Methylene Blue Incorporated Donor-Acceptor g-C3N4 Nanosheet Photocatalyst for H2 Production
Fangxin Yin ,Pinquan Qin ,Jingsan Xu ,Shaowen Cao ,*
1 State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,Wuhan University of Technology,Wuhan 430070,China.
2 School of Science,Wuhan University of Technology,Wuhan 430070,China.
3 School of Chemistry and Physics,Queensland University of Technology,Brisbane,QLD 4000,Australia.
Abstract: Photocatalytic hydrogen production is a promising strategy for utilizing inexhaustible solar energy as a source of clean energy.Graphitic carbon nitride (g-C3N4) is a widely used photocatalytic material in photocatalytic hydrogen production because of its simple preparation process,suitable band structure,and high stability.However,the low charge carrier separation efficiency and small specific surface area of pristine g-C3N4 restrict its photocatalytic activity.It has been demonstrated that the construction of intramolecular donor-acceptor (D-A) systems and ultra-thin nanosheet structures are effective strategies for enhancing the photocatalytic activity of g-C3N4.Herein,an intramolecular D-A structured g-C3N4 nanosheet photocatalyst is synthesized through the thermal copolymerization of dicyandiamide and methylene blue (MB),followed by thermal exfoliation.X-ray diffraction,Fourier transform infrared spectrometry,solid-state 13C nuclear magnetic resonance,and X-ray photoelectron spectroscopy analyses reveal that MB is successfully incorporated into the g-C3N4 framework and well retained after thermal exfoliation.The resulting D-A system induces intramolecular charge transfer from the donor units (MB segment) to the acceptor units (tri-s-triazine rings) and extends the absorption edge to approximately 500 nm.The ultra-thin nanosheet structure produced by thermal exfoliation shortens the charge transfer distance from the interior to the surface of g-C3N4 and reduces the charge transfer resistance,which increases the charge carrier separation efficiency.Furthermore,the introduction of MB generates a flaky structure during copolymerization,which promotes thermal exfoliation and results in a remarkably increased specific surface area.The transient photocurrent response,electrochemical impedance spectra,and time-resolved photoluminescence decay spectra reveal that the charge transfer and separation of g-C3N4 are further promoted by integrating the intramolecular DA system and ultra-thin nanosheet structure.Density functional theory calculations further demonstrate that MB donates electrons to tri-s-triazine rings (electron acceptor).Moreover,the highest occupied molecular orbit of D-A structured g-C3N4 is mostly distributed around the MB segment,while the lowest unoccupied molecular orbit is distributed around tri-s-triazine rings,resulting in spatially separated photogenerated electron-hole pairs.Through integrating the intramolecular D-A system and ultra-thin nanosheet structure,the obtained photocatalyst exhibits enhanced charge carrier separation,an extended absorption edge,and enlarged specific surface area.As a consequence,the D-A structured g-C3N4 nanosheet shows a considerably improved photocatalytic hydrogen production activity (2275.6 μmol·h-1·g-1),which is 5.30,2.60,and 1.30 times that of bulk g-C3N4,D-A structured bulk g-C3N4,and g-C3N4 nanosheet,respectively.This work offers a valuable strategy for developing D-A-modified photocatalytic materials for solar energy conversion.
Key Words: Donor-acceptor; Methylene blue; g-C3N4 nanosheet; Photocatalytic hydrogen production
1 Introduction
Over the past few decades,the growing energy consumption and alarming environmental concern have become a great challenge for sustainable development1–3.Due to solar energy being inexhaustible,converting it into chemical energy by photocatalysis is a promising approach to address these issues4.Hydrogen (H2) energy is considered as an ideal clean energy owing to its carbon-free and high energy density5.Since Fujishima and Honda reported a photoelectrochemical hydrogen production process from water in 19726,various semiconductors,such as TiO27,ZnO8,CdS9,g-C3N410,and WO311have been used as photocatalysts for hydrogen production.Among these photocatalysts,graphitic carbon nitride (g-C3N4) grabs considerable attention owing to its simple preparation process,suitable band structure,and excellent stability12.However,pristine g-C3N4does not have excellent photocatalytic performance owing to the low separation efficiency of photogenerated charge carriers,low specific surface area,and narrow absorption range13.Given these issues,multifarious methods have been developed to enhance the photocatalytic activity of g-C3N4,such as engineering nanostructures14,doping with elements15,constructing heterojunctions16,and copolymerizing with organic compounds17.Among these strategies,the construction of heterojunctions can efficiently facilitate the photogenerated charge carriers’ separation by spatially separating electrons and holes.Nevertheless,the effective separation only occurs around the interface,and the carriers in other parts tend to recombine.
