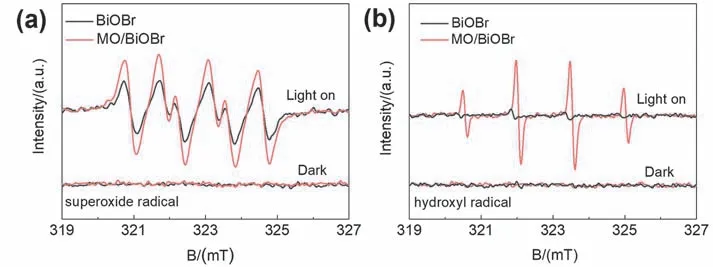
Fig.7 ESR spectra of (a) DMPO-•O2- and (b) DMPO-•OH for BiOBr and MO/BiOBr composites.
To investigate photocatalytic reaction mechanism of MO/BiOBr composites,the energy band structure of MnO2and BiOBr was investigated by Mott-Schottky measurement.Tauc plot combined with XPS VB spectra were conducted to further explore the photogenerated carriers’ separation and transfer mechanism.The band gap (Eg) of BiOBr and MnO2was calculated as 2.65 and 2.04 eV by Tauc plot (Fig.8a,b) of the relationship between (αEphoton)1/2andEphoton55.The flat band position of BiOBr is extrapolated as -0.32 V from the Mott-Schottky plot (Fig.8d).And the Fermi level of BiOBr can be approximately close to the flat band position56.Consequently,the corresponding valence band (EVB) of BiOBr is calculated as 1.74 eV (Fig.8c).The conduction band positions (ECB) of BiOBr are calculated to be -0.91 eV by the formulaECB=EVB-Eg,respectively.Furthermore,ECBof MnO2is estimated to be 0.64 eV by the equations (ECB=X-Ee- 0.5Eg,whereXof 5.96 eV (absolute electronegativity) andEe= 4.5 eV (Ee,the free energy of electrons of the semiconductor on the hydrogen scale)57.So,ECBof MnO2is about 2.68 eV.According to energy band structure,type II heterojunction based on BiOBr and MnO2might not meet the requirements redox potential for •OH generation and CO2reduction,where not identical with ESR spectra,photocatalytic CIP removal and CO2reduction result.The mechanism for boosting photocatalytic CIP degradation and CO2reduction performanceviaZ-scheme MO/BiOBr heterostructure is demonstrated in Fig.8e under illumination.Above all,photogenerated electrons generated BiOBr and MnO2is excited in VB and transfer to CB,respectively.Subsequently,photogenerated electrons from CB of MnO2would transfer to VB of BiOBr.In this process,the photoexcited electrons on the CB of MnO2were trapped to reduce Mn4+to Mn3+,and then Mn3+can be converted to Mn4+again by the photogenerated holes in the VB of BiOBr23,58.The Mn3+active sites can act as an electron transport chain between BiOBr and MnO2,and simultaneously enhance the separation and transfer of photogenerated carriers by Mn3+/Mn4+redox couple contributing to the enhancement of photocatalytic CIP oxidation and CO2reduction.

Fig.8 Tauc plots of (a) BiOBr and (b) MnO2,(c) valence band XPS spectra of BiOBr,(d) Mott-Schottky plots of BiOBr;(e) schematic illustration of electron-hole separation on the type II and Z-scheme heterojunction based on BiOBr and MnO2.
As theECBof BiOBr is more negative thanE0(O2/•O2-),thus photogenerated electrons originated from reduce O2to •O2-for further oxidating the surface adsorbed CIP59.CO2can be simultaneously reduced to CO by the CB of BiOBr.On the other hand,holes retained on the VB of MnO2not only can directly degrade CIP to small molecules but also oxidizes OH-to •OH for further CIP oxidation60.Furthermore,the electron transport chain formed by Mn3+/Mn4+redox couple can contribute to the formation of Z-scheme MO/BiOBr heterojunction and enhance the photogenerated charge separation and utilization for maintaining the strong redox capability of both BiOBr and MnO2,which is beneficial for boosting the CIP removal and CO2conversion.
The photoelectrochemical measurement was performed on BiOBr,MnO2and MO/BiOBr composites for demonstrating the accelerated carrier migration attributed to the Z-scheme heterojunction.Compared with MnO2and BiOBr,MO/BiOBr composites exhibit higher photocurrent intensity (Fig.9a),which indicates that the construction of MnO2can accelerate carrier separation and transport of MO/BiOBr composites61.The Nyquist radius arc of MO/BiOBr composites in EIS spectroscopy is relatively small (Fig.9b),which further indicates that MO/BiOBr composites exhibited diminished interfacial electron transfer efficiency.Based on the PEC measurement,the formation of Z-scheme heterojunction could promote the effective charge separation in MO/BiOBr composites for boosting the photocatalytic performance.