Recently,constructing intramolecular donor-acceptor (D-A)system in the g-C3N4polymer framework has been proven to improve charge carriers’ separation efficiency and enhance the light absorption capacity18.Under irradiation,these asymmetric structures at the molecular level would induce polarization,which can induce intramolecular charge transfer (ICT) from donor units to acceptor units,accompanied by the efficient separation of photogenerated charge carriers19,20.Meanwhile,there will be a new ICT absorption at a longer wavelength,leading to the expansion of light absorption21.For instance,Cheetal.22reported that copolymerizing melamine-formaldehyde resin with urea could construct intramolecular g-C3N4-based DA conjugated copolymers in which the enhanced activity originates from the enlarged light absorption and the accelerated separation of photogenerated electron-hole pairs.Fanetal.23designed a series of donor-acceptor g-C3N4photocatalysts by incorporating fundamental aromatic rings into the g-C3N4frameworks,and the obtained copolymer showed remarkably enhanced photocatalytic hydrogen evolution due to the ICT.Moreover,thermal exfoliating g-C3N4into ultra-thin nanosheets with large specific surface area is an effective and environmentally friendly method to enhance photocatalytic performance24–28.The thinner nanosheet structure can shorten the charge migration distance from the catalyst interior to the surface and reduce charge-transfer resistance,efficiently promoting the separation of charge carriers29–33.Thus,integrating the D-A system with ultra-thin nanosheet structure to synergistically improve the separation of charge carriers is a promising strategy.
Methylene blue (MB),a common organic dye,is a derivative of quaternary ammonium salts that can be easily decomposed at high temperature to form imine groups and other reactive functional groups,which are beneficial for organic molecular modification.Herein,viathermal copolymerization of dicyandiamide and MB followed by a thermal exfoliation step,an intramolecular D-A structured g-C3N4nanosheet photocatalyst is successfully prepared.The D-A structured g-C3N4nanosheet possesses enhanced separation ability of charge carriers,strong visible-light absorption,and large specific surface area at the same time.As expected,the D-A structured g-C3N4nanosheet shows the highest H2evolution rate (2275.6 μmol·h-1·g-1),which is 5.30,2.60,and 1.30 times that of bulk g-C3N4,D-A structured bulk g-C3N4,and g-C3N4nanosheet,respectively.It is noteworthy that the introduction of MB not only successfully constructs the D-A system in g-C3N4but also promotes the thermal exfoliation process due to the flaky structure generated by copolymerization.The specific surface area of the D-A structured g-C3N4nanosheet photocatalyst reaches 60.8 m2·g-1,which is 1.97 times larger than g-C3N4nanosheet.In addition,the apparent quantum efficiency (AQE)reaches 1.1% at 420 nm.
2 Experimental section
Synthesis of CN and CN-MBx.Typically,3 g of dicyandiamide and a certain amount of MB were adequately blended and well ground.Following that,the mixture was loaded into a crucible with a lid and the crucible was transferred to a muffle furnace.The calcination process took place at 550 °C for 4 h,ramping up at a rate of 5 °C·min-1.Finally,the synthesized samples were denoted as CN-MBx(x= 5,10,15),in whichx(mg) indicates the mass of the added MB.For the pristine g-C3N4(denoted as CN),the synthesis procedure was the same as that of CN-MBx,except that MB was not added.
Synthesis of TCN and TCN-MBx.The TCN-MBxwas fabricated by air-assisted thermal exfoliation,in which the obtained CN-MBx(500 mg) was transferred to an open crucible and annealed at 550 °C for 2 h in air with an increasing rate of 5 °C·min-1.The obtained catalysts were denoted as TCN-MBx.For the TCN,the synthesis procedure was the same as that of TCN-MBx,except that CN replaced CN-MBx.
3 Results and discussion
3.1 Morphology and structure characterizations
The fabrication process and possible structure of TCN-MBxphotocatalyst are illustrated in Scheme 1.The possible reaction path for incorporating MB into the g-C3N4frameworks in the thermal copolymerization process is proposed in Fig.S1.As shown in Fig.1a,the field emission scanning electron microscope (FESEM) image of CN shows a thick bulk structure.After dicyandiamide copolymerization with MB and thermal exfoliation,the obtained TCN-MB10catalyst changes to a thin nanosheet structure with numerous pores (Fig.1b).The processes of morphology changes are further analyzed by highmagnification FESEM images of CN,CN-MB10,TCN,TCNMB10in Fig.S2.As can be seen,when MB is copolymerized with dicyandiamide,the main part of the catalyst remains the bulk structure but the surface changes to an apparent flaky structure.After thermal exfoliation,the bulk structure further changes to the ultra-thin nanosheet structure,and the porous structure is formed.Such structure evolution driven by thermal exfoliation is further demonstrated by transmission electron microscope (TEM) observation,showing the change from the bulk structure of CN-MB10(Figs.1c and S3a) to the ultra-thin nanosheet structure of TCN-MB10(Figs.1d and S3b).
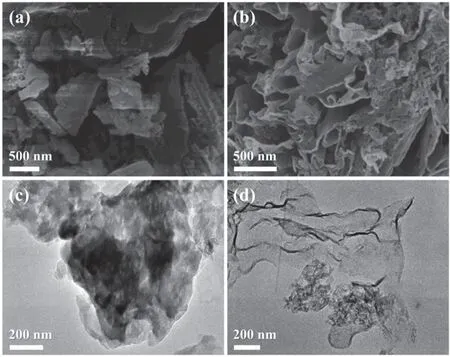
Fig.1 FESEM images of (a) CN and (b) TCN-MB10.TEM images of (c) CN-MB10 and (d) TCN-MB10.

Scheme 1 Schematic illustration of the fabrication of porous TCN-MBx D-A conjugated polymers.
As depicted in Brunauer-Emmett-Teller (BET) surface analysis (Fig.S4 and Table S1),the four samples show type-IV isotherm and H3hysteresis loop,reflecting the existence of slitlike mesopores34.CN shows a lowSBETvalue of 8.7 m2·g-1,mainly due to the strong layer stacking evidenced by FESEM.CN-MB10shows a higherSBETvalue of 9.9 m2·g-1than that of CN,which may be ascribed to the change of catalyst surface from the bulk to flaky structure.Compared with CN,TCN exhibits an increasedSBETvalue of 30.8 m2·g-1,which is ascribed to the reduced layer stacking and the formation of porous structure caused by thermal exfoliation.It is noteworthy that TCN-MB10shows anSBETvalue of 60.8 m2·g-1,which is 1.97 times that of TCN.This change may be attributed to the surface flake structure generated by the thermal copolymerization of dicyandiamide and MB,which is conducive to the entry of air into g-C3N4,thus promoting the thermal exfoliation process and further improving the specific surface area.The large surface area and the porous structure can provide more reaction active sites and diffusion channels,which are beneficial to photocatalytic hydrogen production.
As depicted in X-ray diffraction (XRD) (Fig.2a),all samples display similar XRD patterns with two prominent diffraction peaks,revealing that the introduction of MB and the thermal exfoliation do not change the main structure of the g-C3N4framework.The peak at 13.0° (100) originates from the periodic tri-s-triazine unit in-plane,and the peak at 27.3° (002) represents the stacking of the conjugated aromatic system of g-C3N435.Compared with CN,the (002) peaks of CN-MBxslightly shift to a smaller angle,indicating the enlarged interlayer distance after MB incorporation.Conversely,the (002) peaks of TCN and TCN-MB10shift to a larger angle,indicating that the heating during the thermal exfoliation process results in denser packing and thus shortens the interlayer distance36.Note that the (002)peaks of TCN-MB10and TCN become much weaker than those of CN-MB10and CN,indicating that the thermal exfoliation significantly reduces the interlayer stacking of g-C3N4,corresponding to the change of bulk structure into nanosheet structure evidenced by FESEM and TEM.Moreover,the thermogravimetry analysis (TGA) in Fig.S5 shows that the asprepared photocatalysts have high thermal stability (over 400 °C) after the incorporation of MB and thermal exfoliation.