LC-MS measurement was employed for various reaction intermediates in the photocatalytic CIP oxidation process and the tangible molecular architecture was estimated in accordance with mass spectrum (Fig.10a).According to DMPO spintrapping ESR spectra of MO/BiOBr composites,MO/BiOBr composites can be photoexcited for oxidating dissolved O2and OH-to •O2-and •OH by h+generation.•O2-and •OH radicals can participate in CIP degradation and function as strong electrophiles for various intermediates generation.Consequently,the CIP degradation pathway by MO/BiOBr composites was reckoned as two pathways with eleven intermediates.As shown in Fig.11,the cleavage reaction of piperazine N ring was stepwise deduced in pathway I.C3―C4 bond in piperazine ring can be susceptible to attack by •OH and•O2-,which leads to the oxidation of C―H bond to ―C=O bond with the emergence of P1 (m/z= 348)viaelectron migration process62.Subsequently,cleavage reaction of piperazine ring might carry out the generation of P2 (m/z= 362) contributed to the further oxidation.In the subsequent reaction process,P2 is step-by-step oxidated to P3 (m/z= 334),P4 (m/z= 306) and P5(m/z= 263) by successive carbonyl elimination and ultimate CNH2oxidation.Since then,P5 can convert to P6 (m/z= 245)by F removal,and followed the aforementioned process,P6 can be oxidated to P7 (m/z= 217) and P8 (m/z= 204) by the removal of ―COO and ―C(CH3)2group,respectively63,64.In pathway II,CIP molecules are probably attacked by •OH for producing P9 (m/z= 348) by hydroxylation reaction.Subsequently,quinolone ring breakage of P9 forms to P10 (m/z= 320) through decarboxylation and hydroxylation reaction.Simultaneously,F atom is substituted by hydroxyl through hydroxylation reaction,which is conducive to P11 (m/z= 318) formation62.Ultimately,P7,P8 and P11 is oxidized and decomposed into small molecules until CO2and H2O.

Fig.10 (a) Mass spectra of CIP degradation process; (b) Daphnia Magna LC50 (48 h),(c) Fathead Minnow LD50 (96 h),(d) mutagenicity and (e) bioconcentration factor of CIP and their degradation intermediates;(f) in situ FTIR spectra of MO/BiOBr composites in the CO2 reduction process
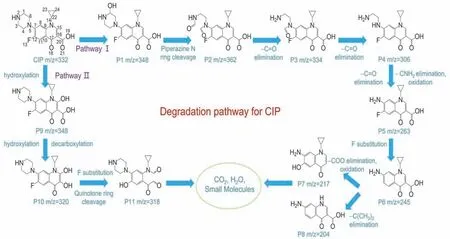
Fig.11 Possible CIP degradation pathway of MO/BiOBr composites.
For investigating the environmental friendliness of CIP removal process by MO/BiOBr composites,the true physiological toxicity of corresponding intermediates was predictedviaToxicity Estimation Software (T.E.S.T.) by calculating the Daphnia Magna lethal concentration 50% (LC50,48 h),Fathead Minnow lethal dose 50% (LD50,96 h),mutagenicity and bioaccumulation factor of CIP and various intermediates65,66.The Daphnia Magna LC50(48 h) for manifesting CIP acute toxicity is estimated as “toxic” level,which reckons as about 2.84 mg·L-1(Fig.10b).It is noteworthy that all degradation intermediates except P6 possess higher LC50numerical value than that of CIP,and the LC50value of P1,P2,P9,P10 and P11 is even estimated as “harmful”.Similarly,the Fathead Minnow LD50(96 h) numerical value of all degradation intermediates except P4,P5 and P9 is obviously higher than that of CIP (Fig.10c).The above calculation demonstrates that that photocatalytic CIP removal of MO/BiOBr composites is advantageous to reducing acute toxicity of CIP.CIP is judged to possess positively mutagenic (Mutagenicity = 0.62 > 0.5),the CIP oxidation process significantly diminishes the mutagenicity of various intermediates until “mutagenicity negative” excluding P1,P2,P9,and P10 (Fig.10d).Fig.10e demonstrated that the constructed photocatalytic system by MO/BiOBr composites achieves the reduction of bioaccumulation factor for most intermediates except P7,P9 and P10.The calculation and analysis indicate that the CIP oxidation procedure can partly achieve low toxicity and show a bright future for large-scale application.
The photocatalytic CO2reduction process of MO/BiOBr composites were investigated byinsituFTIR spectra.As demonstrated in Fig.10f,various intermediates can be distinguished during the CO2conversion process,including b-CO32-(1271 cm-1),m-CO32-(1318,1339,1361,1521 cm-1),p-CO32-(1395 cm-1),HCO3-(1420,1436,1488,1507,1650,1684 cm-1),HCOOH (1456 cm-1),COOH*(1541 cm-1),HCOO-(1698,1716 cm-1),and HCOO2-(1750,1771 cm-1)29,67–69,respectively.Wherein,the COOH*is regarded as vital intermediates for CO2to CO conversion.
4 Conclusions
Z-scheme MO/BiOBr heterojunction was successfully constructedviamechanically assisted ball-milling process.MO/BiOBr composites possess enhanced carries separation and migration performance by photoelectrochemical measurements and PL spectroscopy.Simultaneously,the Z-scheme heterojunction formed by MnO2and BiOBr possesses remarkable advantages as space separation of active centers,which achieved by the Mn3+/Mn4+redox couple and staggered energy band positions of MnO2and BiOBr.Accordingly,MO/BiOBr composites demonstrates superior photocatalytic CIP removal and CO2conversion performance.The CIP oxidation efficiency of MO/BiOBr composites is promoted to 77.3% at 60 min,which is 1.28-fold than that of BiOBr (60.2%).On the other hand,photocatalytic CO2reduction rate of MO/BiOBr composites (20.02 µmol·g-1·h-1) simultaneously elevated to 2.2-fold than that of BiOBr (9.08 µmol·g-1·h-1).LCMS andinsituFTIR spectra was measured for investigating the various intermediates generated in photocatalytic CIP removal and CO evolution process.Consequently,this manuscript proposes an efficient method for Z-scheme heterojunction construction for boosting the antibiotics oxidation and fuel synthesis driven by solar energy.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Holey Graphene for Sodium-Ion Battery Anode Material
- Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution
- Methylene Blue Incorporated Donor-Acceptor g-C3N4 Nanosheet Photocatalyst for H2 Production