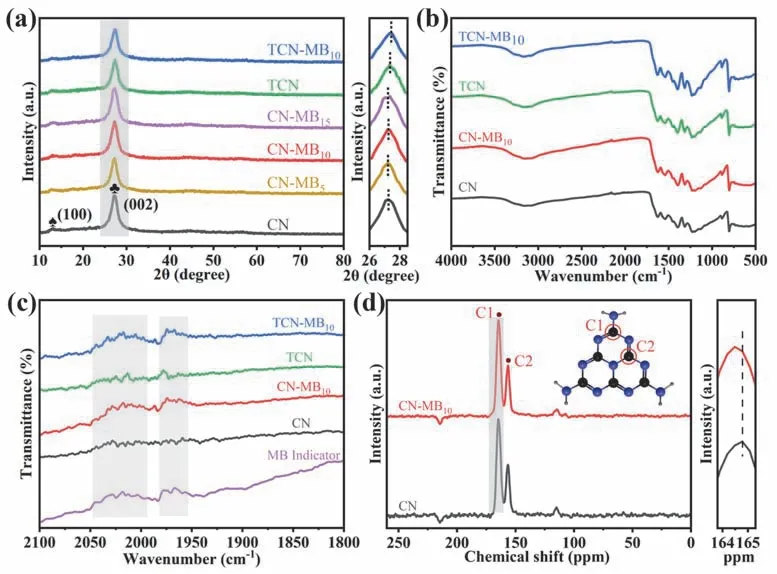
Fig.2 (a) XRD patterns and (b,c) FTIR spectra of CN,CN-MB10,TCN,and TCN-MB10; (d) 13C NMR spectra of CN and CN-MB10.
Meanwhile,Fourier transform infrared (FTIR) spectra of CN,CN-MB10,TCN,and TCN-MB10in Fig.2b show similar characteristic peaks,which further proves that the chemical structure of g-C3N4is well retained.The peak at 810,1300–1700,and 3000–3500 cm-1can be assigned to heptazine rings,C―N heterocycles,and N―H group,respectively37,38.In the magnified FTIR spectra (Fig.2c),MB,CN-MB10,and TCNMB10exhibit two small peaks at around 1970 and 2020 cm-1which cannot be observed in the spectra of CN and TCN.This result indicates that these two peaks in CN-MB10and TCN-MB10originate from MB,indicating that the structure of MB molecule has been incorporated into the g-C3N4framework.Solid-state13C nuclear magnetic resonance (NMR) spectra are measured to further investigate the structure of CN-MBxphotocatalysts.As depicted in Fig.2d,the C1 peak location of CN-MB10is shifted in comparison with CN,which further reveals that the structure of MB molecule has been incorporated into the g-C3N4framework39,40.In addition,the C2 peak location of CN-MB10is unchanged compared with CN,indicating that the incorporation site of MB is the C1 of tri-s-triazine rings.
As depicted in X-ray photoelectron spectroscopy (XPS) (Fig.3a),CN,CN-MB10,TCN,and TCN-MB10primarily consist of C,N,and O elements.As depicted in the high-resolution C 1sspectra (Fig.3b),the obtained samples display two signals at around 288.06 and 284.80 eV,which are attributed to N―C=N and C―C,respectively41–43.The N―C=N signals of CNMB10,TCN,and TCN-MB10remain nearly unchanged,revealing the stable molecular structure after MB modification and thermal exfoliation.The N 1sXPS spectra (Fig.3c) are deconvolved into three peaks at around 398.57,399.94,and 401.14 eV,corresponding to C―N=C,N―(C)3,and ―NH,respectively44.Compared with CN and TCN,the C―N=C signals and N―(C)3signals of CN-MB10and TCN-MB10shift to a lower location.This shift should be attributed to the increase of electron density,indicating that MB acts as the electron donor to afford electrons to tri-s-triazine rings (electron acceptor) in CN-MB10.Noticeably,the elemental analysis in Table S2 and the high-resolution S 2pspectra in Fig.3d show the increased C/N atomic ratio from CN (0.81) to CN-MB10(0.83) and the existence of an S―C bond,indicating that MB is successfully incorporated into the g-C3N4framework and well retained after the thermal exfoliation.The C/N atomic ratio decreases from CN-MB10(0.83) to TCN-MB10(0.80),which is contrary to the increase from CN (0.81) to TCN (0.82),indicating the possible presence of carbon vacancies.The S―O signal also appears in the high-resolution S 2pspectra,which may originate from the oxidation of MB at high temperature.
3.2 Photoelectrochemical properties
The optical properties of the obtained catalysts are investigated by UV-Vis diffuse reflectance spectra (DRS).In Fig.S6,CN-MBxshows increasing light absorption with rising MB content compared with CN,which is beneficial to utilize solar energy.As depicted in Fig.4a,the absorption edge of CN,CN-MB10,TCN,and TCN-MB10are 460,497,451,and 488 nm.Despite the blue shift of the intrinsic absorption edge caused by quantum confinement effect after the thermal exfoliation,the optical absorption capacity of TCN-MB10is still higher than that of CN,suggesting that MB molecule in the g-C3N4framework is well retained after the thermal exfoliation.Notably,in the visible light region,the UV-Vis DRS of CN-MBxand TCN-MB10show apparent “shoulder”- and “tail”-like features,which could be attributed to an extra electron-transition mode originating from ICT23.Correspondingly,the band gap of CN,CN-MB10,TCN,and TCN-MB10are calculated to be 2.47,2.24,2.57,and 2.25 eV according to the plots of (αhν)1/2vs.(hν) in Fig.4b.In addition,as depicted in Fig.4c,the Mott-Schottky plots of the four samples show positive slopes,indicating that they are typical n-type semiconductors45.Correspondingly,the flat-band potentials (Efb) of CN,CN-MB10,TCN,and TCN-MB10are-1.50,-1.53,-1.58,and -1.60 V (vs.Ag/AgCl),which can be converted to -1.30,-1.33,-1.38,and -1.40 V (vs.SHE),respectively.The conduction band (CB) of n-type semiconductor is generally close to theEfb.Therefore,the CB of CN,CN-MB10,TCN,and TCN-MB10are -1.30,-1.33,-1.38,and -1.40 V.According to these analyses,the band alignments of the four samples are depicted in Fig.4d.TCN-MB10has a narrower band gap and a more negative CB than that of CN,representing a broader light absorption and a stronger thermodynamic reduction ability of the photogenerated electrons,which is conducive to photocatalytic hydrogen production.

Fig.4 (a) UV-Vis DRS,(b) plots of (αhν)1/2 vs.(hν),(c) Mott-Schottky plots,and (d) band alignments of CN,CN-MB10,TCN,and TCN-MB10.
The photoluminescence (PL) spectra are measured to investigate the separation of charge carriers.As depicted in Figs.5a and S7,CN and TCN show a strong emission peak due to the high recombination rate of charge carriers.However,the PL peak intensity decreases sharply after copolymerization with MB,indicating that constructing an intramolecular D-A system in g-C3N4can efficiently promote the photogenerated charge carriers’ separation.The electrochemical impedance spectra(EIS) and transient photocurrent response (PCR) are employed to evaluate charge carriers’ properties further.As depicted in Fig.5b,CN-MB10,TCN,and TCN-MB10have a higher photocurrent intensity than that of CN,indicating a more efficient charge separation31,46.Note that TCN-MB10has the highest photocurrent intensity of the four samples,which means the charge separation is further improved by integrating the intramolecular D-A system and ultra-thin nanosheet structure.Meanwhile,in the samples’ EIS plots (Fig.5c),TCN-MB10displays the smallest arc radius,corresponding to the lowest charge-transfer resistance47–49.
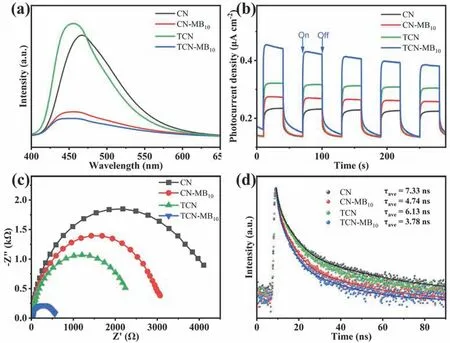
Fig.5 (a) PL spectra,(b) PCR,(c) EIS,and (d) TRPL decay spectra of CN,CN-MB10,TCN,and TCN-MB10.
In addition,the time-resolved photoluminescence (TRPL)decay spectra and triple-exponential fitted lifetimes are further investigated to evaluate the lifetimes of charge carriers.As depicted in Fig.5d and Table S3,the average lifetime of CNMB10(4.74 ns),TCN (6.13 ns),and TCN-MB10(3.78 ns) is shorter than that of CN (7.33 ns),which is ascribed to the more rapid and effective charge transfer of CN-MB10,TCN,and TCNMB1050.The enhanced charge transfer of the MB incorporated g-C3N4and the thermal exfoliated g-C3N4is probably ascribed to the ICT process induced by the D-A structure and the reduced charge-transfer resistance caused by the ultra-thin nanosheet structure,respectively.Noticeably,TCN-MB10has the shortest average lifetime,demonstrating that the intramolecular D-A system and ultra-thin nanosheet structure can synergistically promote charge carriers' transfer and separation.
As seen in Fig.S8,electron paramagnetic resonance (EPR) of all four samples show a Lorentzian line nearg= 2.0035,corresponding to the unpaired electrons on thesp2C atom of the aromatic rings51.CN-MB10and TCN have a higher EPR signal intensity than that of CN,illustrating that a higher concentration of unpaired electrons is formed after copolymerization with MB or thermal exfoliation,which is conducive to photocatalytic hydrogen production52.
3.3 Photocatalytic activity and stability
The as-prepared photocatalysts’ activity is assessed by photocatalytic hydrogen evolution reaction (HER) with 10%(volume fraction) triethanolamine (TEOA) as an electron sacrificial agent and 3% (w) Pt as a co-catalyst.In Fig.6a,CN exhibits a low HER rate of 428.7 μmol·h-1·g-1owing to its low charge carriers’ separation efficiency and poor light adsorption.All CN-MBxsamples exhibit higher photocatalytic HER rates than that of CN.Moreover,CN-MB10reaches a high HER rate of 873.1 μmol·h-1·g-1,which is over 2.03 times higher than that of CN.Nevertheless,a further rise in the amount of MB results in a decrease in photocatalytic activity,which can probably be attributed to the faster recombination of charge carriers caused by excess defects.The enhancement of the photocatalytic performance of CN-MBxoriginates from the enhanced charge separation and light absorption caused by the construction of the intramolecular D-A system in g-C3N4.Meanwhile,TCN also exhibits an increased photocatalytic HER rate of 1750.3 μmol·h-1·g-1,which is attributed to the more efficient charge separation and the larger specific surface area.TCN-MB10exhibits the highest HER rate of 2275.6 μmol·h-1·g-1,which is over 5.30,2.60,and 1.30 times that of CN,CN-MB10,and TCN.The remarkably enhanced HER rate of TCN-MB10can be ascribed to the more effective charge separation,the broader light absorption,and the larger specific surface area.However,it can be found that the D-A structured g-C3N4nanosheet (TCNMB10) does not have such a remarkable enhancement of the HER rate compared to pure g-C3N4nanosheet (TCN) after the thermal exfoliation.These results may be attributed to that the introduction of MB causes the immoderate thermal etching and forms excess carbon vacancies in agreement with the XPS analysis,which become the recombination center of photogenerated carriers and are prejudiced to the HER.
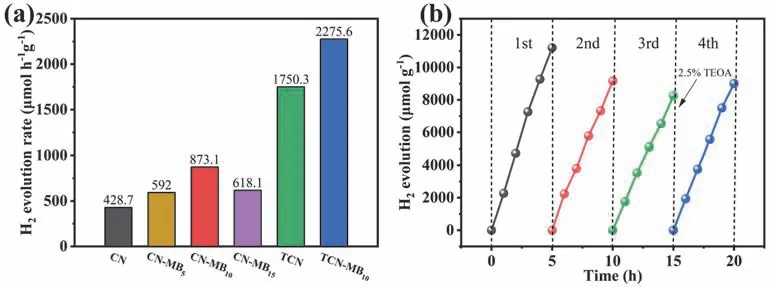
Fig.6 (a) Hydrogen evolution rates of CN,CN-MB,TCN,and TCN-MB10.(b) Stability test of TCN-MB10 photocatalyst.
To evaluate the hydrogen evolution performance in different water matrices,we configure a 3.5% (w,mass fraction) NaCl aqueous solution to simulate seawater.As can be seen in Fig.S9,the HER rate of TCN-MB10in the simulated seawater is 1175.1 μmol·h-1·g-1,indicating that the D-A structured g-C3N4nanosheet photocatalyst has good stability in different water matrices.No H2is detected in the absence of Pt deposits and dark conditions on TCN-MB10.The AQE of TCN-MB10photocatalyst reaches 1.1% at 420 nm (Table S4).As shown in the stability test of Fig.6b,the HER rate of TCN-MB10decreases slightly in the second and third rounds but recovers rapidly after the re-addition of 2.5% (volume fraction) TEOA,indicating that the reduced HER rate is caused by the decrease of sacrificial agent concentration.
3.4 DFT calculations and possible photocatalytic mechanism
To further disclose the relationship between the enhanced photocatalytic performance and the D-A structure,the density functional theory (DFT) calculations with ViennaAb-initioSimulation Package (VASP) are conducted to investigate the electronic band structures of CN and CN-MB1053.According to the Bader charge analysis shown in Fig.S10,the MB segment(electron donor) tends to provide electrons to tri-s-triazine rings(electron acceptor) in CN-MB10,resulting in a transfer charge of 0.229e.
The calculated partial density of state (PDOS) and total density of state (TDOS) of CN and CN-MB10are plotted in Fig.7a,b.Note that the calculated band gap of CN-MB10(1.95 eV) is also narrower than that of CN (2.61 eV),which is in agreement with the UV-Vis DRS analysis.In addition,the spatial distribution of charge density over the HOMO and LUMO is shown in Fig.7c.For the pristine g-C3N4,the HOMO is primarily distributed around the combination of nitrogenpzorbits,while the LUMO is mostly localized at the C―N bond orbits36.It can be predicted that the uniform distribution of HOMO and LUMO in the pure g-C3N4caused by the high symmetry of the planar structure will result in the low charge carrier separation.However,the electron cloud of CN-MB10has been redistributed after D-A modification,and the HOMO is mostly concentrated on the MB segment.In contrast,the LUMO is mostly concentrated on tri-s-triazine rings.Under light irradiation,the electrons on the HOMO of the donor unit are excited and then driven to the LUMO of the acceptor unit,while the holes are left on the HOMO of the donor unit,which is the induced ICT process.Thus,the D-A structure eventually leads to a spatial separation of photogenerated electron-hole pairs and achieves improved photocatalytic activity.

Fig.7 (a,b) PDOS and TDOS profiles of CN and CN-MB10.(c) The spatial distribution of charge density over the HOMO and LUMO of CN and CN-MB10.
Based on all experimental results and DFT calculations,the possible mechanism for the significantly improved photocatalytic activity of TCN-MB10is suggested and depicted in Fig.8.Under light irradiation,the photogenerated electrons and holes of pristine g-C3N4are on close atoms,leading to the high recombination rate.However,after constructing the D-A system in the g-C3N4framework,the induced ICT leads to the spatial separation of photogenerated electron-hole pairs and the light absorption is extended.Meanwhile,after thermally exfoliating g-C3N4from bulk into ultra-thin nanosheets,the charge migration distance from the interior to the surface of photocatalyst is shortened and the charge transfer resistance is reduced,which lead to the enhanced separation of charge carriers.In addition,the introduction of MB promotes the thermal exfoliation process,remarkably increasing the specific surface area.Thus,the enhanced charge carriers’ separation,the extended light absorption,and the enlarged specific surface area ultimately lead to a significant increase of photocatalytic activity.

Fig.8 Schematic illustration of the proposed possible mechanism of the improved photocatalytic activity.
4 Conclusions
In summary,we have successfully prepared the D-A structured g-C3N4nanosheet photocatalyst through thermal copolymerization of dicyandiamide and MB followed by thermal exfoliation.Based on the experimental results,the obtained TCN-MB10photocatalyst has the promoted separation of charge carriers,extended light absorption,and enlarged specific surface area owing to the donor-acceptor system and ultra-thin nanosheet structure.Consequently,TCN-MB10shows the highest photocatalytic hydrogen production activity (2275.6 μmol·h-1·g-1),which is over 5.30,2.60,and 1.30 times that of CN,CN-MB10,and TCN,respectively.This work provides a valuable idea for designing D-A-modified photocatalytic materials.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- 欢迎订阅《大学化学》
- 欢迎订阅《物理化学学报》
- Construction of Z-Scheme MnO2/BiOBr Heterojunction for Photocatalytic Ciprofloxacin Removal and CO2 Reduction
- Holey Graphene for Sodium-Ion Battery Anode Material
- Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO
- Constructing a CeO2/ZnxCd1-xIn2S4 S-Scheme Hollow Heterostructure for Efficient Photocatalytic H2 Evolution

